Abstract
Objectives
Depot medroxyprogesterone acetate (Depo-Provera) is the most commonly used injectable hormone contraceptive in Sub-Sahara Africa where HIV incidence is high. We determined the impact of Depo-Provera on cervical immune cells and mediators in healthy women.
Methods
In this longitudinal study, vaginal, endocervical, and rectal swabs were collected at baseline (visit 1), one month (visit 2), and three months (visit 3) after Depo-Provera injection. Cervical cells were collected by cytobrush and immune markers on cervical CD4+ T cells were analyzed by multicolor flow cytometry at 3 different visits. The levels of immune mediators in cytobrush supernatants as well as vaginal, cervical, and rectal secretions from swabs were analyzed by multiplex assays and ELISA.
Results
Compared to baseline levels we found a significant increase in the frequency of cervical CCR5+CD4+ T cells and a significant decrease in the frequency of cervical central memory CD4+ T cells. Depo-Provera treatment had little effect on expression of immune mediators in rectal mucosa but significantly suppressed numerous immune mediators at cervicovaginal mucosa. Levels of MCP-1, G-CSF, IL-6, IL-10, GM-CSF, and IP-10 were significantly decreased in both vaginal and cervical secretions after Depo-Provera injection. In cervical samples collected by cytobrush, we found reduced levels of 22 of 25 immune mediators after Depo-Provera injection. Changes in immune mediators differed between vaginal and cervical mucosa, demonstrating compartment-specific responses.
Conclusion
Depo-Provera altered immune profiles of cervical CD4+ T cells and suppressed host immune response at cervicovaginal mucosa, suggesting its likely effect on transmission of sexually transmitted infections including HIV.
Introduction
Depo-Provera (depot medroxyprogesterone acetate) has been associated with increased risks of HIV acquisition (reviewed in [1, 2]). Findings concerning the effect of Depo-Provera on the cervicovaginal immune cells and milieu have been inconsistent (reviewed in [2]). Increased numbers of cervical CCR5+CD4+ T cells were found in Depo-Provera users in a cross-sectional study [3], but this finding conflicted with another cross-sectional study indicating significant changes in CCR5+CD4+ T cells in the endometrium but not the cervix [4]. Chandra et al showed an overall increase in all immune cells from vaginal tissues 12 weeks after Depo-Provera injection [5]. However, there are no reports investigating the impact of Depo-Provera on expression of HIV preference markers on cervical CD4+ T cells longitudinally.
The effect of Depo-Provera on the level of immune mediators in the human female reproductive tract (FRT) has not been consistent [2]. Among cross-sectional studies, some showed an increase in proinflammatory cytokines including MIP-1α, MIP-1β, IL-6, IL-8, IP-10, and RANTES in Depo-Provera users [6], whereas others indicated a decrease in IFNα, CXCL10, MCP-1, and G-CSF [7]. Higher levels of RANTES and lower levels of antimicrobial peptides such as HBD2 and SLPI are found in Depo-Provera users compared to non-users [8], and the presence of sexually transmitted infections (STIs) enhance these alterations in immune profiles [9]. A longitudinal study with a small cohort of 15 women with self-reported contraceptive methods showed a sustained decline in IL-6, IL-8, and IL-1RA in vaginal secretions one year following Depo-Provera use [10].
To address the immediate impact of Depo-Provera on immune responses at different compartments of lower FRT and rectal mucosa, we collected cervical cells and vaginal, endocervical, and rectal secretions from women before (visit 1), one month following (visit 2), and three months following (visit 3) Depo-Provera injection. The three-month schedule was chosen because serum Depo-Provera concentrations are known to decrease over time and return to near baseline three months after Depo-Provera injection [11, 12]. We examined changes in immunological markers associated with preferential HIV infection of cervical CD4+ T cells, including integrin α4β7, CCR5 (HIV co-receptor), and CD38 (activation), and characterized cervical CD4+ T cell subsets in addition to analyzing the mucosal immune mediators.
Methods
Study design and sample collection
Participants were women attending Rutgers New Jersey Medical School (NJMS) clinics located in Newark, New Jersey, which offer a broad range of women’s health related services including general OB/GYN, high risk obstetrics, surgery, colposcopy, nutrition, social service, and family planning. Women who sought birth control were presented with all birth control options. Those who chose Depo-Provera and met the primary screening criteria were invited to participate in this study. Those who provided informed consent were recruited and compensated. Enrollment eligibility criteria are detailed in our previous studies [13, 14]. Briefly, healthy, non-pregnant women aged 18–35 with no HIV or other sexually transmitted infections, no immunosuppressive conditions, no use of hormonal contraception, and reporting no sexual intercourse for 3 days before the enrollment visit were deemed eligible. In total, 39 of 67 recruited healthy subjects who met the eligibility criteria were included in this study. Among these, 27 participants returned for all visits, 6 participants returned only for visit 2, and 6 participants returned only for visit 3. All subjects were Black (n=24) or Hispanic (n=15). The median age was 26 (interquartile range (IQR), 21.5–30.5).
None of the participants had used any form of hormone contraception during the 10 months prior to recruitment. Cervical cell samples were collected before Depo-Provera use, one month and 3 months post Depo-Provera injection by sweeping the endocervix with a Medscand Cytobrush Plus GT (Cooper Surgical), which was immediately immersed in 3 mL of RPMI-1640 without serum as described previously [15]. Cervical cells were collected and analyzed by flow cytometry as we previously described [13, 16]. Vaginal, endocervical, and rectal swabs were collected using sterile HydraFlock swabs (Puritan). Immune mediators from the swabs were eluted in 0.7 mL of PBS. Supernatants from cytobrush and swabs were aliquoted and stored at −80°C. Immune mediators were analyzed by multiplex assay (EMD Millipore) or ELISA (RANTES, Sigma-Aldrich).
Statistical analysis
Wilcoxon matched-pairs signed rank test was used to compare values for each subject between study visits (GraphPad Software, Inc.); p<0.05 was considered statistically significant.
Results
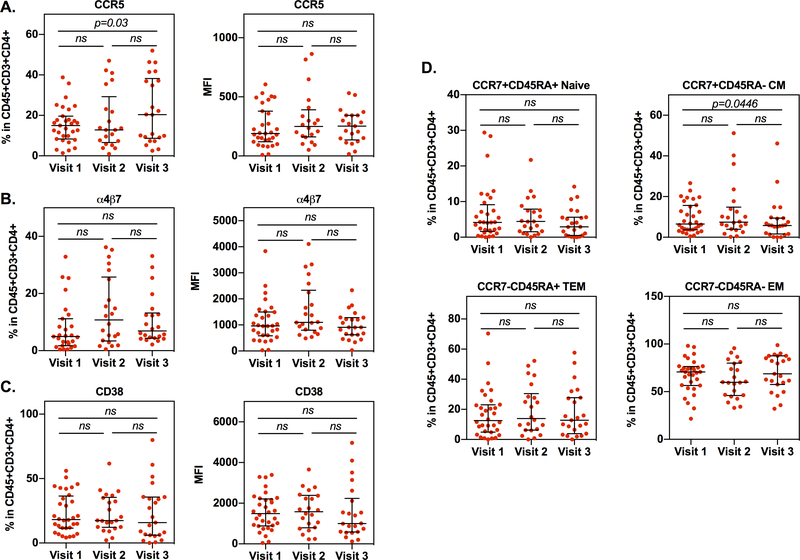
Analysis of immune markers on cervical cells revealed a significant increase in the frequency of CCR5+CD4+ T cells between visit 1 and visit 3 (Figure 1A). The mean fluorescence intensity (MFI) of CCR5 increased from visit 1 to visit 2 and visit 3, although this change did not reach significance. The frequency and expression level of cervical CD4+ T cells expressing integrin α4β7 increased slightly, although not significantly, from visit 1 to visit 2 (Figure 1B). The frequency and expression of integrin α4β7+CD4+ T cells at visits 1 and 3 were comparable. The frequency and expression level of cervical CD4+ T cells expressing the activation marker CD38 remained unchanged (Figure 1C). Analysis of cervical CD4+ T cell subsets including naïve (CCR7+CD45RA+), central memory (CM) (CCR7+CD45RA-), effector memory (EM) (CCR7-CD45R-), and terminal effector memory (TEM) (CCR7-CD45RA+) showed a significant decrease in the frequency of CM CD4+ cells at visit 3 compared to visit 1 (Figure 1D).
Figure 1: Depo-Provera administration alters expression of cell surface markers associated with HIV susceptibility on cervical cells.
Cervical cells were collected from healthy women before and after treatment with Depo-Provera. Live cells (Zombie UV fixable viability kit, BioLegend) were gated and lymphocytes were selected per their forward-scatter verses side-scatter light-scattering properties. CD4+ T cells were identified as CD3+ and CD4+ positive cells within the CD45+ population. Expression of integrin α4β7, CCR5, CD38, CCR7, and CD45RA on CD4+ T cells were further analyzed. Cell surface expression levels of integrin α4β7 (A), HIV co-receptor CCR5 (B), activation marker CD38 (C), and T cell subsets (D) were analyzed. The percentage positive and MFI of cells within the CD45+CD3+CD4+ T population were measured by flow cytometry. Wilcoxon matched-pairs signed rank test was used to compare values from before Depo-Provera (visit 1), one month after Depo-Provera (visit 2), and three months after Depo-Provera (visit 3). Each dot represents an individual donor (median, IQR). p≤0.05 was considered significant; ns, not significant.
Determination of the levels of immune mediators in supernatants of the cervical specimens collected by cytobrush as well as endocervical and vaginal secretions from swabs indicated that Depo-Provera injection led to general immune suppression in the cervicovaginal mucosa (Table 1). For example, in the cervical supernatants from cytobrushes, 22 analyte levels from visit 2 and 19 from visit 3 were significantly decreased compared to visit 1. The levels of multiple cytokines and chemokines in endocervical and vaginal secretions were also reduced although the immune suppressive effect of Depo-Provera administration was less pronounced. Pairwise comparisons among different visits showed that the levels of IL-1β, IL-4, IL-6, IL-7, IL-8, IL-10, IL-12p40, IL-12p70, IL-15, IL-17, TNFα, MCP-1, G-CSF, GM-CSF, MIP-1α, MIP-1β, IP-10, IFNα2, IFNγ, Eotaxin, VEGF, and EGF were significantly decreased from visit 1 to visit 2. The significant decrease in these immune mediators was sustained at visit 3 except for IL-12p40, IL-12p70, IP-10, IFNγ, Eotaxin, and EGF. The level of IL-1α was decreased at visit 2 but then increased significantly from visit 2 to visit 3. The level of RANTES was increased, although not significantly, from visit 1 to visit 2, but was significantly decreased from visit 2 to visit 3. In endocervical secretions, the G-CSF level was significantly decreased from visit 1 to visit 2, whereas IL-17 was significantly increased. Similar to the results from the cytobrush supernatants, there was a non-significant increase in RANTES at visit 2 compared to visit 1 but a significant decrease from visit 2 to visit 3. IL-7 was increased at visit 2 and then decreased at visit 3, but only changes between visit 2 and visit 3 were significant. The levels of GM-CSF, MIP-1α, and IL-15 were significantly decreased from visit 1 to visit 3. In vaginal secretions, the levels of MCP-1, G-CSF, and IP-10 were significantly decreased from visit 1 to visit 2; there was a significant reduction of IL-6, IL-7, IL-10, MCP-1, G-CSF, and GM-CSF from visit 1 to visit 3. Interestingly, EGF levels were significantly increased at visit 3 compared to visit 1 or visit 2 in vaginal secretions. The effect of Depo-Provera administration was less pronounced in rectal secretions (Supplementary Table 1). The IL-10 level was significantly decreased from visit 1 to visit 2, whereas the level of IP-10 was significantly decreased from visit 1 to visit 3. There was a significant decrease in the MIP-1β level from visit 2 to visit 3 in rectal secretions.
Table 1.
The levels of immune mediators in cytobrush supernatants, endocervical and vaginal secretions from women before (visit 1), one month (visit 2) and 3 months (visit 3) after Depo-Provera treatment.
| Cytobrush | |||||||||
| Mediator pg/mL | Visit 1 | Visit 2 | Visit 3 | P Value | |||||
| Median | IQR | Median | IQR | Median | IQR | V1 vs V2 | V2 vs V3 | V1 vs V3 | |
| IL-1α | 66.49 | (29.24–140.72) | 19.8 | (9.09–82.53) | 41.45 | (17.74–73.45) | 0.0525 | 0.0266 | 0.0329 |
| IL-1β | 12.925 | (3.69–30.46) | 2.63 | (1.7–17.7) | 2.9 | (1.89–4.2) | 0.0395 | 0.799 | 0.0012* |
| IL-4 | 12.87 | (7.86–20.46) | 9.29 | (4.76–13.03) | 8.18 | (4.28–11.37) | 0.0048 | 0.5949 | 0.0037 |
| IL-6 | 90.18 | (30.42–280.34) | 22.34 | (10.82–45.13) | 31.73 | (9.2–66.76) | 0.0002* | 0.924 | 0.0018* |
| IL-7 | 10.26 | (7.92–15.57) | 7.37 | (4.68–10.41) | 6.44 | (3.61–10.08) | 0.0032 | 0.5678 | 0.006 |
| IL-8 | 1484.65 | (487.86–2798.51) | 427.115 | (245.79–698.75) | 401.865 | (211.1–713.43) | 0.0096 | 0.924 | 0.0065 |
| IL-10 | 13.885 | (7.97–25.4) | 6.46 | (2.44–14.05) | 4.08 | (3.3–20.66) | 0.0086 | 0.3926 | 0.0115 |
| IL-12p40 | 13 | (8.17–24.07) | 8.06 | (5.26–19.17) | 9.19 | (5.69–18.51) | 0.039 | 0.7216 | 0.1601 |
| IL-12p70 | 9.26 | (5.74–14.36) | 4.51 | (2.96–9.91) | 6.21 | (4.65–14.02) | 0.0053 | 0.4304 | 0.2965 |
| IL-13 | 3.62 | (2.98–6.53) | 3.23 | (1.9–4.64) | 3.025 | (2.12–4.22) | 0.2901 | 0.8518 | 0.0213 |
| IL-15 | 4.41 | (3.33–5.65) | 2.53 | (1.47–3.99) | 2.43 | (1.45–3.33) | 0.0009* | 0.8288 | 0.0012* |
| IL-17 | 3.51 | (2.44–6.29) | 2.43 | (1.6–3.92) | 2.77 | (0.85–4.8) | 0.0022 | 0.8207 | 0.0461 |
| TNFα | 4.15 | (2.38–9.65) | 2.7 | (1.85–5.58) | 2.045 | (1.32–4.2) | 0.0138 | 0.9457 | 0.0491 |
| MCP-1 | 424.06 | (261.69–802.56) | 134.35 | (33.96–349.35) | 103.71 | (50.82–262.76) | 0.0179 | 0.1601 | 0.013 |
| G-CSF | 1885.81 | (797.30–3019.62) | 451.04 | (113.65–863.52) | 628.355 | (223.97–1088.69) | <0.0001* | 0.3294 | 0.0088 |
| GM-CSF | 6.88 | (4.82–9.71) | 3.35 | (2.83–5.75) | 3.22 | (1.55–4.49) | <0.0001* | 0.4883 | 0.0006* |
| MIP-1α | 33.75 | (15.9–51.69) | 14.91 | (10.61–31.95) | 33.925 | (12.64–55.84) | 0.0061 | 0.1956 | 0.0215 |
| MIP-1β | 26.17 | (20.08–64.04) | 20.7 | (12.1–29.77) | 24.2 | (10.63–37.42) | 0.0002* | 0.5446 | 0.0029 |
| RANTES | 5.360 | (2.91–346.94) | 17.333 | (6.63–41.8) | 3.798 | (1.88–11.29) | 0.9341 | 0.0101 | 0.0415 |
| IP-10 | 296.2 | (146.01–701.43) | 185.53 | (99.32–491.12) | 151.26 | (68.3–327.93) | 0.0151 | 0.5028 | 0.0564 |
| IFNα2 | 40.34 | (26.37–53.48) | 28.77 | (20.68–39.16) | 23.02 | (14.71–33.46) | 0.0032 | 0.8987 | 0.002* |
| IFNγ | 6.62 | (3.11–8.4) | 3.745 | (2.15–5.24) | 4.3 | (1.88–6.42) | 0.0043 | 0.3247 | 0.0799 |
| EOTAXIN | 12.62 | (8.15–15.29) | 8.64 | (6.31–14.15) | 9.805 | (5.64–12.37) | 0.0449 | 0.7467 | 0.1819 |
| VEGF | 106.995 | (75.68–127.07) | 59.605 | (50.84–103.09) | 82.43 | (46.23–105.31) | 0.0007* | 0.2726 | 0.0088 |
| EGF | 11.53 | (6.56–16.55) | 8.515 | (5.11–11.62) | 10.14 | (5.3–11.44) | 0.0053 | 0.9032 | 0.0599 |
| Endocervical Secretions | |||||||||
| Mediator pg/mL | Visit 1 | Visit 2 | Visit 3 | P Value | |||||
| Median | IQR | Median | IQR | Median | IQR | V1 vs V2 | V2 vs V3 | V1 vs V3 | |
| IL-1α | 252.48 | (71.81–718.56) | 216.95 | (45.59–469.62) | 305.27 | (126.41–657.54) | 0.546 | 0.1207 | 0.5838 |
| IL-1β | 21.85 | (9.32–217.87) | 25.85 | (8.97–81.51) | 24.09 | (14.94–65.31) | 0.2872 | 0.8736 | 0.2643 |
| IL-4 | 21.83 | (14.52–26.51) | 23.91 | (20.21–41.44) | 22.72 | (13.75–32.28) | 0.2661 | 0.2182 | 0.6528 |
| IL-6 | 339.43 | (56.11–903.53) | 168.165 | (78.47–502.51) | 145.84 | (38.98–335.44) | 0.5824 | 0.4389 | 0.0551 |
| IL-7 | 13.74 | (11.00–17.99) | 17.875 | (12.03–20.65) | 13.07 | (9.57–16.87) | 0.4742 | 0.0195 | 0.6379 |
| IL-8 | 3759.92 | (877.41–7532.78) | 2128.28 | (1085.35–6163.68) | 1368.74 | (764.04–4827.54) | 0.594 | 0.5646 | 0.1427 |
| IL-10 | 44.015 | (10.04–78.93) | 34.86 | (15.66–70.3) | 21.21 | (5.92–32.36) | 0.7883 | 0.5235 | 0.1208 |
| IL-12p40 | 24.07 | (17.41–31.31) | 26.53 | (18.47–48.71) | 28.66 | (13.08–42.95) | 0.1917 | 0.4559 | 0.2872 |
| IL-12p70 | 10.955 | (7.22–14.12) | 12.32 | (9.08–18.82) | 11.4 | (8.44–16.24) | 0.2522 | 0.1659 | 0.2838 |
| IL-13 | 6.74 | (4.35–9.40) | 8.85 | (5.34–13.68) | 7.78 | (4.64–10.08) | 0.1638 | 0.0564 | 0.8858 |
| IL-15 | 7.42 | (3.90–9.42) | 8.44 | (5.78–10.15) | 6.16 | (2.43–8.32) | 0.6915 | 0.0576 | 0.0484 |
| IL-17 | 4.69 | (3.44–11.50) | 8.09 | (4.24–14.09) | 5.565 | (2.62–15.69) | 0.0285 | 0.9821 | 0.3131 |
| TNFα | 13.665 | (3.55–39.63) | 10.65 | (6.36–38.45) | 8.53 | (3.29–19.32) | 0.5647 | 0.3748 | 0.4525 |
| MCP-1 | 677.91 | (264.42–3031.71) | 630.38 | (157.06–1995.48) | 349.205 | (94.13–700.69) | 0.7676 | 0.065 | 0.1199 |
| G-CSF | 3142.97 | (1592.84–4338.69) | 1494.5 | (372.39–3409.92) | 1564.67 | (618.49–2625.39) | 0.0005* | 0.4732 | 0.173 |
| GM-CSF | 13.45 | (7.07–23.58) | 10.99 | (6.45–14.66) | 8.95 | (6.28–13.12) | 0.0802 | 0.1174 | 0.0319 |
| MIP-1α | 44.41 | (16.80–97.71) | 30.195 | (18.87–79.29) | 31.545 | (11.42–44.51) | 0.714 | 0.1982 | 0.0395 |
| MIP-1β | 41.03 | (23.84–101.09) | 60.26 | (26.75–98.99) | 49.3 | (21.94–81.88) | 0.8532 | 0.0684 | 0.0691 |
| RANTES | 7.121 | (1.69–554.98) | 17.062 | (8.41–35.16) | 3.487 | (1.19–6.3) | 0.7496 | 0.0037 | 0.0691 |
| IP-10 | 504.55 | (122.39–1441.37) | 623.3 | (253.5–1558.07) | 329.92 | (214.88–968.09) | 0.5824 | 0.7502 | 0.0787 |
| IFNα2 | 53.46 | (38.57–72.84) | 66 | (53.48–83.34) | 43.43 | (39.93–69.24) | 0.1145 | 0.0564 | 0.6348 |
| IFNγ | 7.92 | (5.83–13.38) | 9.77 | (6.43–15.6) | 8.49 | (5.23–12.51) | 0.3182 | 0.2579 | 0.2305 |
| EOTAXIN | 16.115 | (11.37–22.52) | 17.795 | (13.52–24.57) | 18.275 | (12.26–20.64) | 0.2099 | 0.6578 | 0.4434 |
| VEGF | 151.415 | (98.51–243.17) | 164.565 | (132.62–229.12) | 157.47 | (99–258.41) | 0.2448 | 0.9161 | 0.856 |
| EGF | 19.83 | (12.64–27.86) | 20.46 | (12.04–34.16) | 24.11 | (17.04–29.04) | 0.2996 | 0.1193 | 0.1186 |
| Vaginal Secretions | |||||||||
| Mediator pg/mL | Visit 1 | Visit 2 | Visit 3 | P Value | |||||
| Median | IQR | Median | IQR | Median | IQR | V1 vs V2 | V2 vs V3 | V1 vs V3 | |
| IL-1α | 393.16 | (154.07–654.91) | 296.015 | (133.97–702.31) | 433.97 | (135.99–1022.65) | 0.7493 | 0.2405 | 0.6893 |
| IL-1β | 22.98 | (4.93–108.05) | 28.67 | (9.38–135.39) | 14.035 | (2.88–67.96) | 0.9007 | 0.3205 | 0.406 |
| IL-4 | 14.86 | (9.32–20.44) | 16.715 | (9.64–21.54) | 11.65 | (6.27–18.38) | 0.7451 | 0.423 | 0.065 |
| IL-6 | 13.995 | (8.43–105.62) | 13.6 | (6.69–47.5) | 5.325 | (1.62–34.49) | 0.4108 | 0.2345 | 0.026 |
| IL-7 | 11.61 | (6.64–15.05) | 11.29 | (8.11–15.05) | 9.375 | (4.97–13.58) | 0.1638 | 0.5202 | 0.0335 |
| IL-8 | 1614.74 | (915.03–3899) | 1291.04 | (660–3153.52) | 1059.135 | (272.78–3311.33) | 0.9553 | 0.9406 | 0.7112 |
| IL-10 | 6.09 | (3.30–25.43) | 8 | (5.06–10.94) | 2.87 | (2.12–8.62) | 0.6617 | 0.1678 | 0.0008* |
| IL-12p40 | 14.985 | (7.99–23.70) | 16.805 | (8.77–23.85) | 10.22 | (4.66–27.82) | 0.427 | 0.9168 | 0.9403 |
| IL-12p70 | 7.31 | (4.22–11.97) | 8.1 | (4.72–12.27) | 7.38 | (2.9–9.44) | 0.5995 | 0.2226 | 0.4578 |
| IL-13 | 4.75 | (3.09–7.28) | 5.95 | (4.97–8.19) | 4.78 | (2.26–7.57) | 0.2713 | 0.1341 | 0.6915 |
| IL-15 | 3.27 | (1.91–5.74) | 3.46 | (2.42–6.06) | 2.205 | (1.22–3.97) | 0.7903 | 0.0701 | 0.0826 |
| IL-17 | 3.25 | (2.26–5.86) | 3.23 | (1.46–7.7) | 1.75 | (1.39–5.31) | 0.5821 | 0.6544 | 0.3554 |
| TNFα | 5.87 | (1.93–23.80) | 5.92 | (2.91–12.25) | 1.93 | (1.06–11.92) | 0.6277 | 0.3737 | 0.0993 |
| MCP-1 | 280.97 | (36.05–472.43) | 70.36 | (18.06–211.78) | 20.775 | (10.95–84.32) | 0.0136 | 0.2774 | 0.0103 |
| G-CSF | 415.955 | (115.19–1364.75) | 179.665 | (74.18–334.52) | 47.245 | (34.32–196.79) | 0.0187 | 0.2112 | 0.0138 |
| GM-CSF | 6.87 | (4.08–10.09) | 5.095 | (3.7–10.32) | 3.63 | (2.16–6.35) | 0.2182 | 0.9273 | 0.0019 |
| MIP-1α | 9.8 | (5.97–27.45) | 9.555 | (5.78–13.64) | 5.97 | (2.76–24.02) | 0.4846 | 0.8053 | 0.0751 |
| MIP-1β | 18.965 | (9.81–30.98) | 16.9 | (11.72–21.59) | 10.68 | (7.11–20.63) | 0.4414 | 0.1793 | 0.0594 |
| RANTES | 3.359 | (1.21–15.05) | 3.258 | (1.51–13.55) | 2.414 | (1.35–14.02) | 0.5944 | 0.1424 | 0.7683 |
| IP-10 | 205.115 | (35.96–513.31) | 65.065 | (33.78–249.16) | 151.605 | (20.71–500.04) | 0.0385 | 0.8382 | 0.9368 |
| IFNα2 | 40.35 | (30.12–56) | 43.09 | (29.99–56.03) | 36.2 | (23.4–50.5) | 0.5188 | 0.41 | 0.7712 |
| IFNγ | 6.72 | (4.70–8.78) | 6.04 | (3.73–9.7) | 5.36 | (3.06–7.65) | 0.3305 | 0.615 | 0.6827 |
| EOTAXIN | 15.86 | (11.37–20.68) | 17.325 | (13.44–21.52) | 12.82 | (7.17–16.79) | 0.4227 | 0.3205 | 0.178 |
| VEGF | 101.235 | (1.90–4) | 87.34 | (66.94–122.16) | 74.18 | (44.69–133.04) | 0.1569 | 0.6556 | 0.1291 |
| EGF | 26.14 | (15.34–50.78) | 26.055 | (16.17–64.15) | 67.69 | (28.55–153.28) | 0.5083 | 0.0001* | 0.0015* |
P value <0.05 after adjusted by false discovery rates for multiple tests.
Discussion
Depo-Provera use was associated with a significantly increased frequency of cervical CCR5+CD4+ T cells at visit 3, and there was a trend toward an increase in the percentage and intensity of integrin α4β7 expression on cervical CD4+ T cells at visit 2. These kinetics are different from those in PBMCs, in which we previously observed an increase (non-significant) in CCR5+CD4+ T cells at visit 2 and a significant increase in the frequency and expression of integrin α4β7 on peripheral CD4+ cells [13]. The effect of Depo-Provera on CD4+ T cell subsets in the cervix and blood also differed with a significant decrease in CD4+ CM T cells in the cervical lumen but not blood at visit 3 [13]. In agreement with the increase in cervical CCR5+CD4+ T cells, immunostaining of vaginal biopsies from women pre- and 3 months following Depo-Provera showed increased numbers of CCR5+ cells [4, 5]. However, a decrease in CD3+ T cells but no change in HLA-DR+, and CCR5+ cells was found in vaginal biopsies from long-term users (24 months) [17]. It remains to be determined whether CCR5+CD4+T cells in the cervical lumen persist after long-term Depo-Provera treatment.
Depo-Provera use was associated with reduced levels of IL-6, IL-8, and IL-1RA in vaginal secretions from swabs 12 months after Depo-Provera treatment [10]. The levels of IFNα, CXCL10, MCP-1, and G-CSF were decreased in cervicovaginal fluids of Depo-Provera users in a cross-sectional study [7]. An increase in the endocervical levels of MCP-1 and IFNα (cytobrush) but a decrease in IL-1β and IL-6 in Depo-Provera users was reported in another small cross-sectional study [4]. Other cross-sectional studies with different inclusion criteria (e.g., the presence of STIs) indicated elevated levels of immune mediators in cervicovaginal lavage or secretions collected with cotton swabs [4, 6, 18]. Sample collection and handling methods, BMI, ethnic background (between controls and Depo-users), and STI history may contribute to the discrepancies [9, 19]. Our current longitudinal study demonstrates general immune suppression at different sites of the lower FRT. The extent of immune suppression was more pronounced in samples collected by cytobrush, possibly due to the enrichment of immune cells. At visit 2, there was a significant decrease in IL-1β, IL-6, IL-8, and IL-15, which were involved in innate immune response and regulation of T and NK cells. Reduction of mediators persisted at visit 3, a time when the serum concentration of Depo-Provera was decreased, suggesting a prolonged effect of Depo-Provera. MCP-1 and G-CSF, which are important for immune cell recruitment, exhibited the most significant reduction at both visits 2 and 3 in vaginal secretions. Decreases in G-CSF levels were observed in all samples from the lower FRT, indicating the possibility of host defense immunosuppression in response to Depo-Provera, which may contribute to an increase risk of STIs including HIV.
Expression of a few factors was induced in response to Depo-Provera. In the vaginal compartment at visit 3, we found a significant increase in EGF, which is important for vaginal epithelial growth and differentiation [20]. This may explain previously reported findings indicating no significant change in vaginal epithelial thickness in Depo users [5, 17, 21, 22]. At visit 2, in the endocervical compartment, IL-17, which is critical for mucosal immunity, was significantly induced. Similar to previous findings [9], there was an increase, albeit non-significant, in RANTES.
Based on our findings, we conclude that Depo-Provera alters markers on cervical CD4+ T cells and mucosal immune milieu in a compartment and time-specific manner. Future investigations into the mechanisms by which Depo-Provera modulates immune responses in the FRT will be relevant to HIV transmission, and will have implications for HIV prevention.
Supplementary Material
Acknowledgments
We thank the subjects who participated in the study, and Jeanette Rios for assistance in patient recruitment.
Funding
This work is supported by NIH grant R01AI110372.
Footnotes
Competing interests
The authors declare that they have no competing interest.
Declarations
Ethics approval and consent to participate
The study (Pro2013003407) was approved by Rutgers, New Jersey Medical School Institutional Review Board.
Consent for publication
not applicable
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information file.
References
- 1.Hel Z, Stringer E, Mestecky J. Sex steroid hormones, hormonal contraception, and the immunobiology of human immunodeficiency virus-1 infection. Endocr Rev 2010; 31(1):79–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hapgood JP, Kaushic C, Hel Z. Hormonal Contraception and HIV-1 Acquisition: Biological Mechanisms. Endocr Rev 2018; 39(1):36–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrne EH, Anahtar MN, Cohen KE, Moodley A, Padavattan N, Ismail N, et al. Association between injectable progestin-only contraceptives and HIV acquisition and HIV target cell frequency in the female genital tract in South African women: a prospective cohort study. Lancet Infect Dis 2016; 16(4):441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith-McCune KK, Hilton JF, Shanmugasundaram U, Critchfield JW, Greenblatt RM, Seidman D, et al. Effects of depot-medroxyprogesterone acetate on the immune microenvironment of the human cervix and endometrium: implications for HIV susceptibility. Mucosal Immunol 2017; 10(5):1270–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandra N, Thurman AR, Anderson S, Cunningham TD, Yousefieh N, Mauck C, et al. Depot medroxyprogesterone acetate increases immune cell numbers and activation markers in human vaginal mucosal tissues. AIDS Res Hum Retroviruses 2013; 29(3):592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deese J, Masson L, Miller W, Cohen M, Morrison C, Wang M, et al. Injectable Progestin-Only Contraception is Associated With Increased Levels of Pro-Inflammatory Cytokines in the Female Genital Tract. Am J Reprod Immunol 2015; 74(4):357–367. [DOI] [PubMed] [Google Scholar]
- 7.Michel KG, Huijbregts RP, Gleason JL, Richter HE, Hel Z. Effect of hormonal contraception on the function of plasmacytoid dendritic cells and distribution of immune cell populations in the female reproductive tract. J Acquir Immune Defic Syndr 2015; 68(5):511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrison C, Fichorova RN, Mauck C, Chen PL, Kwok C, Chipato T, et al. Cervical inflammation and immunity associated with hormonal contraception, pregnancy, and HIV-1 seroconversion. J Acquir Immune Defic Syndr 2014; 66(2):109–117. [DOI] [PubMed] [Google Scholar]
- 9.Fichorova RN, Chen PL, Morrison CS, Doncel GF, Mendonca K, Kwok C, et al. The Contribution of Cervicovaginal Infections to the Immunomodulatory Effects of Hormonal Contraception. MBio 2015; 6(5):e00221–00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roxby AC, Fredricks DN, Odem-Davis K, Asbjornsdottir K, Masese L, Fiedler TL, et al. Changes in Vaginal Microbiota and Immune Mediators in HIV-1-Seronegative Kenyan Women Initiating Depot Medroxyprogesterone Acetate. J Acquir Immune Defic Syndr 2016; 71(4):359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishell DR Jr. Pharmacokinetics of depot medroxyprogesterone acetate contraception. J Reprod Med 1996; 41(5 Suppl):381–390. [PubMed] [Google Scholar]
- 12.Jeppsson S, Gershagen S, Johansson ED, Rannevik G. Plasma levels of medroxyprogesterone acetate (MPA), sex-hormone binding globulin, gonadal steroids, gonadotrophins and prolactin in women during long-term use of depo-MPA (Depo-Provera) as a contraceptive agent. Acta Endocrinol (Copenh) 1982; 99(3):339–343. [DOI] [PubMed] [Google Scholar]
- 13.Tasker C, Davidow A, Roche NE, Chang TL. Depot medroxyprogesterone acetate administration alters immune markers for HIV preference and increases susceptibility of peripheral CD4(+) T cells to HIV infection. Immunohorizons 2017; 1(9):223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang L, Hao Y, Hu J, Kelly D, Li H, Brown S, et al. Differential effects of depot medroxyprogesterone acetate administration on vaginal microbiome in Hispanic White and Black women. Emerg Microbes Infect 2019; 8(1):197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sperling R, Kraus TA, Ding J, Veretennikova A, Lorde-Rollins E, Singh T, et al. Differential profiles of immune mediators and in vitro HIV infectivity between endocervical and vaginal secretions from women with Chlamydia trachomatis infection: a pilot study. J Reprod Immunol 2013; 99(1–2):80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding J, Tasker C, Lespinasse P, Dai J, Fitzgerald-Bocarsly P, Lu W, et al. Integrin alpha4beta7 Expression Increases HIV Susceptibility in Activated Cervical CD4+ T Cells by an HIV Attachment-Independent Mechanism. J Acquir Immune Defic Syndr 2015; 69(5):509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell CM, McLemore L, Westerberg K, Astronomo R, Smythe K, Gardella C, et al. Long-term effect of depot medroxyprogesterone acetate on vaginal microbiota, epithelial thickness and HIV target cells. J Infect Dis 2014; 210(4):651–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guthrie BL, Introini A, Roxby AC, Choi RY, Bosire R, Lohman-Payne B, et al. Depot Medroxyprogesterone Acetate Use Is Associated With Elevated Innate Immune Effector Molecules in Cervicovaginal Secretions of HIV-1-Uninfected Women. J Acquir Immune Defic Syndr 2015; 69(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dezzutti CS, Hendrix CW, Marrazzo JM, Pan Z, Wang L, Louissaint N, et al. Performance of swabs, lavage, and diluents to quantify biomarkers of female genital tract soluble mucosal mediators. PLoS One 2011; 6(8):e23136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson KG, Takahashi T, Bossert NL, Walmer DK, McLachlan JA. Epidermal growth factor replaces estrogen in the stimulation of female genital-tract growth and differentiation. Proc Natl Acad Sci U S A 1991; 88(1):21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller L, Patton DL, Meier A, Thwin SS, Hooton TM, Eschenbach DA. Depomedroxyprogesterone-induced hypoestrogenism and changes in vaginal flora and epithelium. Obstet Gynecol 2000; 96(3):431–439. [DOI] [PubMed] [Google Scholar]
- 22.Mauck CK, Callahan MM, Baker J, Arbogast K, Veazey R, Stock R, et al. The effect of one injection of Depo-Provera on the human vaginal epithelium and cervical ectopy. Contraception 1999; 60(1):15–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.