Abstract
PURPOSE:
To characterize the relationship between mean and sectoral variation of anterior chamber angle (ACA) width using anterior segment optical coherence tomography (AS-OCT).
METHODS:
Subjects aged 50 years or older were identified from the Chinese American Eye Study (CHES), a population-based epidemiological study in Los Angeles, CA. Each subject underwent a complete ocular examination including gonioscopy and AS-OCT imaging. Primary angle closure disease (PACD) was defined as inability to visualize pigmented trabecular meshwork in 3 or more quadrants. Four AS-OCT images from one eye per subject were analyzed and parameters describing ACA width were measured at 500 and 750 μm from the scleral spur: angle opening distance (AOD), trabecular iris space area (TISA), and scleral spur angle (SSA). The relationship between mean and sectoral variation of ACA width was assessed using locally-weighted scatterplot smoothing (LOWESS) regression and change-point analyses and Spearman correlation coefficients.
RESULTS:
674 eyes (337 with PACD, 337 without PACD) from 674 subjects were analyzed. Overall, sectoral variation of ACA width decreased as mean ACA width decreased. This relationship was divided into two phases based on the change-point analysis. Sectoral variation of ACA width was strongly and significantly correlated (P < 0.001) with mean ACA width with below parameter-specific change points for most parameters: AOD500 (r = 0.599), AOD750 (r = 0.246), TISA500 (r = 0.734), TISA750 (r = 0.664), SSA500 (r = 0.661), SSA750 (r = 0.394). Correlations were weaker but still significant (P < 0.004) above these change points for most parameters: AOD500 (r = 0.321), AOD750 (r = 0.550), TISA500 (r = 0.122), TISA750 (r = 0.275), SSA500 (r = −0.036), SSA750 (r = 0.313). Correlations to the left and right of the change points strengthened when sectoral variation of ACA width was adjusted for mean ACA width.
CONCLUSIONS:
Correlations between mean and sectoral variation of ACA width strengthen as the severity of angle narrowing worsens. This relationship likely reflects anatomical changes related to chronic angle closure and may be relevant for refining current definitions and management of PACD.
Introduction
The anterior chamber angle (ACA), defined as the junction between the posterior comeal and anterior iris surfaces, contains the trabecular meshwork (TM), the primary site of aqueous humor outflow from the eye. Angle closure occurs when there is appositional or synechial contact between the TM and peripheral iris. Generalized angle closure leads to impaired aqueous humor outflow and elevation of intraocular pressure (IOP), an important risk factor for glaucomatous optic neuropathy.1,2 Therefore, decreased ACA width plays a central role in the pathogenesis of primary angle closure glaucoma (PACG), the most severe form of primary angle closure disease (PACD) and a leading cause of permanent vision loss worldwide.3–5
The width of the ACA varies along its circumferential extent, and narrower sectors of the ACA are more likely to develop localized angle closure and associated anatomical changes, such as peripheral anterior synechiae (PAS).6–10 Gonioscopy is the current clinical standard for evaluating the ACA. However, gonioscopy alone is weakly predictive of which patients with early angle closure will develop more serious sequelae of angle closure, such as PAS and elevated IOP.11,12 Gonioscopic assessments of the ACA also have inherent limitations; gonisocopy is poorly able to quantify mean and sectoral variations of ACA width compared to anterior segment optical coherence tomography (AS-OCT), a non-contact form of in vivo ocular imaging that produces quantitative measurements of anterior segment structures.13–15 Therefore, quantitative AS-OCT measurements of ACA width provide complementary information to gonioscopic assessments of the ACA.
Chronic PACD is precipitated by generalized angle narrowing through a variety of mechanisms, including increased lens vault (LV) and iris curvature (IC), that also predispose the ACA to localized angle closure.16,17 Intuitively, this portion of the chronic angle closure process should lead to reduced sectoral variation of ACA width, since focal angle closure produces “floor” (zero value) sectoral measurements of ACA width that cease to decline with measurements from other sectors that are not yet at the floor. However, the relationship between mean and sectoral variation of ACA width has never been characterized, and the minimum degree of angle narrowing associated with localized angle closure is unknown. In this study, we use AS-OCT data from the Chinese American Eye Study, a population-based study of Chinese Americans, to examine the effect of decreasing ACA width on sectoral variation of ACA width.18
Methods
Ethics committee approval was previously obtained from the University of Southern California Medical Center Institutional Review Board. All study procedures adhered to the recommendations of the Declaration of Helsinki. All study participants provided informed consent at the time of enrollment.
Clinical Assessment
Subjects were identified from the Chinese American Eye Study (CHES), a population-based, cross-sectional study that enrolled 4,582 Chinese participants aged 50 years and older residing in the city of Monterey Park, California.18 As participants of CHES, subjects received a complete eye examination by a trained ophthalmologist including, in order, Goldmann applanation tonometry (GAT), gonioscopy, and AS-OCT imaging. GAT was performed in a lighted environment with the room lights on (27 cd/m2). Gonioscopy was performed with a Posner-type 4-mirror lens (Model ODPSG; Ocular Instruments, Inc., Bellevue, WA, USA) under dark ambient lighting (0.1 cd/m2) by two trained ophthalmologists (DW, CL) masked to other examination findings. A 1-mm light beam was reduced to a narrow slit. Care was taken to avoid light from falling on the pupil and to avoid inadvertent indentation during examination. The gonioscopy lens could be tilted to gain a view of the angle over the convexity of the iris. The angle in each quadrant was graded using the modified Shaffer grading system based on identification of anatomical landmarks: grade 0, no structures visualized; grade 1, non-pigmented TM visible; grade 2; pigmented TM visible; grade 3, scleral spur visible; grade 4, ciliary body visible. Gonioscopic angle closure was defined as a quadrant with grade 0 or 1. PACD was defined as an eye with three or more quadrants of gonioscopic angle closure. AS-OCT imaging was performed with the Tomey CASIA SS-1000 swept-source Fourier-domain device (Tomey Corporation, Nagoya, Japan) under dark ambient lighting (0.1 cd/m2) prior to pupillary dilation.
Inclusion criteria for the study included CHES subjects who received AS-OCT imaging. Exclusion criteria included eyes receiving medications that could affect pupil size and/or ACA width. Subjects with a history of prior eye procedures that could affect ACA width, including laser peripheral iridotomy (LPI) and cataract surgery, or corneal opacities that precluded AS-OCT imaging were also excluded. Subjects were not excluded based on angle status (PACD or non-PACD) as the primary objective of the study was to characterize the effect of angle narrowing in eyes with a wide range of mean ACA widths. However, all eligible subjects with PACD were included since we hypothesized the effect of angle closure on sectoral variation of ACA width would be strongest in eyes with the narrowest angles. An equal number of non-PACD subjects were included so that the general effect of angle narrowing could be characterized in open angle eyes. One eye per subject was selected at random for data analysis using MATLAB (Mathworks, Natick, MA).
Image Analysis
128 two-dimensional cross-sectional AS-OCT images were acquired per eye. AS-OCT data from eyes imaged in the light were analyzed using the Tomey SS OCT Viewer software (version 3.0, Tomey Corporation, Nagoya, Japan) which automatically segmented anterior segment structures and produced measurements of the anterior segment parameters once the scleral spurs were marked. Four images per eye were analyzed to capture the majority of variation of ACA dimensions.7 The first image analyzed was oriented along the horizontal (temporal-nasal) meridian. Additional OCT images were evenly spaced 45 degrees apart from the horizontal meridian.
One observer (AAP) masked to the identities and examination results of the subjects confirmed segmentation of anatomical structures and marked the scleral spurs in each image. The scleral spur was defined as the inward protrusion of the sclera where a change in curvature of the corneoscleral junction was observed.19 Eyes with missing or corrupt images and eyes in which 2 or more of the 8 scleral spurs could not be identified were excluded from the analysis to avoid grossly misrepresenting sectoral variation of ACA width.
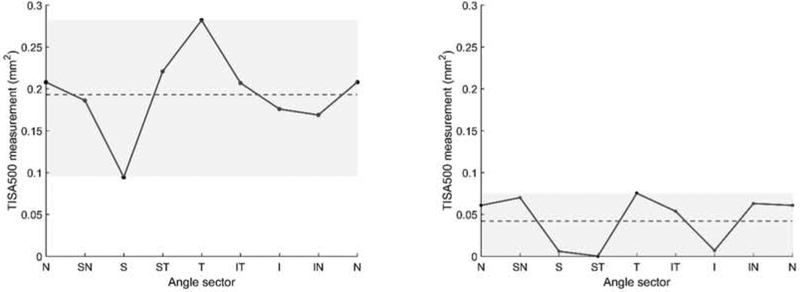
Data from six AS-OCT parameters describing ACA width were measured at 500 and 750 um from the scleral spur: angle opening distance (AOD), trabecular iris space area (TISA), and scleral spur angle (SSA).15 AOD was calculated as the perpendicular distance measured from the trabecular meshwork at 500 or 750 μm anterior to the scleral spur to the anterior iris surface. TISA was defined as the area bounded anteriorly by AOD; posteriorly by a line drawn from the scleral spur perpendicular to the plane of the inner scleral wall to the opposing iris; superiorly by the inner corneoscleral wall; and inferiorly by the iris surface. SSA was defined as an angle measured with the apex at the scleral spur and the arms of the angle passing through a point on the trabecular meshwork 500 or 750 μm from the scleral spur and the point on the iris perpendicularly. Mean ACA width was computed by averaging measurements from each of the four cross-sectional images. Sectoral variation of ACA width was calculated by subtracting the maximum from the minimum sectoral ACA width measurement (Figure 1). Five other AS-OCT parameter were also measured: iris area (IA), anterior chamber depth (ACD), lens vault (LV), anterior chamber width (ACW), and anterior chamber area (ACAr).
Figure 1.
Mean and sectoral variation of TISA500 for representative eyes with high (left) and low (right) mean anterior chamber area (ACA) width. Sectoral variation = maximum − minimum sectoral TISA500. Mean (dotted line) and sectoral variation (height of grey box) of TISA500 are indicated. Angle sectors: N (nasal), SN (superonasal), S (superior), ST (superotemporal), T (temporal), IT (inferotemporal), I (inferior), IN (inferonasal).
Intra-observer reproducibility of measurements was calculated in the form of intraclass correlation coefficients (ICCs). ICCs were calculated for each parameter based on images from 20 open angle and 20 angle closure eyes graded three months apart. This analysis was performed using MATLAB.
Statistical Analyses
LOWESS regression analysis was performed on AS-OCT measurements of mean and sectorial variation of ACA width from individual eyes. We applied a change-point analysis to determine the change point for each LOWESS regression curve, defined as the point at which there is the greatest change in the rate of change of measurement range.20,21 We computed the slope m between all consecutive points (yi,yi+1) of the LOWESS curve. The consecutive tangent slope values for any two pairs of points (xi,yi), (xi+1,yi+1) on the LOWESS curve are given by mi,i+1 = (yi+1 − yi)/(xi+1 − xi). The slope measure takes into account the change in both independent (mean ACA width) and dependent (sectoral variation of ACA width) variables, thus describing the magnitude of change in sectoral variation of ACA width between consecutive measurements of mean ACA width. Finally, we compared the changes in slopes for each pair of consecutive points.
The relationship between mean and range of measurements below each change point was evaluated for each AS-OCT parameter. The distribution of the data points was assessed for normality using the Kolmogorov-Smirnov (K-S) test. Spearman correlation coefficients and their P-values were calculated for each set of measurements. These analyses were repeated for eyes with measurements above each change point.
Analyses were repeated with adjusted sectoral variation of ACA width, defined as unadjusted sectoral variation of ACA width divided by mean ACA width. Analyses were also repeated excluding eyes with evidence of PAS on gonioscopic examination.
Characteristics and ocular biometrics of subjects segregated by the change point for TISA500 were analyzed. The distribution of continuous data was assessed for normality using the Kolmogorov-Smirnov (K-S) test and compared using the Wilcoxan rank-sum test. The proportion of male to female subjects was compared using the Chi-square test. All statistical analyses were performed using MATLAB and conducted at the significance level of 0.05.
Results
508 (11.9%) of the 4,257 CHES subjects who received complete eye exams fit the definition of PACD. 366 (72.0%) of these 508 subjects also received AS-OCT imaging in at least one eye. 29 eyes were excluded due to usage of IOP-lowering or pupil-affecting medications (n = 2), history of LPI or intraocular surgery (n = 18), incomplete or corrupt imaging data (n = 4), eyelid artifacts (n = 3), or poor image quality that precluded identification of the scleral spur in 2 or more images (n = 2). 337 consecutive CHES subjects with open angles who satisfied the inclusion and exclusion criteria were also included in the analysis.
The mean age of all 674 subjects was 61.2 ± 8.2 years (range 50 – 91). 454 (67.4%) of the subjects were female and 220 (32.6%) were male. The mean IOP was 15.7 ± 3.4 mmHg (range 8.7 −45.3).
Intra-observer ICC values for AAP reflected excellent measurement reproducibility for all parameters. The ICC values were: AOD500, 0.90; AOD750, 0.96; TISA500, 0.89; TISA750, 0.92; SSA500, 0.90; SSA750, 0.94.
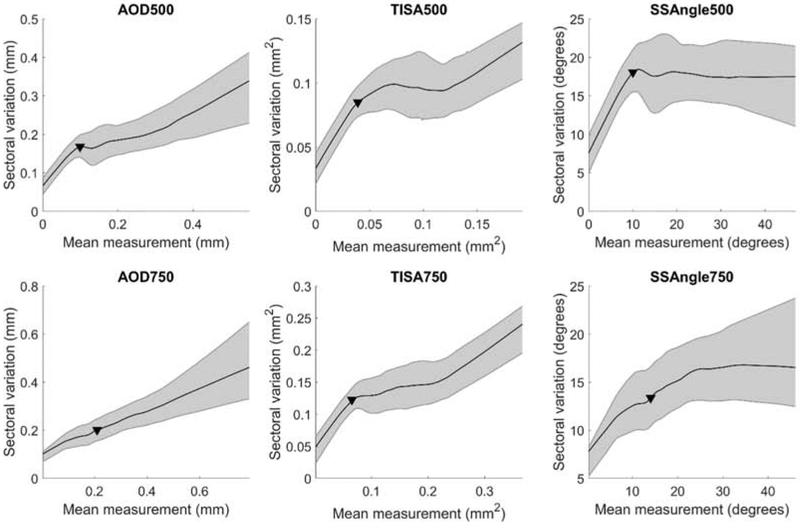
LOWESS curves with 95% confidence intervals were fit to measurements of mean and sectoral variation of ACA width for each parameter (Figure 2). As mean ACA width decreased, sectoral variation of ACA width tended to decrease for all parameters. The change point, indicating the measurement at which the slope of the curve changed most rapidly, was calculated for each parameter (Table 1).
Figure 2.
Relationship between mean and sectoral variation of anterior chamber angle (ACA) width. LOWESS plots with 95% confidence intervals (shaded bars). Solid triangles (▼) indicate change points. AOD = angle opening distance; TISA = trabecular iris space area; SSA = scleral spur angle. 500 and 750 denote measurement at 500 or 750 μm from the scleral spur.
Table 1.
Statistical measures of correlation between mean and sectoral variation of ACA width in eyes below or above parameter-specific change points.
| Parameter | Change-point | Measurement ≤ Change point | Measurement > Change point | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rho | P value | Eyes (N) | PACD (N) | Rho | P value | Eyes (N) | PACD (N) | ||
| AOD500 | 0.099 mm | 0.599 | < 0.001 | 138 | 133 | 0.321 | < 0.001 | 536 | 204 |
| AOD750 | 0.167 mm | 0.246 | < 0.001 | 198 | 188 | 0.55 | < 0.001 | 476 | 149 |
| TISA500 | 0.039 mm2 | 0.734 | < 0.001 | 103 | 96 | 0.122 | 0.004 | 571 | 241 |
| TISA750 | 0.068 mm2 | 0.664 | < 0.001 | 93 | 90 | 0.275 | < 0.001 | 581 | 247 |
| SSA500 | 10.113° | 0.661 | < 0.001 | 108 | 103 | −0.036 | 0.389 | 566 | 234 |
| SSA750 | 13.556° | 0.294 | 0.001 | 133 | 127 | 0.313 | < 0.001 | 541 | 210 |
AOD = angle opening distance; TISA = trabecular iris space area; SSA = scleral spur angle. 500 or 750 denotes distance in μm from the scleral spur.
Boldface values indicate statistical significance of Spearman correlation coefficient at P < 0.05.
Sectoral variation of ACA width tended to decrease as mean ACA width decreased for distance (AOD500, AOD750) and area (TISA500, TISA750) measurements of ACA width. The relationship between mean and sectoral variation of ACA width appeared to be divided into two phases for AOD500, TISA500, and TISA750: sectoral variation of ACA width gradually declined to the right of the change points and rapidly declined to the left of the change points. In contrast, the relationship between the mean and sectoral variation of AOD750 measurements was fairly uniform across all eyes.
The relationship between the mean and sectoral variation of SSA500 measurements differed in that there was little to no relationship between the two sets of measurements until mean ACA width decreased below the change point. A similar flattening of the right-most portion of the curve was seen for SSA750, although the range of measurements gradually decreased as mean ACA width decreased to the right of the change point.
Spearman correlation coefficients were calculated for measurements of mean and sectoral variation of ACA width below and above each parameter-specific change point. For measurements below the change points, correlation coefficients ranged from 0.246 (AOD750) to 0.734 (TISA500) (Table 1). This relationship was significant (p < 0.001) for all parameters. For measurements above the change points, correlation coefficients ranged from −0.036 (SSA500) to 0.550 (AOD750). This relationship was significant (p < 0.004) for all parameters except SSA500 (p = 0.389).
The majority but not all of the of eyes (93% to 97%) to the left of the change points were eyes identified with PACD (Table 1). The proportion of eyes identified with PACD to the left of the change-point compared to the right varied by parameter: AOD500, 0.39; AOD750, 0.56; TISA500, 0.28; TISA750, 0.27; SSA500, 0.31; SSA750, 0.38.
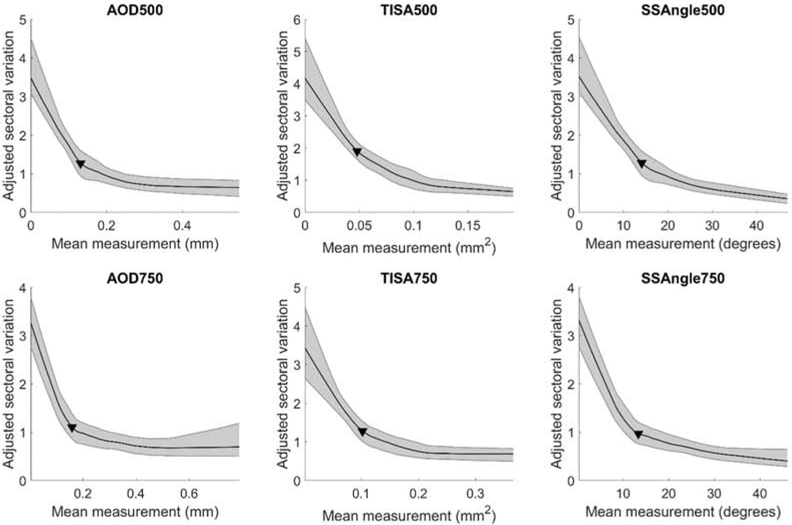
Adjusted sectoral variation of ACA width rapidly increased as mean ACA width decreased (Figure 3). The relationship was stronger (steeper slope) to the right of the parameter-specific change points than to the left. Change points for adjusted sectoral variation measurements tended to greater than for non-adjusted sectoral variation measurements (Table 2). Correlation coefficients were significant (p < 0.001) and greater for measurements below (range −0.607 to −0.728) and above the change points (−0.409 to −0.655) for all parameters except AOD750 (Table 2).
Figure 3.
Relationship between mean and adjusted sectoral variation of ACA width. Mean sectoral variation = (maximum − minimum sectoral measurement) / mean sectoral measurement. Conventions are the same as Figure 1.
Table 2.
Statistical measures of correlation between mean and adjusted sectoral variation of ACA width in eyes below or above parameter-specific change points.
| Parameter | Change-point | Measurement ≤ Change point | Measurement > Change point | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rho | P value | Eyes (N) | PACD (N) | Rho | P value | Eyes (N) | PACD (N) | ||
| AOD500 | 0.131 mm | −0.712 | < 0.001 | 238 | 218 | −0.502 | < 0.001 | 429 | 119 |
| AOD750 | 0.160 mm | −0.607 | < 0.001 | 178 | 169 | −0.409 | < 0.001 | 489 | 168 |
| TISA500 | 0.048 mm2 | −0.728 | < 0.001 | 154 | 142 | −0.681 | < 0.001 | 513 | 195 |
| TISA750 | 0.103 mm2 | −0.677 | < 0.001 | 239 | 219 | −0.494 | < 0.001 | 428 | 118 |
| SSA500 | 14.181° | −0.722 | < 0.001 | 228 | 208 | −0.655 | < 0.001 | 439 | 129 |
| SSA750 | 13.376° | −0.621 | < 0.001 | 228 | 216 | −0.555 | < 0.001 | 439 | 121 |
AOD = angle opening distance; TISA = trabecular iris space area; SSA = scleral spur angle. 500 or 750 denotes distance in μm from the scleral spur.
Boldface values indicate statistical significance of Spearman correlation coefficient at P < 0.05.
The relationship between mean and sectoral variation of ACA width in eyes without PAS closely resembled the relationship observed in all eyes (Supplementary Figure 1). Change points and strength of correlations above and below the change points were also similar between the two groups (Supplementary Table 1).
The correlation between measurements of mean and sectoral variation of ACA width was strongest for TISA500 below its change point. Therefore, the TISA500 change point was used to segregate subjects with PACD into two separate groups: 103 below the change point and 234 above the change point (Table 3). Iris area (IA), anterior chamber depth (ACD), and anterior chamber width (ACW) differed significantly between the two groups (p < 0.05). Age, gender, IOP, LV, and ACAr did not differ significantly between the two groups (p > 0.35).
Table 3.
Characteristics and ocular biometrics of subjects with PACD segregated by the change point for TISA500.
| TISA500 ≤ Change-point | TISA500 > Change-point | P-value | |||
|---|---|---|---|---|---|
| Mean | STD | Mean | STD | ||
| Eyes (N) | 103 | 234 | |||
| Age (years) | 63.143 | 8.708 | 63.02 | 8.941 | 0.715 |
| Gender (M/F) | 36/97 | 48/153 | 0.349 | ||
| IOP (mmHg) | 15.914 | 3.496 | 16.243 | 4.099 | 0.646 |
| IA (mm2) | 1.555 | 0.210 | 1.599 | 0.201 | 0.048 |
| ACD (mm) | 2.014 | 0.357 | 2.125 | 0.356 | 0.009 |
| LV (mm) | 0.789 | 0.222 | 0.778 | 0.218 | 0.981 |
| ACW (mm) | 10.787 | 1.655 | 11.182 | 1.374 | 0.014 |
| ACA (mm2) | 14.914 | 2.097 | 15.270 | 2.583 | 0.392 |
IOP = intraocular pressure; IA = iris area; ACD = anterior chamber depth; LV = lens vault; ACW = anterior chamber width; ACAr = anterior chamber area.
P-values indicate statistical significance from Wilcoxan rank-sum test.
P-value from Chi-squared test.
The number of instances that the minimum sectoral measurement of TISA500 occurred in each sector was calculated for eyes to the left of the change point. The number of instances varied by sector: nasal (N = 17), superonasal (N = 49), superior (N = 54), superotemporal (N = 40), temporal (N = 11), inferotemporal (N = 12), inferior (N = 47), inferonasal (N = 15). The minimum “floor” measurement of TISA500 did not exceed 0.005 m2 in any sector.
Discussion
In this cross-sectional study, we examined the effect of angle narrowing on sectoral variation of ACA width in a cohort of Chinese Americans. Our data show that for most parameters, there is a strong correlation between mean and sectoral variation of ACA width in eyes below parameter-specific change points. This correlation is weak in eyes with mean ACA width above these thresholds for all angle parameters except AOD750. All correlations strengthen when sectoral variation of ACA width is adjusted for mean ACA width. To our knowledge, this is the first population-based study to characterize the relationship between mean and sectoral variation of ACA width in individual eyes. We believe this relationship reflects the progression of chronic angle closure and may have important implications for refining current definitions and management of PACD.
Quantitative studies of ACA width provide insight into not only what anatomic or physiologic changes are associated with the chronic angle closure process, but when these changes are likely to occur. Sectoral variation of ACA width varies widely between individual eyes but is strongly correlated with mean ACA width below parameter-specific change points. This relationship closely resembles the previously reported relationship between mean ACA width and IOP; below parameter-specific change points, IOP is strongly correlated with mean ACA width.21 In fact, change points for decreased sectoral variation of ACA width and increased IOP are closely related (within 20%) for all ACA parameters except SSA750. This finding suggests that these two events are closely related in the progression of chronic angle closure, and patients with mean ACA width approaching or below these change points are at higher risk for anatomical and physiological changes associated with chronic angle closure. However, longitudinal studies comparing clinical outcomes of patients with ACA width either above or below these change points are necessary to clarify their utility in clinical practice for prognosticating the progression of PACD and development of PACG.11
Our findings support the hypothesized natural history of chronic angle closure, progressing from generalized angle narrowing to localized angle closure and then finally generalized angle closure. This process begins with angle narrowing and mean ACA width decreasing toward a change point. Then, when mean ACA width reaches the change point, the narrowest sectors of the ACA begin to develop localized angle closure, reflected in near-zero minimum sectoral measurements but otherwise width-appropriate sectoral variation. Finally, as angle closure becomes generalized, sectoral variation of ACA width rapidly declines as all sectoral measurements converge toward the “floor” (zero value). Further supporting this hypothesis is our finding that minimum measurements more commonly occur in narrower sectors of the ACA (superior and inferior), which matches previous reports that PAS are most commonly observed in the narrowest (superior) portion of the angle.8–10 Therefore, proximity of mean ACA width to the change points described in the study may be associated with or even predict onset or progression of localized anatomical changes and chronic angle closure.
It is important to evaluate the significance of the change points in the context of established definitions and risk factors for PACD. While the majority of PACD is comprised of primary angle closure suspect (PACS), the majority of untreated PACS does not progress to primary angle closure (PAC).11 This suggests that current definitions of PACS are too broad to be clinically useful. The change points described in this study could be one method to refine current definitions of PACD and risk-stratify patients for disease progression. More eyes with PACD fell to the right of the change points than to the left, suggesting there is little to no relationship between ACA width and localized anatomical changes associated with angle closure in most eyes with PACD. In addition, there were significant differences among AS-OCT measurements of biometric risk factors for PACD, including IA, ACD, and ACW when the subjects with PACD were segregated based on the TISA500 change point.16,17,22 This finding suggests that these biometric risk factors may be useful for identifying patients with higher risk of advanced PACD. However, quantitative analysis of AS-OCT images is currently time-consuming and expertise-dependent. Therefore, automated methods that support accurate and rapid assessment of biometric risk factors for PACD are required before these findings can realistically be adopted into clinical practice.
Mean ACA width was more strongly correlated with adjusted compared to unadjusted sectoral variation of ACA width, especially to the right of the change points. This supports that greater sectoral variation of ACA width in these eyes is primarily attributable to greater overall ACA width. This portion of the curve can be interpreted as the baseline effect of angle narrowing on ACA width, and its relatively flat slope suggests that the effect is relatively static and weak. Conversely, the relatively steep slope of the portion of the curve to the left of the change points suggests that angle closure has a dynamic and dramatic effect on sectoral variation of ACA width. This relationship demonstrates that the effects of angle closure on sectoral variation of ACA width supersedes the effects of generalized angle narrowing.
It is feasible that PAS, whether focal or generalized, could have contributed to the observed relationship between mean and sectoral variation of ACA width. Therefore, we excluded all eyes with PAS on gonioscopy and re-examined this relationship. The results of the sub-analysis were similar to our primary results, which suggests that PAS does not strongly affect sectoral variation of ACA width, at least when measured using AS-OCT images taken the dark.
In general, correlations between area measurements of mean and sectoral variation of ACA width (ARA and TISA) are stronger compared to distance (AOD) and angle (SSA) measurements. We believe this indicates that area parameters are more robust representations of ACA width compared to distance and angle parameters, which are more easily influenced by focal variations in iris contour. In addition, correlations tended to be stronger for parameters measured at 500 μm from the scleral spur compared to parameters measured at 750 μm. This supports that iridotrabecular contact starts posteriorly and migrates anteriorly in the majority of angle closure eyes. AOD750 was the only parameter for which correlations were stronger above the change point compared to below, which may be related to the finding that AOD750 is best-correlated with gonioscopic angle closure.13,23 Interestingly, there was no correlation between mean and sectoral variation of SSA measurements above its change point. This suggests that variations in the geometric configuration between the iris and TM at 500 μm from the scleral spur does not change, even if the distance and area bounded by the cornea and iris continues to increase.
Our study has some limitations. First, ACA width was measured in only 8 sectors per eye, which could have led to loss of detectable sectoral variation of ACA width in some eyes. A previous study of predominantly open angle eyes showed that measurements in 8 sectors approximates the 64-sector range of measurements within 10%, but this has not been studied specifically in eyes with PACD.7 Second, the generalizability of the change points to individuals of other ethnicities or imaged under different lighting conditions is unclear. Third, one grader marked the scleral spur in all of the AS-OCT images. While inter-grader variability in marking the scleral spur could result in a different set of change points, we suspect this difference would be small since there is excellent inter-grader measurement reproducibility among modern OCT devices.24 Finally, while there is a strong relationship between mean and sectoral variation of ACA width on a population level, we are unable to assess the variability of change points in individual eyes. This question can only be answered by a longitudinal study tracking sectoral variation of ACA width in individual eyes as angle narrowing progresses over time.
In summary, sectoral variation of ACA width is a new AS-OCT parameter that is strongly correlated with mean ACA width, but only once mean ACA width decreases below parameter-specific change points. These change points likely reflect localized anatomical changes secondary to generalized angle narrowing and may help to identify patients with PACS who have higher risk for progression to PAC and PACG. However, longitudinal studies are necessary to demonstrate the clinical significance of these quantitative AS-OCT measurements for prognosticating progression of PACD.
Supplementary Material
Supplementary Figure 1. Relationship between mean and sectoral variation of ACA width in eyes without peripheral anterior synechiae (PAS). LOWESS plots with 95% confidence intervals (shaded bars). Solid triangles (▼) indicate change points. AOD = angle opening distance; TISA = trabecular iris space area; SSA = scleral spur angle. 500 and 750 denote measurement at 500 or 750 μm from the scleral spur.
Supplementary Table 1. Statistical measures of correlation between mean and sectoral variation of ACA width in eyes without PAS below or above parameter-specific change points.
Sectoral variation of anterior chamber angle width is a new anterior segment OCT parameter that is correlated with angle narrowing and reflects localized anatomical changes that are related to chronic angle closure.
Acknowledgements
This work was supported by grants U10 EY017337, P30 EY029220, and K23 EY029763 from the National Eye Institute, National Institute of Health, Bethesda, Maryland and an unrestricted grant from Research to Prevent Blindness, New York, NY.
We would like to thank Drs. Dandan Wang (D.W.) and Carlos L. Gonzalez (C.L.G.) for performing eye examinations, including gonioscopy and AS-OCT imaging, during CHES.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: A review. JAMA - J Am Med Assoc. 2014;311(18):1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun X, Dai Y, Chen Y, et al. Primary angle closure glaucoma: What we know and what we don’t know. Prog Retin Eye Res. 2017;57:26–45. [DOI] [PubMed] [Google Scholar]
- 3.Quigley H, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. [DOI] [PubMed] [Google Scholar]
- 5.Nongpiur ME, Ku JYF, Aung T. Angle closure glaucoma: A mechanistic review. Curr Opin Ophthalmol. 2011;22(2):96–101. [DOI] [PubMed] [Google Scholar]
- 6.Tun TA, Baskaran M, Perera SA, et al. Sectoral variations of iridocorneal angle width and iris volume in Chinese Singaporeans: A swept-source optical coherence tomography study. Graefe’s Arch Clin Exp Ophthalmol. 2014;252(7):1127–1132. [DOI] [PubMed] [Google Scholar]
- 7.Xu BY, Israelsen P, Pan BX, Wang D, Jiang X, Varma R. Benefit of measuring anterior segment structures using an increased number of optical coherence tomography images: The Chinese American Eye Study. Investig Ophthalmol Vis Sci. 2016;57(14):6313–6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inoue T, Yamamoto T, Kitazawa Y. Distribution and morphology of peripheral anterior synechiae in primary angle-closure glaucoma. J Glaucoma. 1993;2(3):171–176. [PubMed] [Google Scholar]
- 9.Lee JY, Kim YY, Jung HR. Distribution and Characteristics of Peripheral Anterior Synechiae in Primary Angle-Closure Glaucoma. Korean J Ophthalmol. 2010;20(2):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorairaj SK, Tello C, Liebmann JM, Ritch R. Narrow Angles and Angle Closure. Arch Ophthalmol. 2007;125(6):734. [DOI] [PubMed] [Google Scholar]
- 11.He M, Jiang Y, Huang S, et al. Laser peripheral iridotomy for the prevention of angle closure: a single-centre, randomised controlled trial. Lancet. 2019;393(10181):1609–1618. [DOI] [PubMed] [Google Scholar]
- 12.Thomas R, George R, Parikh R, Muliyil J, Jacob A. Five year risk of progression of primary angle closure suspects to primary angle closure: A population based study. Br J Ophthalmol. 2003;87(4):450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu BY, Pardeshi AA, Burkemper B, et al. Quantitative Evaluation of Gonioscopic and EyeCam Assessments of Angle Dimensions Using Anterior Segment Optical Coherence Tomography. Transl Vis Sci Technol. 2018;7(6):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu BY, Pardeshi AA, Burkemper B, et al. Differences in Anterior Chamber Angle Assessments Between Gonioscopy, EyeCam, and Anterior Segment OCT: The Chinese American Eye Study. Transl Vis Sci Technol. 2019;8(2):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung CKS, Weinreb RN. Anterior chamber angle imaging with optical coherence tomography. Eye. 2011;25(3):261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nongpiur ME, He M, Amerasinghe N, et al. Lens vault, thickness, and position in chinese subjects with angle closure. Ophthalmology. 2011;118(3):474–479. [DOI] [PubMed] [Google Scholar]
- 17.Wang B, Sakata LM, Friedman DS, et al. Quantitative Iris Parameters and Association with Narrow Angles. Ophthalmology. 2010;117(1):11–17. [DOI] [PubMed] [Google Scholar]
- 18.Varma R, Hsu C, Wang D, Torres M, Azen SP. The Chinese American eye study: Design and methods. Ophthalmic Epidemiol. 2013;20(6):335–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho SW, Baskaran M, Zheng C, et al. Swept source optical coherence tomography measurement of the iris-trabecular contact (ITC) index: A new parameter for angle closure. Graefe’s Arch Clin Exp Ophthalmol. 2013;251(4):1205–1211. [DOI] [PubMed] [Google Scholar]
- 20.Mazhar K, Varma R, Choudhury F, McKean-Cowdin R, Shtir CJ, Azen SP. Severity of diabetic retinopathy and health-related quality of life: The Los Angeles Latino eye study. Ophthalmology. 2011;118(4):649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu BY, Burkemper B, Lewinger JP, et al. Correlation between Intraocular Pressure and Angle Configuration Measured by OCT. Ophthalmol Glaucoma. 2018;1(3):158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nongpiur ME, Sakata LM, Friedman DS, et al. Novel association of smaller anterior chamber width with angle closure in Singaporeans. Ophthalmology. 2010;117(10):1967–1973. [DOI] [PubMed] [Google Scholar]
- 23.Narayanaswamy A, Sakata LM, He MG, et al. Diagnostic performance of anterior chamber angle measurements for detecting eyes with narrow angles: An anterior segment OCT study. Arch Ophthalmol. 2010;128(10):1321–1327. [DOI] [PubMed] [Google Scholar]
- 24.Marion KM, Maram J, Pan X, et al. Reproducibility and Agreement between 2 Spectral Domain Optical Coherence Tomography Devices for Anterior Chamber Angle Measurements. J Glaucoma. 2015;24(9):642–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Relationship between mean and sectoral variation of ACA width in eyes without peripheral anterior synechiae (PAS). LOWESS plots with 95% confidence intervals (shaded bars). Solid triangles (▼) indicate change points. AOD = angle opening distance; TISA = trabecular iris space area; SSA = scleral spur angle. 500 and 750 denote measurement at 500 or 750 μm from the scleral spur.
Supplementary Table 1. Statistical measures of correlation between mean and sectoral variation of ACA width in eyes without PAS below or above parameter-specific change points.