Abstract
Purpose:
Ophthalmologists commonly perform glaucoma surgery to treat progressive glaucoma. Few studies have examined the stability of OCT neuroretinal rim parameters after glaucoma surgery for ongoing detection of glaucoma progression.
Design:
Longitudinal cohort study.
Participants:
20 eyes (16 subjects) with primary open angle glaucoma who had undergone a trabeculectomy.
Methods:
We calculated the change in OCT parameters (minimum rim area (MRA), minimum rim width (MRW), Bruch’s membrane opening (BMO) area, mean cup depth (MCD), anterior lamina cribrosa surface depth (ALCSD), prelaminar tissue thickness (PLTT), retinal nerve fiber layer thickness (RFNLT) during an interval from the visit before the surgery to the visit after the surgery, a span of approximately 6-months. We also calculated changes in the same eyes over two separate 6-month intervals that did not contain trabeculectomy to serve as control. We compared these intervals using a generalized linear model (with compound symmetry correlation structure), accounting for the correlation between time intervals for the same eye.
Main outcomes measures:
MRW, MRA, angle above the reference plane for MRW and MRA, BMO area, MCD, mean ALCSD, PLTT, RNFLT and visual field parameters (mean deviation (MD), pattern standard deviation (PSD), and visual field index (VFI)).
Results:
The intervals containing trabeculectomy showed a significant decrease in intraocular pressure (−9.2 mmHg, p<.001) when compared to control intervals. Likewise, the following neuroretinal rim parameters showed significant changes with trabeculectomy: increased MRW (+6.04μm, p=.001), increased MRA (+0.014mm2, p=.024), increased angle above reference plane of MRW (+2.64°, p<.001), decreased MCD (−11.6μm, p=.007), and decreased mean ALCSD (−18.91μm, p=.006). This is consistent with an increase in rim tissue thickness and a more anterior position of the ILM and ALCS relative to the BMO plane. Conversely, RNFLT change was not significantly different between trabeculectomy and control intervals (p=.37).
Conclusion:
Trabeculectomy resulted in anatomical changes to the ONH rim associated with reduced glaucomatous cupping. The RNFL thickness may be a more stable measure of disease progression that clinicians can use to monitor across time intervals containing glaucoma surgery.
Keywords: peripapillary, retinal nerve fiber layer thickness, optical coherence tomography, retinal nerve fiber layer, glaucoma, glaucoma progression, optic disc, optic nerve, lamina cribrosa, glaucoma surgery
Précis:
IOP reduction after trabeculectomy was associated with a significant improvement in OCT-based optic nerve head rim parameters, which correspond to reduced “cupping”.
Introduction
Ophthalmologists perform glaucoma surgery to lower the intraocular pressure (IOP) in progressive glaucoma.1,2 IOP lowering creates dynamic changes in the anatomy of the optic nerve head (ONH).3-14 These dynamic changes include thickening of the neuroretinal rim as demonstrated by stereoscopic disc photography and confocal scanning laser ophthalmoscopy.5,6,11-15 Optical coherence tomography (OCT) enables three dimensional (3D) quantification of optic nerve head structures and has demonstrated decreased lamina cribrosa depth and increased lamina cribrosa thickness after trabeculectomy.8-10 After trabeculectomy, OCT has demonstrated variable results in retinal nerve fiber layer (RNFL) thickness with RNFL thickening3 or no significant RNFL thickness change.4,16,17 A pair of recent studies used an OCT-derived -Bruch’s membrane opening (BMO) - to quantify the optic nerve head rim tissue parameters of minimum rim width (MRW) and minimum rim area (MRA) and showed that both increased significantly after trabeculectomy16 and ab-interno trabeculectomy.17 However, those studies did not evaluate lamina cribrosa depth or prelaminar tissue thickness parameters.
We sought to perform a comprehensive assessment of optic nerve head structural conformational changes resulting from trabeculectomy using OCT. Our study is also the first such comprehensive study to include all of these OCT-derived optic nerve head parameters (Table 1) and to incorporate intra-subject control intervals in the study design. Clinicians and researchers can use the results of this study to understand the fundamental changes in optic disc anatomy that occur with IOP lowering resulting from glaucoma surgery, and to evaluate OCT-based optic nerve head measurements before and after glaucoma surgery for ongoing assessment for progressive glaucoma.
Table 1. Previous studies analyzing neuroretinal rim parameters after glaucoma surgery.
| Author (Year) | PMID | Sample size (N of patients) |
Follow-up (months) |
Imaging technique | Control group |
Parameter(s) measured | Results |
|---|---|---|---|---|---|---|---|
| Krzyżanowska-BP (2018) | 29423838 | 30 | 6 | SD-OCT | - | LCD, PTA | Decreased, NSC |
| Reis AS (2012) | 22807291 | 22 | 6 | SD-OCT | - | LCD, PTT | Decreased |
| Shin JW (2017) | 29183045 | 31 | 3 | OCT-A, OCT-EDI | - | LCD | Decreased |
| Lee EJ (2012) | 22464141 | 35 | 6 | SD-OCT | - | LCD, PTT | Decreased, Increased |
| Lee EJ (2013) | 23218823 | 100 | 6 | SD-OCT | - | LCD | Decreased |
| Lee EJ (2013) | 23838772 | 28 | 24 | SD-OCT | - | LCD | Decreased |
| Lee EJ (2015) | 26298719 | 34 | 2.5 | SD-OCT | - | LCD | Decreased |
| Lee SH (2016) | 27654427 | 39 | 6 | SD-OCT | - | LCCI, LCCD | Decreased, Decreased |
| Kadziauskiené A (2018) | 29961552 | 112 | 12 | SD-OCT | - | LCD | Decreased |
| Quigley H (2017) | 28494490 | 27 | - | SD-OCT | - | LCD | Variable |
| Park HY (2014) | 24204049 | 37 | 1 | SD-OCT | - | LCD | Decreased |
| Yoshikawa M (2014) | 24398100 | 73 | 3 | SS-OCT | - | LCD, PLTT | Decreased, Increased |
| Kiessling D (2019) | 30483950 | 65 | 6 | SD-OCT | - | MRW, RNFLT | Increased, NSC |
| Girard MJ (2016) | 26992836 | 12 | 1.5 | SD-OCT | External | LCD | Decreased |
| Chang PT (2007) | 17466378 | 21 | 1.5 | TD-OCT | - | RNFLT | NSC |
| Gietzelt C (2018) | 30053469 | 88 | 12 | SD-OCT | - | MRW, MRA, RNFLT, | Increased, Increased, NSC |
| Current manuscript | - | 16 | - | SD-OCT | Internal | MRW, MRA, MRW angle, MRA angle, BMO area, PTT, MCD, ALCSD, RNFLT | Increased, Increased, Increased, NSC, NSC, NSC, Decreased, Decreased, NSC |
LCD: Lamina cribrosa depth; PTA: prelaminar tissue area; PLTT: prelaminar tissue thickness; LCCI: Lamina cribrosa curvature index; LCCD: Lamina cribrosa curve depth; NSC: no significant change.
Methods
Subjects:
We included participants enrolled in the ongoing, prospective Portland Progression Project at Legacy Devers Eye Institute (Portland, Oregon). The Legacy Health Institutional Review Board (Portland, OR) approved and monitored the study. Participants provided written informed consent before undergoing any study-related testing. All aspects of the study adhered to the tenets of the Declaration of Helsinki.
At enrollment, participants had optic discs suspicious for glaucoma (i.e., large cup-to-disc ratio, cup-to-disc asymmetry ≥0.2 between eyes, history of disc hemorrhage, rim notching, and nerve fiber layer thinning or defect); ocular hypertension (i.e., untreated intraocular pressure ≥22 mmHg on ≥2 occasions); and ≥1 additional risk factor for glaucoma (e.g., first-degree family history of primary open-angle glaucoma).18 Exclusion criteria at study entry included participants with visual acuity worse than 20/40 or other ocular conditions that could affect visual acuity or visual fields.18 These features were necessary for initial enrollment in the Portland Progression Project (P3) study. However, all patients in this paper ultimately progressed, manifesting uncontrolled glaucoma requiring trabeculectomy. They were tested every six months with a series of tests including visual acuity, tonometry, standard automated perimetry, and OCT scans.18
We retrospectively identified 20 eyes from 16 participants who underwent trabeculectomy for failure to achieve target IOP. Eighteen (18/20, 90%) had a primary trabeculectomy and 2 eyes (2/20, 10%) had a repeat trabeculectomy at a second site occurring 18 and 26 years after their initial surgery, respectively. Experienced glaucoma specialists performed fornix-based trabeculectomy in all of these eyes.
Data acquisition:
The patients performed perimetry using a standard protocol (Humphrey Field Analyzer, SITA standard algorithm, Size III stimulus, 24-2 test pattern, Carl Zeiss Meditec, Dublin, CA). Trained operators used a spectral domain OCT to obtain peripapillary RNFL thickness measurements (Spectralis, Heidelberg Engineering, Heidelberg, Germany). Briefly, the operator centered the standard circumpapillary scan on the optic nerve head and focused images to maximize clarity. Each circle scan included 1536 A-scans at a radius of 6 degrees from the center of the optic nerve head. The final image was the average of 9 collected sweeps obtained with real-time eye-tracking. Next, the operators used the Heidelberg Eye Explorer Software tool (Software version 5.6.1.0, Heidelberg Engineering, Heidelberg, Germany) to achieve an accurate delineation of the anterior RNFL boundary (between the vitreous and the internal limiting membrane) and the posterior boundary of the RNFL 19 (between the RNFL and the ganglion cell layer) when there were obvious errors in the automated results.20,21 Then, all B-scans using these manually refined segmentation data were exported in the ‘align’ mode for subsequent offline analysis. Average RNFL thickness was computed from the 1536 samples (one per A-line) along the circumpapillary B-scan.
On the same visit, the operator used the OCT to capture 24 radial B-scans (spaced every 7.5 degrees) centered on the optic nerve head. Raw data were exported from the instrument for subsequent B-scan image segmentation and 3D morphometric quantifications performed using custom software, as follows. Three investigators (JM, FS, DSS), masked to time point, manually marked the positions of the internal limiting membrane (ILM) and the pair of Bruch’s membrane opening (BMO) points on each B-scan. The investigators completed the marking individually. They did not mark features when unclear (e.g., when completely obscured by shadowing from the major blood vessels). A separate investigator (BF), also masked to time point, adjudicated any discrepancies in marks in consultation with the initial grader(s). These marks were used to calculate the minimum rim width (MRW) and the minimum rim area (MRA).3 MRW represents the minimum distance from the inner opening of the BMO to the internal limiting membrane (ILM) through which optic nerve axons must pass; MRA represents the minimum area. The angle of the vectors defining MRW and MRA (i.e., the angles above the reference plane at which these minima occurred, averaged across B-scans) were also tabulated.22 In similar fashion, investigators also manually marked the anterior lamina cribrosa surface in four B-scans (i.e., every 45 degrees, radially). If reduced image quality prevented measurement on the exact 45-degree meridian, then the researchers measured at the next sequential scan. This methodology solved the vast majority of such cases. Using the latter segmentation, we computed mean anterior lamina cribrosa surface depth (ALCSD) relative to the 3D reference plane fit to the BMO; mean cup depth (MCD), defined as the average depth of the ILM within the ONH below a plane fit to the ring of ILM points directly above or just inside BMO; and prelaminar tissue thickness (PLTT), defined as the perpendicular distance from ALCS to the point of intersection with ILM (the value reported is the average perpendicular distance from B-scans containing anterior lamina cribrosa surface markings). All parameters were analyzed and reported as global averages (i.e., using all available samples from each OCT scan; 48 samples for MRW and MRA, 1536 for RNFLT, etc.).
All OCT scans were acquired in follow-up mode using the instrument’s real-time eye tracking algorithm to ensure that all B-scans were captured at the same location as the first scan in the series.
Data Analysis:
We used R (www.R-project.org, version 3.5.0) to perform all analyses. Data were analyzed as global average values of RNFL thickness and ONH rim parameters for each eye and time point. We compared the change in IOP, RNFL thickness, MRA, MRW, MCD, ALCSD, PLTT, visual field indices (mean deviation (MD), pattern standard deviation (PSD), and visual field index (VFI)) as well as parameters thought to be stable in progressive glaucoma (e.g. BMO area) from the visit before the surgery to the visit after the surgery. We identified two additional time intervals (comprising four separate visit dates total) that did not have any surgical (or other) interventions to serve as the ‘control intervals’. Each of these control intervals spanned a similar time period of approximately 6 months; and were used to account for changes from aging and/or glaucoma progression within that eye. The main outcome was the difference in changes determined within the trabeculectomy interval compared to changes determined from the control intervals. These were compared using a generalized linear model. We used a compound symmetry correlation structure to adjust for the inclusion of multiple visits for each eye and the correlation between eyes of a particular subject (as both eyes were included for 4/16 participants).
Results
Table 2 lists the baseline characteristics of the participants. Participants had open angle glaucoma with an average mean deviation on visual field testing of −4.9 ± 3.7 dB, a mean IOP of 20.3 ± 4.4 mmHg and a mean preoperative number of medications of 2.1 (median 2, range 1 to 3). The average central corneal thickness was 538.9 ± 33.9 μm. The mean baseline global average RNFL thickness was 64.4 ± 11.5 μm. The mean duration of the trabeculectomy intervals was 8.6 months (median 7.5, range 4.9-23.2 months). The period of time between the preoperative OCT and the surgery had a mean, range, and IQR of 73.6, 13-164 and 55 days, respectively. The period of time between the postoperative OCT and the surgery had a mean, range and IQR of 189.9, 79-640 and 132 days, respectively. For the control intervals, the mean duration was 6.3 months (median 6.3, range 4.2-8.2 months). 72.7% and 27.3% of the control intervals were dates prior and after surgery, respectively.
Table 2. Baseline characteristics of participants.
| Parameter | Mean (SD or %), range |
|---|---|
| N of eyes (N of patients) | 20 (16) |
| Age | 66.38 (9.27), 46 to 80 |
| Gender | |
| Female | 7 (43.8%) |
| Race | |
| Caucasian | 16 (100%) |
| Intraocular pressure (mmHg) | 20.3 (4.4), 14 to 29 |
| Preoperative N of glaucoma medications (total N of drugs) | 2.1, 1 to 3 |
| Central Corneal Thickness (μm) | 538.9 (33.9), 471 to 617 |
| Retinal Nerve Fiber Layer Thickness (μm) | 64.4 (11.5), 47 to 95 |
| Minimum Rim Width (μm) | 178.0 (44.5), 106 to 268 |
| Minimum Rim Area (mm2) | 0.767 (0.179), 0.50 to 1.15 |
| Angle above reference plane of Minimum Rim Width (°) | 41.8 (11.5), 20 to 60 |
| Angle above reference plane of Minimum Rim Area (°) | 35.8 (7.9), 20 to 47 |
| Bruch’s Membrane Opening Area (mm2) | 1.84 (0.32), 1.0 to 2.4 |
| Mean Deviation (dB) | −4.9 (3.70), −0.7 to −13.0 |
| Pattern Standard Deviation (dB) | 6.91 (4.33), 1.7 to 15.2 |
| Visual Field Index (%) | 85.1 (12.6), 61 to 99 |
Baseline values belong to the first time point of the interval that contains trabeculectomy.
In those patients who have had more than one surgery, the age listed is the age at the time of the first surgery.
SD = standard deviation.
The first column of Table 3 lists the change for each OCT parameter that occurred within the interval containing trabeculectomy. After trabeculectomy, IOP decreased an average of 9.2 mmHg (p<.001) from the preoperative IOP. The mean number of medications decreased to 0.5 (median 0, range 0 to 3, p<.001). Peripapillary RNFL thickness decreased significantly from zero (by an average value of −2.01 μm, p=.033). The angle above the reference plane of MRW increased significantly from zero (+2.64 degrees, p=.005). Changes in MRW (+6.04 μm, p=.057), MRA (+0.014 mm2, p=.283), angle above the reference plane of MRA (+1.16 °, p=.133), BMO area (−0.034 mm2, p=.126), MCD (−11.6 μm, p=.057), mean ALCSD (−18.91 μm, p=.059) and PLTT (+13.2 μm, p=.179) did not differ significantly from zero. Finally, the visual field indices (MD, PSD, and VFI) did not exhibit any significant change during the interval containing trabeculectomy (p>.1 for all).
Table 3. Change in IOP and ONH characteristics of participants with and without trabeculectomy.
One-time interval contained the trabeculectomy (with optical coherence tomography measurements prior to and following the surgery). Two time intervals did not contain the trabeculectomy.
| Change during interval containing trabeculectomy (95% CI) |
Change during intervals not containing trabeculectomy (95% CI) |
P-value comparing intervals with and without trabeculectomy |
|
|---|---|---|---|
| Intraocular pressure (mmHg) | −9.2 (−11.7 to −6.8) p < 0.001 |
+0.2 (−1.1 to +1.4) p = 0.814 |
<0.001 |
| Retinal Nerve Fiber Layer Thickness (μm) | −2.01 (−3.72 to −0.29) p = 0.033 |
−0.74 (−1.64 to +0.17) p = 0.119 |
.375 |
| Minimum Rim Width (μm) | +6.04 (+0.2 to +11.9) p = 0.057 |
−4.43 (−7.38 to −1.47) p = 0.006 |
0.001 |
| Minimum Rim Area (mm2) | +0.014 (−0.011 to +0.040) p = 0.283 |
−0.021 (−0.043 to +0.002) p = 0.083 |
0.024 |
| Angle above reference plane of Minimum Rim Width (°) | +2.64 (+1.00 to +4.29) p = 0.005 |
−0.74 (−1.53 to +0.04) p = 0.072 |
<0.001 |
| Angle above reference plane of Minimum Rim Area (°) | +1.16 (−0.29 to +2.61) p = 0.133 |
−0.19 (−0.99 to +0.62) p = 0.656 |
0.113 |
| Bruch’s Membrane Opening Area (mm2) | −0.034 (−0.075 to +0.008) p = 0.126 |
−0.01 (−0.05 to +0.03) p = 0.625 |
0.292 |
| Mean Cup Depth (μm) | −11.6 (−22.8 to −0.4) p = 0.057 |
−1.0 (−3.3 to +1.3) p = 0.411 |
0.007 |
| Mean Anterior Lamina Cribrosa Surface Depth (μm) | −18.91 (−37.38 to −0.43) p = 0.059 |
−0.02 (−5.24 to +5.20) p = 0.995 |
0.006 |
| Prelaminar Tissue Thickness (μm) | 13.2 (−5.4 to +31.9) p = 0.179 |
−5.5 (−16.1 to +5.1) p = 0.319 |
0.131 |
| Mean Deviation (dB) | −0.26 (−0.98 to +0.45) p = 0.478 |
−0.22 (−0.80 to +0.36) p = 0.459 |
0.972 |
| Pattern Standard Deviation (dB) |
−0.2 (−1.1 to +0.7) p = 0.633 |
+0.4 (+0.0 to +0.7) p = 0.042 |
0.150 |
| Visual Field Index (%) | −0.9 (−3.2 to +1.4) p = 0.445 |
−0.2 (−1.9 to +1.5) p = 0.823 |
0.661 |
Confidence intervals and p-values are from generalized least squares models adjusting for the presence of multiple time points and fellow eyes. P-values in the first two columns represent the significance of the change against zero.
The second column of Table 3 reports change in these parameters observed during the control intervals (i.e. not containing trabeculectomy) and the p-values listed in the that column refer to the probability that each change is different from zero.
P-values in the third column represent the statistical significance of the difference between the magnitude of change observed in the interval containing trabeculectomy versus the magnitude of change observed in the control intervals. When comparing these differences, MRW and MRA were both significantly more positive (p=.001 and .024, respectively); while ALCSD and MCD were both significantly more negative (i.e. the lamina surface and the ILM became less deep) (p=.006 and .007, respectively) after trabeculectomy as compared to control. PLTT followed a similar pattern but did not achieve statistical significance. This pattern overall is consistent with an increase in rim tissue thickness and a more anterior position of the ILM and ALCS relative to the BMO plane. MRW angle differed significantly (p<.001) between trabeculectomy and control intervals, while that of MRA angle did not reach statistical significance (p=.113). The magnitude of peripapillary RNFL thickness change in the trabeculectomy interval was not significantly different from the change observed in the control intervals (p=.459). This suggests that RNFL thinning occurred in both intervals and with similar magnitudes.
In the time intervals containing trabeculectomy, no statistically significant associations were found between the magnitude of IOP reduction and the amount of change in any of the parameters (p>.05 for all). Two patients had hypotony (IOP≤6mmHg) the day after surgery, and four had a postoperative IOP spike ≥5 mmHg. However, there were no cases in which these persisted on the date at which postoperative OCT was obtained.
In those cases where control intervals were obtained after trabeculectomy, there was no significant difference in the amount of change in any OCT parameter compared to cases in which control intervals were obtained before surgery; all parameters had p>.1.
Discussion
We hypothesized that trabeculectomy would alter the optic nerve head rim tissue parameters in a manner favorable to ameliorate glaucomatous structural deformation. We sought to study this because clinicians and researchers may use this information to understand the fundamental changes in optic nerve head anatomy that occur with IOP lowering resulting from glaucoma surgery; and to evaluate OCT based optic disc measurements before and after glaucoma surgery for ongoing progression of glaucoma. This study had several key findings: in the intervals containing trabeculectomy (versus those that did not), 1) the optic nerve head thickness measurements of MRW and MRA increased, 2) ALCSD and MCD decreased, and 3) there was no difference in RNFL thickness change.
Trabeculectomy affects the minimum rim width (MRW) and minimum rim area (MRA). We found that MRW and MRA decreased during the control intervals not containing trabeculectomy, consistent with glaucomatous progression of neuroretinal rim thinning and/or aging. After trabeculectomy and successful IOP lowering, the MRW and MRA significantly increased compared to the control intervals. In other words, we noted improved anatomic configuration (less deformation) with IOP lowering in glaucomatous eyes. Gietzelt et al. also found a significant increase in MRW and MRA post-trabeculectomy compared to baseline, up to 1 year follow-up (p=.010 and .034, respectively).16
Prior research has attempted to evaluate the clinical utility of these parameters. The MRW and MRA have been characterized as more anatomically accurate measurements of the optic nerve head 23,24; this may indicate they are more anatomically susceptible to deformational changes from IOP fluctuations.16 Sharma and colleagues 25 found that an acute IOP elevation in glaucomatous eyes created a reduction in the MRW; this same phenomenon was not seen in normal eyes. Our findings similarly show that MRW and MRA may be more mechanically susceptible to reversal of deformation of ONH connective tissues (secondary to relief of compressive and stretch forces on the neuroretinal rim tissue) that have little influence on the peripapillary RNFL at a distance from the ONH.
A previous study from our group published results looking at the angle at which the MRW and MRA occur in monkey eyes with induced chronic intraocular pressure elevations.22 In this prior study, the eyes prior to the IOP elevation had shallow cups with larger MRW and MRA angles closer to ‘vertical’. After chronic IOP elevation, the angles decreased and became more ‘horizontal’ as the optic nerve head became cupped. Our study findings are consistent with these previous observations. Prior to the trabeculectomy, the subjects had cupped nerves with smaller values for these (i.e. more horizontal) angles, but after trabeculectomy these angles increased and became more ‘vertical’.
The MRW and MRA increase after trabeculectomy were accompanied by a decrease in MCD and ALCSD, creating the clinical impression of reduced optic nerve head deformation (less ‘cupping’). These findings are in line with observations published by Lee et al, who reported a significant reduction in the posterior displacement of the lamina cribrosa and an increase in the prelaminar tissue following glaucoma surgery, using enhanced depth imaging SD-OCT of the optic nerve head.8-10 Further research will determine the amount of increase in neuroretinal rim parameters associated with stable glaucoma after glaucoma interventions.
The RNFL thinned in the trabeculectomy interval to an extent similar to that of the intervals not containing surgery. This result likely reflects disease progression rather than an effect of the surgery and has been previously reported.16,17 However, this result contradicts other prior studies that demonstrated an increase in the RNFL thickness after surgery to lower IOP.3,26 It is important to note that the study by Yamada et al is limited due to the use of a scanning laser polarimeter to measure the RNFL without correction for the corneal polarization axis,27 which may have been altered by surgery. Our data also contradicts findings reported in 2002 by Aydin et al,3 who used a noncommercial prototype OCT device with lower resolution.
Our data does support more recent studies that evaluated RNFL changes on OCT, including findings reported by Chang et al from 2007.4 This group used a Stratus OCT and found no significant change in the RNFL thickness after IOP reduction by either medical or surgical methods. They examined 21 eyes of which 8 had IOP reduction with eye drops only while the rest received a trabeculectomy or tube shunt placement. They did not find any RNFL thickness difference after trabeculectomy. Our analysis showed there was a decrease in the RNFL thickness after trabeculectomy, but after comparing the change during the trabeculectomy interval against the control intervals in the same eyes, we found no differences. Thus, it is possible that this decrease in RNFL thickness would have occurred because of continued glaucoma progression and/or aging rather than as a result of the trabeculectomy itself. This is consistent with results of experiments conducted under controlled laboratory conditions using manometry, which indicate that RNFL thickness is unaffected by acute IOP level at the distance away from the optic nerve head where it is typically measured using a circumpapillary B-scan (1.7 mm from the center of the optic nerve head).28 We also limited our selection of participants to those who underwent trabeculectomy, given its ability to consistently yield lower pressures compared with medications alone.
Our study included two eyes undergoing their second trabeculectomy. The first case showed the same pattern as the aggregate results reported for the group, while the data for the second case were not as clear, arguably because there was not much difference between the trabeculectomy interval and the other two control intervals for that eye. However, with just two such eyes, we cannot draw any firm conclusions about the generalizability of this.
Lastly, our study looked at the short-term changes in the visual field parameters after a trabeculectomy. There was no significant change in the mean deviation, pattern standard deviation, or the visual field index. This contradicts prior studies showing improvement in visual field parameters after trabeculectomy.29-32 These previous studies had longer follow-up while our study included only 8.6 months between visual fields. This is a potential limitation since longer follow-up may have yielded significant visual field changes but our study was focused on the optic nerve head anatomical changes revealed by OCT scans.
Our study was underpowered to perform sub-analyses to determine the effect of the magnitude of IOP reduction on ONH parameters. Future studies with a larger sample size may reveal these associations. Finally, in one third of the cases, control OCT images were not available for a time period prior to surgery (so that both control intervals for these eyes occurred after surgery). However, we think that the results are nonetheless robust because sub-analyses showed there were no significant differences in the amount of change in any OCT parameter between those control intervals that were before surgery versus control intervals that were after trabeculectomy; all parameters had p>0.1.
In summary, this study examined the effects of IOP-lowering by trabeculectomy on a comprehensive set of OCT parameters in patients with open-angle glaucoma. We found that optic nerve head rim tissue thickness measurements, including the minimum rim width and minimum rim area were increased (improved) following trabeculectomy and lamina cribrosa depth and mean cup depth were decreased (improved) following trabeculectomy. We found that RNFL thickness was not significantly affected by lowered pressure in the setting of trabeculectomy. RNFL thickness may be a more stable measure of disease progression that can be monitored across time intervals containing glaucoma surgery.
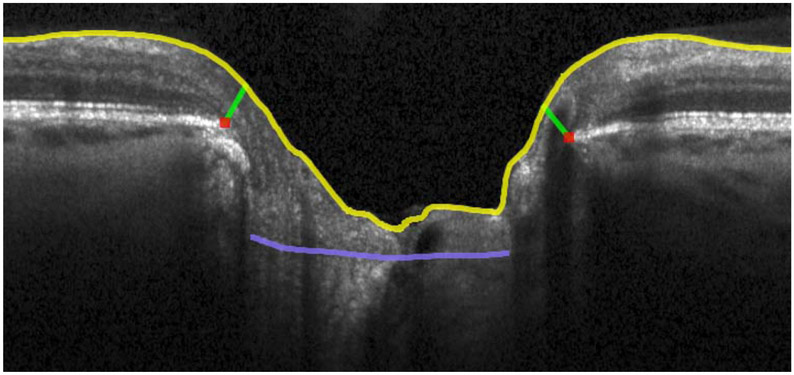
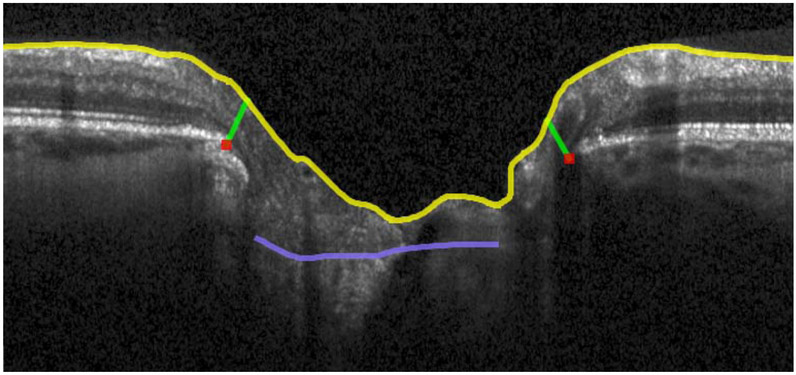
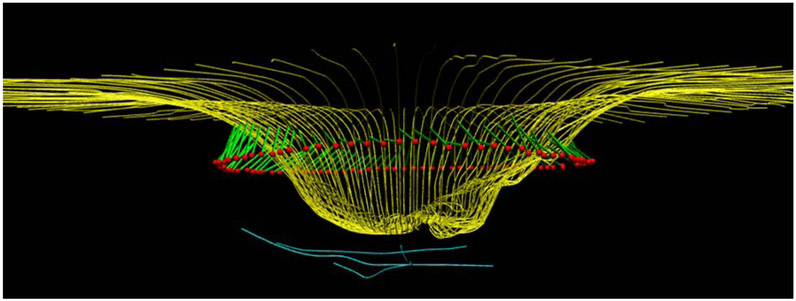
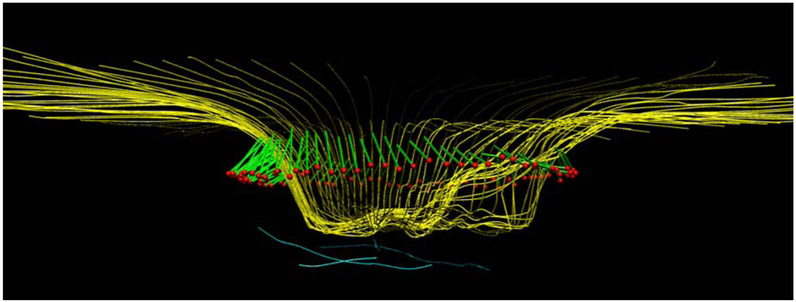
Figure 1.
OCT radial scans of the ONH illustrating changes in MRW, MCD and ALCSD between pre- and post-trabeculectomy scans. 1a: B-scan from the timepoint preceding trabeculectomy. 1b: B-scan from the timepoint following trabeculectomy. 1c: 3D reconstruction of ILM, BMO, MRW and ALCS pre-trabeculectomy. 1d: 3D reconstruction of ILM, BMO, MRW and ALCS post-trabeculectomy. There is a statistically significant improvement in all these ONH parameters which corresponds to a clinical impression of reduced "cupping". Yellow lines: ILM; red dots: BMO; light blue lines: anterior lamina cribrosa surface; green lines: MRW.
MCD: mean cup depth; ALCS: anterior lamina cribrosa surface; ALCSD: anterior lamina cribrosa surface depth; MRW: minimum rim width. BMO: Bruch's membrane opening.
Acknowledgments
Financial Support: The Good Samaritan Foundation, Portland, Oregon, and the National Eye Institute grant number R01 EY 019674, Bethesda, Maryland.
Footnotes
Conflict of Interest: no conflicting relationship exists for any author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Cairns JE. Trabeculectomy. Preliminary report of a new method. American journal of ophthalmology. 1968;66(4):673–679. [PubMed] [Google Scholar]
- 2.Hitchings R Initial treatment for open-angle glaucoma- medical, laser, or surgical? Surgery is the treatment of choice for open-angle glaucoma. Archives of ophthalmology (Chicago, Ill: 1960). 1998;116(2):241–242. [DOI] [PubMed] [Google Scholar]
- 3.Aydin A, Wollstein G, Price LL, Fujimoto JG, Schuman JS. Optical coherence tomography assessment of retinal nerve fiber layer thickness changes after glaucoma surgery. Ophthalmology. 2003;110(8):1506–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang PT, Sekhon N, Budenz DL, Feuer WJ, Park PW, Anderson DR. Effect of lowering intraocular pressure on optical coherence tomography measurement of peripapillary retinal nerve fiber layer thickness. Ophthalmology. 2007;114(12):2252–2258. [DOI] [PubMed] [Google Scholar]
- 5.Greenidge KC, Spaeth GL, Traverso CE. Change in appearance of the optic disc associated with lowering of intraocular pressure. Ophthalmology. 1985;92(7):897–903. [DOI] [PubMed] [Google Scholar]
- 6.Irak I, Zangwill L, Garden V, Shakiba S, Weinreb RN. Change in optic disk topography after trabeculectomy. American journal of ophthalmology. 1996;122(5):690–695. [DOI] [PubMed] [Google Scholar]
- 7.Katz LJ, Spaeth GL, Cantor LB, Poryzees EM, Steinmann WC. Reversible optic disk cupping and visual field improvement in adults with glaucoma. American journal of ophthalmology. 1989;107(5):485–492. [DOI] [PubMed] [Google Scholar]
- 8.Lee EJ, Kim TW. Lamina Cribrosa Reversal after Trabeculectomy and the Rate of Progressive Retinal Nerve Fiber Layer Thinning. Ophthalmology. 2015;122(11):2234–2242. [DOI] [PubMed] [Google Scholar]
- 9.Lee EJ, Kim TW, Weinreb RN. Reversal of lamina cribrosa displacement and thickness after trabeculectomy in glaucoma. Ophthalmology. 2012;119(7):1359–1366. [DOI] [PubMed] [Google Scholar]
- 10.Lee EJ, Kim TW, Weinreb RN, Kim H. Reversal of lamina cribrosa displacement after intraocular pressure reduction in open-angle glaucoma. Ophthalmology. 2013;120(3):553–559. [DOI] [PubMed] [Google Scholar]
- 11.Quigley HA. The pathogenesis of reversible cupping in congenital glaucoma. American journal of ophthalmology. 1977;84(3):358–370. [DOI] [PubMed] [Google Scholar]
- 12.Quigley HA. Childhood glaucoma: results with trabeculotomy and study of reversible cupping. Ophthalmology. 1982;89(3):219–226. [DOI] [PubMed] [Google Scholar]
- 13.Robin AL, Quigley HA. Transient reversible cupping in juvenile-onset glaucoma. American journal of ophthalmology. 1979;88(3 Pt 2):580–584. [DOI] [PubMed] [Google Scholar]
- 14.Shirakashi M, Nanba K, Iwata K. Reversal of cupping in experimental glaucoma. Ophthalmologica Journal international d'ophtalmologie International journal of ophthalmology Zeitschrift fur Augenheilkunde. 1991;202(4):194–201. [DOI] [PubMed] [Google Scholar]
- 15.Lesk MR, Spaeth GL, Azuara-Blanco A, et al. Reversal of optic disc cupping after glaucoma surgery analyzed with a scanning laser tomograph. Ophthalmology. 1999;106(5):1013–1018. [DOI] [PubMed] [Google Scholar]
- 16.Gietzelt C, Lemke J, Schaub F, et al. Structural Reversal of Disc Cupping After Trabeculectomy Alters Bruch Membrane Opening-Based Parameters to Assess Neuroretinal Rim. American journal of ophthalmology. 2018;194:143–152. [DOI] [PubMed] [Google Scholar]
- 17.Kiessling D, Christ H, Gietzelt C, et al. Impact of ab-interno trabeculectomy on Bruch's membrane opening-based morphometry of the optic nerve head for glaucoma progression analysis. Graefes Arch Clin Exp Ophthalmol. 2019;257(2):339–347. [DOI] [PubMed] [Google Scholar]
- 18.Mansberger SL, Menda SA, Fortune BA, Gardiner SK, Demirel S. Automated Segmentation Errors When Using Optical Coherence Tomography to Measure Retinal Nerve Fiber Layer Thickness in Glaucoma. American journal of ophthalmology. 2017;174:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spectralis HRA+OCT User Guide Software Version 5.1. 2010:26, 55–56. In. [Google Scholar]
- 20.Gardiner SK, Boey PY, Yang H, Fortune B, Burgoyne CF, Demirel S. Structural Measurements for Monitoring Change in Glaucoma: Comparing Retinal Nerve Fiber Layer Thickness With Minimum Rim Width and Area. Investigative ophthalmology & visual science. 2015;56(11):6886–6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardiner SK, Demirel S, Reynaud J, Fortune B. Changes in Retinal Nerve Fiber Layer Reflectance Intensity as a Predictor of Functional Progression in Glaucoma. Investigative ophthalmology & visual science. 2016;57(3):1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fortune B, Reynaud J, Hardin C, Wang L, Sigal IA, Burgoyne CF. Experimental Glaucoma Causes Optic Nerve Head Neural Rim Tissue Compression: A Potentially Important Mechanism of Axon Injury. Investigative ophthalmology & visual science. 2016;57(10):4403–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chauhan BC, O'Leary N, AlMobarak FA, et al. Enhanced detection of open-angle glaucoma with an anatomically accurate optical coherence tomography-derived neuroretinal rim parameter. Ophthalmology. 2013;120(3):535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gardiner SK, Ren R, Yang H, Fortune B, Burgoyne CF, Demirel S. A method to estimate the amount of neuroretinal rim tissue in glaucoma: comparison with current methods for measuring rim area. American journal of ophthalmology. 2014;157(3):540–549.e541–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma S, Tun TA, Baskaran M, et al. Effect of acute intraocular pressure elevation on the minimum rim width in normal, ocular hypertensive and glaucoma eyes. The British journal of ophthalmology. 2018;102(1):131–135. [DOI] [PubMed] [Google Scholar]
- 26.Yamada N, Tomita G, Yamamoto T, Kitazawa Y. Changes in the nerve fiber layer thickness following a reduction of intraocular pressure after trabeculectomy. Journal of glaucoma. 2000;9(5):371–375. [DOI] [PubMed] [Google Scholar]
- 27.Greenfield DS, Knighton RW, Feuer WJ, Schiffman JC, Zangwill L, Weinreb RN. Correction for corneal polarization axis improves the discriminating power of scanning laser polarimetry. American journal of ophthalmology. 2002;134(1):27–33. [DOI] [PubMed] [Google Scholar]
- 28.Fortune B, Yang H, Strouthidis NG, et al. The effect of acute intraocular pressure elevation on peripapillary retinal thickness, retinal nerve fiber layer thickness, and retardance. Investigative ophthalmology & visual science. 2009;50(10):4719–4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Folgar FA, de Moraes CG, Prata TS, et al. Glaucoma surgery decreases the rates of localized and global visual field progression. American journal of ophthalmology. 2010;149(2):258–264.e252. [DOI] [PubMed] [Google Scholar]
- 30.Gandolfi SA. Improvement of visual field indices after surgical reduction of intraocular pressure. Ophthalmic surgery. 1995;26(2):121–126. [PubMed] [Google Scholar]
- 31.Tsai CS, Shin DH, Wan JY, Zeiter JH. Visual field global indices in patients with reversal of glaucomatous cupping after intraocular pressure reduction. Ophthalmology. 1991;98(9):1412–1419. [DOI] [PubMed] [Google Scholar]
- 32.Yildirim E, Bilge AH, Ilker S. Improvement of visual field following trabeculectomy for open angle glaucoma. Eye (London, England). 1990;4 (Pt 1):103–106. [DOI] [PubMed] [Google Scholar]