Abstract
Nodal Epstein-Barr virus (EBV)-positive cytotoxic T-cell lymphoma (CTL) is a primary nodal peripheral T-cell lymphoma (PTCL) characterized by a cytotoxic phenotype and EBV on the tumor cells. This disease reportedly accounts for 21% of PTCL not otherwise specified (NOS). However, few nodal EBV+ lymphomas have been documented in detail. Nodal EBV+ CTL and nasal-type NK/T-cell lymphoma (NKTL) both exhibit cytotoxic molecule expression and EBV positivity on the tumor cells; however, nodal EBV+ CTL is characterized as a systemic disease without nasopharyngeal involvement, and exhibits a CD8+/CD56− phenotype distinct from NKTL. The clinicopathological uniqueness of nodal EBV+ CTL is further supported by its T-cell origin in most reported cases. In the 2008 WHO classification, it was unclear whether nodal EBV+ CTL should be classified as PTCL or NKTL. However, based on additional data, the 2017 revision classifies nodal EBV+ CTL as PTCL. In the present review, we focus on the clinicopathological characteristics of nodal EBV+ CTL, discuss the relationship between chronic active EBV infection and nodal EBV+ lymphoma, and highlight future perspectives regarding the treatment of this disease.
Keywords: cytotoxic molecule, Epstein–Barr virus, nodal cytotoxic T-cell lymphoma, T-cell receptor phenotype, programmed cell-death ligand 1
INTRODUCTION
Peripheral T-cell lymphoma not otherwise specified (PTCL-NOS) accounts for the largest proportion of mature T-cell and natural killer (NK)-cell neoplasms in the 2017 World Health Organization (WHO) classification.1 We have found evidence that nodal cytotoxic molecule (CM)-positive PTCL-NOS [(also called cytotoxic T-cell lymphoma (CTL)] constitutes a unique category. This group encompasses a wide spectrum of presentations, ranging from indolent disease in a minority to an aggressive neoplasm in the majority; in 45-51% of CTL cases, the pathogenesis is closely associated with Epstein-Barr virus (EBV).2,3 These biological properties are in clear contrast to those of nodal CM-negative T-cell neoplasms, which predominantly comprise PTCL-NOS, angioimmunoblastic T-cell lymphoma, and adult T-cell leukemia (ATLL, positive for human T-cell leukemia virus type 1)—although a small subset of patients (2%) may exhibit EBV-positive bystander lymphocytes.2 This suggests that EBV is detected as the result of immunological deterioration in the tumor microenvironment, but that it plays little role in the pathogenesis of the latter neoplasm.
Among mature T/NK-cell tumors, the detection of EBV on tumor cells is essential for the diagnosis of nasal type NK/T-cell lymphoma (NKTL), aggressive NK-cell leukemia, or EBV+ T/NK-cell lymphoproliferative diseases of childhood—all of which exhibit CM expression and frequent extranodal involvement.4 In most NKTL cases, the prototypic disease initially presents in the upper aerodigestive tract. This disease can also involve a wide variety of extranodal sites, including the skin and gastrointestinal tract, and may exhibit secondary lymph node lesions. Indeed, NKTL affecting middle-aged or elderly patients may feature de novo symptoms with clonal and rapid expansion of neoplastic EBV+ T/NK cells.5
Patients with nodal EBV+ CTL present with lymphadenopathy without nasopharyngeal involvement. However, this nodal lymphoma and NKTL share some biological properties, including CM expression and EBV positivity. We first elucidated the clinicopathological characteristics of nodal EBV+ CTL, which is characterized by diffuse monomorphic infiltration of large cells that often have a centroblastoid appearance, TCR expression, and gene rearrangement.6-8 These findings indicate that nodal EBV+ CTL should be considered as a separate entity from NKTL; however, it remains controversial whether this disease is a variant of PTCL-NOS.
In this mini-review, we summarize the clinicopathological characteristics of nodal EBV+ CTL, with particular focus on its differences from NKTL, to further our understanding of this disease. We also discuss future perspectives regarding the treatment of nodal EBV+ disease. Nodal CTL without EBV-harboring tumor cells is beyond the scope of the present review, but it will be discussed in a future review, including the subject of indolent CD5+ cytotoxic nodal T/NK-cell lymphoproliferative disease affecting patients <60 years old, which was newly identified by Yamashita et al.3
DEFINITION
Nodal EBV+ CTL is defined as a primary nodal peripheral T-cell lymphoma characterized by a cytotoxic phenotype and EBV-harboring tumor cells (Table 1).7 In all of the nodal EBV+ lymphoma cases, lymph node swelling was observed on initial presentation, or lymphadenopathy was the most prominent lesion at the time of diagnosis. The lymphoma cells are positive for at least one T-cell antigen (e.g., CD3, CD4, CD5, or CD8), in addition to constant expression of cytotoxic molecules and related antigens, including granzyme B, granzyme M, perforin, and/or T-cell intracellular antigen 1 (TIA1).9 The category of nodal EBV+ CTL should exclude patients with upper aerodigestive tract involvement.
Table 1. Clinicopathological features of nodal EBV+ CTL and NKTL.
| Nodal EBV+ CTL | NKTL | |
|---|---|---|
| Main lesion | Lymph node | Extranodal site |
| Nasal involvement | – | +/– |
| CM expression | + | + |
| EBV association | + | + |
| CD8 | +/– | –/+ |
| CD56 | –/+ | +/– |
EBV, Epstein-Barr virus; CTL, cytotoxic T-cell lymphoma; NKTL, NK/T-cell lymphoma of nasal type; CM, cytotoxic molecule
EPIDEMIOLOGY
Most nodal EBV+ CTL cases have been reported from East Asia, including Japan and Korea, which a geographical distribution similar to that of other EBV+ T/NK-cell neoplasms, i.e., NKTL and chronic active EBV infection (CAEBV) of the T- and NK-cell type.4,6,7,10-16 Despite several reports on nodal EBV+ CTL,2,6-8,10-14,17-20 its incidence remains to be clarified. Asano et al. found that the nodal EBV+ lymphoma accounted for 21% of nodal PTCL-NOS.2 With a cut-off value of > 25% EBER-positive neoplastic cells, EBV positivity of PTCL-NOS was reported to be 5-31%.21,22 On the other hand, EBV was detected in 6% of PTCL-NOS with a cut-off value of > 50% EBER-positive neoplastic cells.21
The clinicopathological features of nodal EBV+ CTL in previous studies (more than 10 cases) are summarized in Table 2. Nodal EBV+ CTL can develop across a wide range of ages, in infants to the elderly, but it predominantly affects middle-aged and elderly people, with a reported median age of 61-64 years.3,6,10,14 Male predominance has been described, with a M:F ratio of 1.5-3.8:1.3,6,7,10 Some cases may be associated with autoimmune diseases and immunosuppressive treatment.7
Table 2. Summary of major reports* on the clinicopathological features of nodal EBV+ CTL.
| Author | Yamashita et al.3 | Ng et al.6 | Jeon et al.10 | Kato et al.7 | Kato et al.8 |
|---|---|---|---|---|---|
| Reference | Cancer Science. 2018 | Haematologica. 2018 | Hum Pathol. 2015 | The American Journal of Surgical Pathology. 2015 | Histopathology. 2012 |
| Cases (No.) | 48 | 19 | 15 | 39 | 26 |
| Median age (years) | 62 | 61 | 64 | 61 | 62 |
| Sex (male/female) | 33/15 | 15/4 | 9/6 | 26/13 | 15/11 |
| Clinical stage III/IV (n [%]) | 40/46 (86) | 15/17 (88) | 13/15 (87) | 34/39 (87) | 23/26 (88) |
| IPI High-intermediate/High (n [%]) | 29/45 (64) | NA | 13/15 (87) | 25/38 (66) | 19/26 (73) |
| Thrombocytopenia (n [%]) | 22/40 (55) | NA | 8/15 (53) | 21/34 (62) | 11/22 (50) |
| Extranodal involvement >1 site (n [%]) | 7/47 (15) | NA | 5/15 (29) | 7/39 (18) | NA |
| Extranodal sites | |||||
| BM (n [%]) | 11/45 (24) | NA | 4/14 (29) | 11/38 (29) | 7/26 (27) |
| Liver (n [%]) | 15/46 (32) | NA | 9/15 (60) | 14/39 (36) | 9/26 (35) |
| Skin and/or soft tissue (n [%]) | 1/46 (2) | NA | 0/15 (0) | 1/39 (3) | 1/26 (4) |
| GI tract (n [%]) | 1/46 (2) | NA | 1/14 (7) | 1/39 (3) | 1/26 (4) |
| Median OS (months) | 8.0 | 2.5 | 3.5 | 4.0 | 6.6 |
| Immunophenotype | |||||
| CD3 (n [%]) | 46/48 (95) | NA | 15/15 (100) | 37/39 (95) | 24/26 (92) |
| CD4 (n [%]) | 9/47 (19) | NA | 3/15 (20) | 6/39 (15) | 7/25 (28) |
| CD5 (n [%]) | 14/47 (29) | NA | NA | 10/39 (26) | 11/25 (44) |
| CD8 (n [%]) | 30/47 (63) | 12/18 (67) | 10/15 (67) | 28/39 (72) | 17/25 (68) |
| CD56 (n [%]) | 6/48 (12) | 4/18 (22) | 1/15 (7) | 6/39 (15) | 0/26 (0)† |
| TCRβ(n [%]) | 18/41 (43) | 9/19 (47) | 9/14 (64) | 18/39 (46) | 11/24 (46) |
| TCRγ and/or δ(n [%]) | 5/41 (12) | 0/11 (0) | 0/8 (0) | 5/39 (13) | NA |
| T-cell type‡(n [%]) | 33/41 (80) | 16/19 (84) | 11/12 (92) | 33/39 (85) | NA |
EBV, Epstein-Barr virus; CTL, cytotoxic T-cell lymphoma; IPI, International Prognostic Index; BM, bone marrow; GI tract, gastrointestinal tract; OS, overall survival; TCR, T-cell receptor; NA, not available
*Studies reporting more than 10 cases of nodal EBV+ CTL were summarized.
†CD56+ nodal EBV+ CTL cases were excluded in the series (Histopathology. 2012;61:186-199).
‡Patients with T-cell type had positive TCR protein expression and/or TCRγ gene rearrangement
CLINICAL FEATURES AND PROGNOSIS
In most cases of nodal EBV+ CTL, the initial presentation involves lymphadenopathy with aggressive clinical features, including high frequencies of advanced clinical-stage disease (Ann Arbor III-IV) (86-88%), B symptoms (72-80%), high or high/intermediate IPI (64-87%), and thrombocytopenia (53-62%).3,6,7,10 Some cases exhibit hepatic involvement (32-60%) or bone marrow involvement (24-29%). Other less commonly involved extranodal organs in our previous series3 of 48 patients with the nodal EBV+ lymphoma were the lungs, peripheral blood, skin and/or soft tissue, gastrointestinal tract, bone, kidney, and adrenal gland (unpublished data). A minority of patients have two or more extranodal lesions (15-29%); this frequency is lower than that in NKTL (30-59%).3,7,10,23 The median overall survival time of nodal EBV+ disease (2.5-8.0 months) is significantly poorer than that of NKTL (26-50 months)3,6,7,10,14,23,24 or PTCL-NOS (16-20 months).2,3,7,25,26
MORPHOLOGY
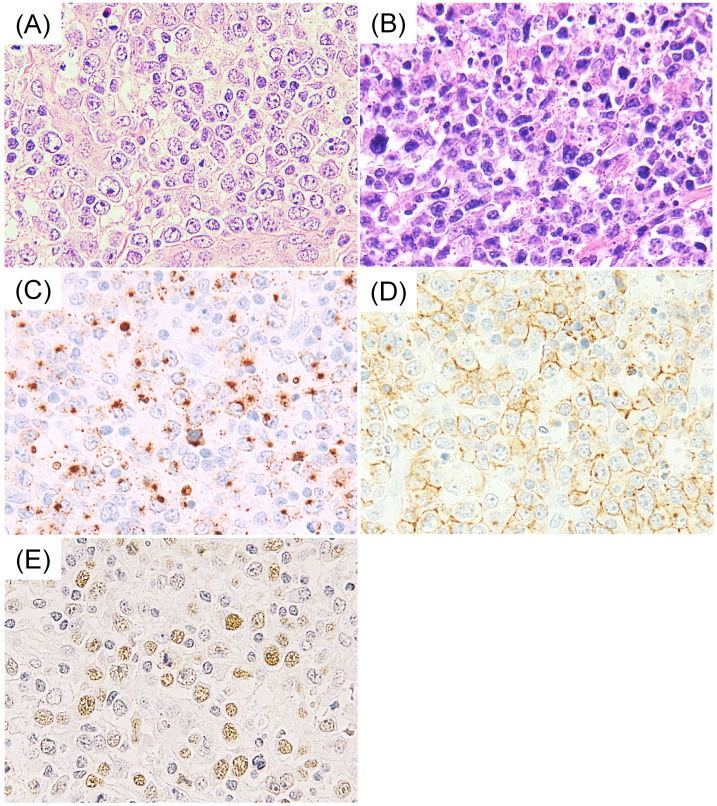
All cases of nodal EBV+ CTL present with high-grade morphology. In addition, we found that half of the cases had a centroblastoid appearance (Fig. 1A), which was originally documented by Kagami et al., emphasizing the challenging differential diagnosis from diffuse large B-cell lymphoma in terms of morphology.27 This unique cytopathological finding is reported in only approximately 15% of extranasal NKTL cases.3,7 Moreover, the centroblastoid appearance is inconsistent with the pleomorphic and elongated nuclei found in prototypic nasal-type tumors (Fig. 1B). Necrosis is more common in extranasal NKTL than in nodal disease.7
Fig. 1.
Histopathological features of nodal EBV+ cytotoxic T-cell lymphoma (CTL). (A) Nodal EBV+ CTL is characterized by a centroblastoid appearance, morphologically resembling diffuse large B-cell lymphoma. (B) In contrast, nasal-type NK/T-cell lymphoma is characterized by pleomorphic and elongated nuclei. (C and D) In nodal EBV+ CTL, the tumor cells are positive for granzyme B (C) and CD8 (D). (E) EBV is detected by in-situ hybridization.
IMMUNOPHENOTYPE
According to the definition, all cases of nodal EBV+ CTL are positive for EBER in-situ hybridization and CMs (Fig. 1C, E). Jeon et al. reported that the percentage of EBER positivity ranged from 40-80% in each case.10 Accordingly, our unpublished data included positivity ranging from 50-90%. Of note, nodal EBV+ CTL is characterized by a CD8+ and CD56− phenotype, and most cases demonstrate positivity for CD3. CD8 positivity is detected in the majority of the cases (63-72%, Fig. 1D), whereas positive CD56 expression is infrequent (12-22%).3,6,7,10,13 This phenotype is in clear contrast to the CD56+ and CD8− phenotype of NKTL.6,23,28-30 These phenotypic differences were also confirmed in the comparison between nodal EBV+ CTL and extranasal NKTL with secondary lymph node involvement.8 A minority of nodal EBV+ lymphoma cases is positive for CD4 (15-20%) and CD5 (26-29%), having lower frequencies than in PTCL-NOS.2,3,7,8,10 Positive CD30 expression has been reported in 37% of cases of nodal EBV+ disease (cut-off value > 30%).3 Positivity for surface CD3 in nodal EBV+ CTL has not been reported in the English literature.
Recently, increasing attention has been paid to the relationship between the T-cell receptor (TCR) phenotype and biological properties among mature T/NK-cell neoplasms.29,31,32 T lymphocytes comprise two distinct lineages that perform non-overlapping roles in immune responses—distinguished by the expression of either αβ or γδ TCR complexes. In terms of localization, αβ T cells are primarily found in secondary lymphoid organs such as lymph nodes and tonsils.33,34 In contrast, γδ T cells account for 2-4% of the T cells within lymph nodes, and up to 50% of the T cells at mucosal sites, particularly in the intestines and skin.34-40 Based on TCRβ positivity, αβ T cells have been detected in 43-64% of nodal EBV+ CTL.3,7,10 On the other hand, based on TCRγ and/or δ positivity, γδ T cells are found in 0-13% of nodal EBV+ disease.3,7,10 Notably, in our previous report, all five patients with TCRγδ+ nodal EBV+ lymphoma exhibited a highly aggressive clinical course and died within 3 months, with a median survival of 2 months.7 Furthermore, all three patients with available clinical information had a medical history of autoimmune disease, and two had undergone previous treatment using immunosuppressive agents.7 Further studies are needed to clarify which is more important for lymphomagenesis of TCRγδ+ nodal EBV+ disease: a history of autoimmune disease or prior use of immunosuppressive agents.
Following advancements in immuno-oncology, increasing numbers of reports have highlighted the neoplastic programmed cell-death ligand 1 (PD-L1) expression on tumor cells in lymphoma entities, including NKTL. We recently reported a small subset of nodal EBV+ CTL (8 of 22 cases; 9%) with neoplastic PD-L1 expression detected by staining with anti-PD-L1 antibody, clone SP142.3 These findings will be discussed in a separate manuscript currently in preparation by Yamashita et al.
CELL OF ORIGIN
Most nodal EBV+ CTL cases (80-92%) are positive for TCR protein expression and/or TCRγ gene rearrangement detected by PCR, i.e., the T-cell type.3,6,7,10 Based on our unpublished data, TCRγ gene rearrangement was found in 71% of the nodal cases. The TCRγ gene rearrangement frequency in nodal EBV+ disease is consistent with findings in other peripheral T-cell lymphomas, including PTCL-NOS, angioimmunoblastic T-cell lymphoma, and adult T-cell leukemia/lymphoma (71-84%).3,41 On the other hand, NKTL generally lacks TCR protein expression and TCR gene rearrangement, with detection rates ranging from 0-14% by immunohistochemistry7,19,29 and 9.5-40% by PCR or Southern blot analysis.6,7,19,23,28,30 These findings additionally support nodal EBV+ CTL being distinct from NKTL.4
Recently, Ng et al. compared the clinicopathological features in T-cell type cases of nodal EBV+ CTL with those in NKTL to clarify whether the differences between nodal EBV+ CTL and NKTL were simply related to lineage.6 They revealed that older age, CD8 expression, and poor outcome remained significantly associated with T-cell type cases of nodal EBV+ lymphoma compared with those of NKTL, confirming that nodal EBV+ CTL is distinct from NKTL regardless of the cell of origin.
RELATIONSHIP WITH EBV+ T/NK-CELL LYMPHOPROLIFERATIVE DISEASES OF CHILDHOOD
The clinical course of patients with nodal EBV+ CTL may include a prodromal phase of chronic active EBV infection (CAEBV). Indeed, in our previous series of 48 patients with nodal EBV+ CTL, 5 patients had a history of EBV+ T/NK-cell lymphoproliferative diseases of childhood (unpublished data).3 Among these 5 patients, the ages at overt EBV+ lymphoma diagnosis were 3, 29, 40, 50, and 55 years. This suggests that nodal EBV+ CTL is related to CAEBV of T- and NK-cell types, systemic form or systemic EBV+ T-cell lymphoma of childhood. We previously reported a case of EBV+ T/NK-cell lymphoma that preceded the clinical presentation of CAEBV.42 The results of TCRγ gene rearrangement analysis suggested that all detectable lymphoma/lymphoproliferative disease (LPD) affecting this patient originated from the same clone throughout the long-term process of EBV+ neoplasia over 18 years, eventually leading to death at 48 years of age. These findings suggest a close relationship between EBV+ T/NK-cell lymphoma and CAEBV in terms of developmental background, especially in younger patients.
Takahashi et al. reported that patients with monoclonal CAEBV-associated NK-cell LPD exhibit clinicopathological features similar to those in younger patients (≤50 years of age) with NKTL and aggressive NK-cell leukemia/lymphoma, and 25% of these patients exhibited hypersensitivity to mosquito bites.43 Recently, Kawamoto et al. reported that patients with adult-onset CAEBV less frequently exhibit hypersensitivity to mosquito bites and hydroa vacciniforme, but more frequently had accompanying hemophagocytic syndrome and exhibited a poorer outcome than pediatric-onset patients (age of onset estimated at <15 years).44 This constellation of findings suggests differences in pathogenesis between young-onset and elderly-onset nodal EBV+ CTL. The pathogenesis of elderly-onset nodal EBV+ CTL may be associated with EBV activation in T cells due to immune senescence with age, even if this is much less frequent than that in B cells.
TREATMENT
There is no clear standard treatment for nodal EBV+ CTL. The prognosis of nodal EBV+ disease is markedly poor when treated using the CHOP regimen.10 In our previous series of the nodal lymphoma, 71% of patients were treated using anthracycline-containing combined chemotherapy;3 however, chemotherapeutic regimens with anthracycline did not improve overall survival (data not shown). Recent reports suggest that l-asparaginase-based regimens, including SMILE (steroid, methotrexate, ifosfamide, l-asparaginase, and etoposide), are effective against advanced-stage NKTL.45-49 Although no report describes the treatment of nodal EBV+ CTL using the SMILE regimen, such treatment may be superior to the CHOP regimen for nodal EBV+ CTL patients. Further investigations are needed to examine the effects of SMILE therapy on nodal EBV+ disease.
Programmed death 1 (PD-1)/PD-L1 pathway inhibitors have recently demonstrated great promise in treating various malignancies, including Hodgkin and non-Hodgkin lymphoma.50-52 Previous studies reported PD-L1 expression in NKTL, and anti-PD-1 immunotherapy is reportedly effective in patients with NKTL.53-55 Ng et al. detected upregulation of PD-L1 mRNA in nodal EBV+ CTL compared with in NKTL.6 This PD-L1 upregulation in the nodal EBV+ disease may have potential therapeutic implications for anti-PD-1 treatment.
CONCLUSION
In this review, we mainly focused on summarizing the clinicopathological characteristics of nodal EBV+ CTL—a systemic disease with an aggressive clinical course and CD8+/CD56− phenotype that is distinct from NKTL and PTCL-NOS. Most cases of nodal EBV+ CTL exhibit a T-cell origin, supporting the clinicopathological uniqueness of this nodal disease. Of note, in our series of Japanese patients with nodal CTLs, EBV presence on tumor cells had no prognostic impact.3 However, EBV detection may be a useful predictive factor for the delineation of therapeutic targets in immuno-oncology-based clinical trials, as the viral agents may be linked with PD-L1 upregulation.56 Overall, nodal EBV+ CTL should be regarded as an aggressive disease that currently presents challenges regarding clinical management and therapeutic approaches. Further investigations are needed to establish appropriate therapeutic strategies against nodal EBV+ CTL.
Footnotes
CONFLICTS OF INTEREST: The authors have declared no conflicts of interest.
REFERENCES
- 1.Vose J, Armitage J, Weisenburger D. International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008; 26: 4124-4130. [DOI] [PubMed] [Google Scholar]
- 2.Asano N, Suzuki R, Kagami Y, et al. Clinicopathologic and prognostic significance of cytotoxic molecule expression in nodal peripheral T-cell lymphoma, unspecified. Am J Surg Pathol. 2005; 29: 1284-1293. [DOI] [PubMed] [Google Scholar]
- 3.Yamashita D, Shimada K, Takata K, et al. Reappraisal of nodal Epstein-Barr Virus-negative cytotoxic T-cell lymphoma: identification of indolent CD5+ diseases. Cancer Sci. 2018; 109: 2599-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swerdlow SH, Campo E, Harris NL, Jaffe ES. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised 4th ed, Lyon, IARC Press. 2017; pp. 353-363, 368-371. [Google Scholar]
- 5.Hamada T, Nakamura S, Ko YH, et al. Epstein-Barr virus-associated T/natural killer-cell lymphomas in the elderly: the first consensus meeting in Kofu 2013. J Dermatol. 2014; 41: 40-42. [DOI] [PubMed] [Google Scholar]
- 6.Ng SB, Chung TH, Kato S, et al. Epstein-Barr virus-associated primary nodal T/NK-cell lymphoma shows a distinct molecular signature and copy number changes. Haematologica. 2018; 103: 278-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato S, Asano N, Miyata-Takata T, et al. T-cell receptor (TCR) phenotype of nodal Epstein-Barr virus (EBV)-positive cytotoxic T-cell lymphoma (CTL): A clinicopathologic study of 39 cases. Am J Surg Pathol. 2015; 39: 462-471. [DOI] [PubMed] [Google Scholar]
- 8.Kato S, Takahashi E, Asano N, et al. Nodal cytotoxic molecule (CM)-positive Epstein-Barr virus (EBV)-associated peripheral T cell lymphoma (PTCL): a clinicopathological study of 26 cases. Histopathology. 2012; 61: 186-199. [DOI] [PubMed] [Google Scholar]
- 9.Krenacs L, Smyth MJ, Bagdi E, et al. The serine protease granzyme M is preferentially expressed in NK-cell, γδ T-cell, and intestinal T-cell lymphomas: evidence of origin from lymphocytes involved in innate immunity. Blood. 2003; 101: 3590-3593. [DOI] [PubMed] [Google Scholar]
- 10.Jeon YK, Kim JH, Sung JY, Han JH, Ko YH, Hematopathology Study Group of the Korean Society of Pathologists Epstein-Barr virus–positive nodal T/NK-cell lymphoma: an analysis of 15 cases with distinct clinicopathological features. Hum Pathol. 2015; 46: 981-990. [DOI] [PubMed] [Google Scholar]
- 11.Ohshima K, Suzumiya J, Sugihara M, et al. Clinical, immunohistochemical and phenotypic features of aggressive nodal cytotoxic lymphomas, including α/β, γ/δ T-cell and natural killer cell types. Virchows Arch. 1999; 435: 92-100. [DOI] [PubMed] [Google Scholar]
- 12.Jung KS, Cho SH, Kim SJ, Ko YH, Kim WS. Clinical features and treatment outcome of Epstein–Barr virus-positive nodal T-cell lymphoma. Int J Hematol. 2016; 104: 591-595. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi E, Asano N, Li C, et al. Nodal T/NK-cell lymphoma of nasal type: a clinicopathological study of six cases. Histopathology. 2008; 52: 585-596. [DOI] [PubMed] [Google Scholar]
- 14.Ha SY, Sung J, Ju H, et al. Epstein–Barr virus-positive nodal peripheral T cell lymphomas: clinicopathologic and gene expression profiling study. Pathol Res Pract. 2013; 209: 448-454. [DOI] [PubMed] [Google Scholar]
- 15.Kimura H, Ito Y, Kawabe S, et al. EBV-associated T/NK–cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood. 2012; 119: 673-686. [DOI] [PubMed] [Google Scholar]
- 16.Okuno Y, Murata T, Sato Y, et al. Defective Epstein–Barr virus in chronic active infection and haematological malignancy. Nat Microbiol. 2019; 4: 404-413. [DOI] [PubMed] [Google Scholar]
- 17.Chan JKC, Sin VC, Wong KF, et al. Nonnasal lymphoma expressing the natural killer cell marker CD56: A clinicopathologic study of 49 cases of an uncommon aggressive neoplasm. Blood. 1997; 89: 4501-4513. [PubMed] [Google Scholar]
- 18.Chim CS, Ma ES, Loong F, Kwong YL. Diagnostic cues for natural killer cell lymphoma: primary nodal presentation and the role of in situ hybridisation for Epstein-Barr virus encoded early small RNA in detecting occult bone marrow involvement. J Clin Pathol. 2005; 58: 443-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gualco G, Domeny-Duarte P, Chioato L, et al. Clinicopathologic and molecular features of 122 Brazilian cases of nodal and extranodal NK/T-cell lymphoma, nasal type, with EBV subtyping analysis. Am J Surg Pathol. 2011; 35: 1195-1203. [DOI] [PubMed] [Google Scholar]
- 20.Attygalle AD, Cabeçadas J, Gaulard P, et al. Peripheral T-cell and NK-cell lymphomas and their mimics; taking a step forward - report on the lymphoma workshop of the XVIth meeting of the European Association for Haematopathology and the Society for Hematopathology. Histopathology. 2014; 64: 171-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karaarslan S, Hekimgil M, Soydan S, Ertan Y, Doğanavşargil B. Evaluation of the role of Epstein-Barr virus in cases of nodal or extranodal T- and NK-cell lymphoma using eber in situ hybridization. Pol J Pathol. 2015; 66: 161-169. [DOI] [PubMed] [Google Scholar]
- 22.Went P, Agostinelli C, Gallamini A, et al. Marker expression in peripheral T-cell lymphoma: A proposed clinical-pathologic prognostic score. J Clin Oncol. 2006; 24: 2472-2479. [DOI] [PubMed] [Google Scholar]
- 23.Li S, Feng X, Li T, et al. Extranodal NK/T-cell lymphoma, nasal type: A report of 73 cases at MD Anderson Cancer Center. Am J Surg Pathol. 2013; 37: 14-23. [DOI] [PubMed] [Google Scholar]
- 24.Lee J, Suh C, Park YH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol. 2006; 24: 612-618. [DOI] [PubMed] [Google Scholar]
- 25.Federico M, Bellei M, Marcheselli L, et al. T cell Project Network. Peripheral T cell lymphoma, not otherwise specified (PTCL-NOS). A new prognostic model developed by the International T cell Project Network. Br J Haematol. 2018; 181: 760-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pellatt J, Sweetenham J, Pickering R, Brown L, Wilkins B. A single-centre study of treatment outcomes and survival in 120 patients with peripheral T-cell non-Hodgkin’s lymphoma. Ann Hematol. 2002; 81: 267-272. [DOI] [PubMed] [Google Scholar]
- 27.Kagami Y, Suzuki R, Taji H, et al. Nodal cytotoxic lymphoma spectrum: A clinicopathologic study of 66 patients. Am J Surg Pathol. 1999; 23: 1184-1200. [DOI] [PubMed] [Google Scholar]
- 28.Au W, Weisenburger DD, Intragumtornchai T, et al. International Peripheral T-Cell Lymphoma Project. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009; 113: 3931-3937. [DOI] [PubMed] [Google Scholar]
- 29.Pongpruttipan T, Sukpanichnant S, Assanasen T, et al. Extranodal NK/T-cell lymphoma, nasal type, includes cases of natural killer cell and αβ, γδ, and αβ/γδ T-cell origin: A Comprehensive Clinicopathologic and Phenotypic Study. Am J Surg Pathol. 2012; 36: 481-499. [DOI] [PubMed] [Google Scholar]
- 30.Ng SB, Lai KW, Murugaya S, et al. Nasal-type extranodal natural killer/T-cell lymphomas: a clinicopathologic and genotypic study of 42 cases in Singapore. Mod Pathol. 2004; 17: 1097-1107. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Herrera A, Song JY, Chuang SS, et al. Nonhepatosplenic γδ T-cell lymphomas represent a spectrum of aggressive cytotoxic T-cell lymphomas with a mainly extranodal presentation. Am J Surg Pathol. 2011; 35: 1214-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka T, Yamamoto H, Elsayed AA, et al. Clinicopathologic spectrum of gastrointestinal T-cell lymphoma: reappraisal based on T-cell receptor immunophenotypes. Am J Surg Pathol. 2016; 40: 777-785. [DOI] [PubMed] [Google Scholar]
- 33.Carding SR, Egan PJ. γδ T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002; 2: 336-345. [DOI] [PubMed] [Google Scholar]
- 34.Lee SY, Stadanlick J, Kappes DJ, Wiest DL. Towards a molecular understanding of the differential signals regulating αβ/γδ T lineage choice. Semin Immunol. 2010; 22: 237-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tripodo C, Iannitto E, Florena AM, et al. Gamma-delta T-cell lymphomas. Nat Rev Clin Oncol. 2009; 6: 707-717. [DOI] [PubMed] [Google Scholar]
- 36.Borst J, van Dongen JJ, Bolhuis RL, et al. Distinct molecular forms of human T cell receptor gamma/delta detected on viable T cells by a monoclonal antibody. J Exp Med. 1988; 167: 1625-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Falini B, Flenghi L, Pileri S, et al. Distribution of T cells bearing different forms of the T cell receptor gamma/delta in normal and pathological human tissues. J Immunol. 1989; 143: 2480-2488. [PubMed] [Google Scholar]
- 38.Groh V, Porcelli S, Fabbi M, et al. Human lymphocytes bearing T cell receptor gamma/delta are phenotypically diverse and evenly distributed throughout the lymphoid system. J Exp Med. 1989; 169: 1277-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haas W, Pereira P, Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993; 11: 637-685. [DOI] [PubMed] [Google Scholar]
- 40.Hayday AC. γδ cells: A right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000; 18: 975-1026. [DOI] [PubMed] [Google Scholar]
- 41.Miyata-Takata T, Takata K, Yamanouchi S, et al. Detection of T-cell receptor γ gene rearrangement in paraffin-embedded T or natural killer/T-cell lymphoma samples using the BIOMED-2 protocol. Leuk Lymphoma. 2014; 55: 2161-2164. [DOI] [PubMed] [Google Scholar]
- 42.Kato S, Miyata T, Takata K, et al. Epstein-Barr virus–positive cytotoxic T-cell lymphoma followed by chronic active Epstein-Barr virus infection–associated T/NK-cell lymphoproliferative disorder: a case report. Hum Pathol. 2013; 44: 2849-2852. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi E, Ohshima K, Kimura H, et al. NK-cell Tumor Study Group. Clinicopathological analysis of the age-related differences in patients with Epstein-Barr virus (EBV)-associated extranasal natural killer (NK)/T-cell lymphoma with reference to the relationship with aggressive NK cell leukaemia and chronic active EBV infection-associated lymphoproliferative disorders. Histopathology. 2011; 59: 660-671. [DOI] [PubMed] [Google Scholar]
- 44.Kawamoto K, Miyoshi H, Suzuki T, et al. A distinct subtype of Epstein-Barr virus-positive T/NK-cell lymphoproliferative disorder: adult patients with chronic active Epstein-Barr virus infection-like features. Haematologica. 2018; 103: 1018-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki R. NK/T cell lymphoma: updates in therapy. Curr Hematol Malig Rep. 2018; 13: 7-12. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki R. Pathogenesis and treatment of extranodal natural killer/T-cell lymphoma. Semin Hematol. 2014; 51: 42-51. [DOI] [PubMed] [Google Scholar]
- 47.Yamaguchi M, Suzuki R, Oguchi M. Advances in the treatment of extranodal NK/T-cell lymphoma, nasal type. Blood. 2018; 131: 2528-2540. [DOI] [PubMed] [Google Scholar]
- 48.Yamaguchi M, Miyazaki K. Current treatment approaches for NK/T-cell lymphoma. J Clin Exp Hematop. 2017; 57: 98-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamaguchi M, Kwong YL, Kim WS, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: The NK-Cell Tumor Study Group study. J Clin Oncol. 2011; 29: 4410-4416. [DOI] [PubMed] [Google Scholar]
- 50.Armand P, Nagler A, Weller EA, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: Results of an International Phase II Trial. J Clin Oncol. 2013; 31: 4199-4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: Preliminary results of a Phase Ib Study. J Clin Oncol. 2016; 34: 2698-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015; 372: 311-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han L, Liu F, Li R, et al. Role of programmed death ligands in effective T-cell interactions in extranodal natural killer/T-cell lymphoma. Oncol Lett. 2014; 8: 1461-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013; 19: 3462-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwong YL, Chan TSY, Tan D, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017; 129: 2437-2442. [DOI] [PubMed] [Google Scholar]
- 56.Green MR, Rodig S, Juszczynski P, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res. 2012; 18: 1611-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]