Abstract
Purpose:
There have been concerns that short-term clinical trials for evaluating new treatments in glaucoma would require prohibitively large sample sizes when using visual field endpoints, given that glaucoma is often a slowly progressive disease. This study sought to determine the required sample size for such trials using event-based analyses, and whether it can be reduced using trend-based analyses.
Design:
Longitudinal, observational study
Participants:
321 eyes of 240 glaucoma participants followed under routine clinical care using 242 visual field for an average of 10 years.
Methods:
Sample size requirements were derived using computer simulations that reconstructed “real-world” visual fields by combining estimates of point-wise variability according to different threshold levels and rates of change obtained from the clinical glaucoma cohort. A clinical trial lasting 2 years with testing every 3 months was simulated, assuming that the new treatment halted visual field change in various percentages of participants (or “responders”). Treatment efficacy was evaluated by: (a) Difference in incidence of point-wise event-based progression (similar to the commercially available Guided Progression Analysis), and (b) Difference in rate of visual field mean deviation (MD) change between groups using linear mixed models (LMMs).
Main Outcome Measures:
Sample size to detect a statistically significance difference between groups.
Results:
Between-group trend-based analyses using LMMs reduced sample size requirements by 85–90% across the range of new treatment effects when compared to the conventional point-wise event-based analysis. To detect the effect of a new treatment that halted progression in 30% of the participants under routine clinical care (equal to a 30% reduction in average rate of MD change) with 90% power, for example, 1924 participants would be required per group using event-based analysis, but only 277 participants per group if LMMs were used.
Conclusions:
The feasibility of future glaucoma clinical trials can be substantially improved by evaluating differences in the rate of visual field change between groups.
Keywords: Visual fields, glaucoma, clinical trials, sample size
Précis:
This study evaluated whether the feasibility of future glaucoma clinical trials using visual field endpoints could be substantially improved by using analytical methods that considers the difference in the rate of functional change between groups.
INTRODUCTION
As glaucoma is generally a slowly progressive disease, there have been concerns regarding the feasibility of conducting expedient clinical trials to evaluate the efficacy of new treatments for slowing or preventing vision loss. It is generally believed that such trials would require very large sample sizes or long follow-up times. For instance, a previously failed clinical trial of a neuroprotective treatment required over two thousand participants to be included over a four-year period.1
Recent results published from the landmark United Kingdom Glaucoma Treatment Study (UKGTS), however, demonstrated that it was possible to detect the beneficial effect of an intraocular-pressure lowering treatment for glaucoma eyes in less than two years, in a clinical trial that included 258 participants per group.2 Using a novel clustered testing paradigm and a pointwise event-based method to assess visual field progression, the UKGTS showed that the risk of incident visual field progression over time was more than halved when subjects were treated with latanoprost compared to placebo. Although the UKGTS estimated a sample size requirement of only 516 participants, these numbers would most likely not be applicable for clinical trials investigating a new treatment compared to an existing treatment, rather than placebo. As a treated population would be expected to have lower incidence of visual field progression than a placebo group, sample size requirements would likely be substantially larger. Due to ethical concerns, it is unlikely that future clinical trials investigating the effect of new therapies on visual field progression in glaucoma would still make use of a placebo arm. Therefore, re-assessment of sample size estimates when the control arm is under current clinical therapy seems necessary.
Despite these concerns, Quigley3 previously suggested that short-term trials may actually be feasible when using rates of visual field mean deviation (MD) as an outcome measure to determine treatment effects. For instance, he suggested that 495 patients would be required per group to detect a 30% reduction in the MD rate of change if visual field testing was performed every 3 months over a 2-year period.3 Comparing the difference in the visual field MD rate of change differs from comparing the incidence of visual field progression as detected by event-based methods, as used in many glaucoma clinical trials to date.1,2,4–8 It can provide increased statistical power to detect treatment differences by using all the information obtained with trend-based analysis. Furthermore, a recent review also supported the use of rate of visual field MD change9 as an outcome measure given the growing body of evidence that underscores the importance of both the level and rate of visual field loss on functional disability.10–17
Although those previous sample size estimates3 provided a useful initial indication on the feasibility of such short-term trials, further refinements to those estimates are required to better reflect what to expect in real-world clinical trials. For instance, the previous sample size estimates3 were derived using rates of visual field change from a study reporting on the natural history of glaucoma,18 assuming that the rates of change in a treated population were simply a percentage of those natural history rates. However, the sample size estimates would likely be more accurate through using real-world rates of visual field change derived from a cohort of glaucoma patients under routine care. In addition, it is not known how sample size estimates using this method would compare to those that derived from point-wise event-based progression analysis, such as the one used by UKGTS. The present study therefore sought to determine whether the sample size required for detecting a significant treatment effect in short-term clinical trials could be reduced by using trend-based analysis of visual field progression between groups when compared to the conventional visual field event-based endpoints.
METHODS
Participants
This study included participants evaluated as part of a longitudinal study designed to investigate structural and functional changes in glaucoma, the Diagnostic Innovations in Glaucoma Study: Functional Impairment. This study was approved by the University of California, San Diego (UCSD) Institutional Review Board (#140276) and adhered with both the Declaration of Helsinki and the Health Insurance Portability and Accountability Act. Written informed consent was obtained from all participants after an explanation of all the test procedures. Participants in this study were reviewed at approximately 6-monthly intervals.
During follow-up, a comprehensive ophthalmologic evaluation was performed for all participants, including a review of their medical history, visual acuity and visual field testing, slit-lamp biomicroscopy and fundus examination, gonioscopy, intraocular pressure measurements and stereoscopic optic disc photography. All participants were required to have open angles on gonioscopy, a best-corrected visual acuity of 20/40 or better, and be 18 years of age or older. Subjects were excluded if they presented any other ocular or systemic disease that could affect the optic nerve or the visual field.
This study included only glaucoma eyes with confirmed visual field damage, being eyes that had ≥ 3 consecutive abnormal visual field tests (defined as having a pattern standard deviation value at P < 0.05, or glaucoma hemifield test being outside normal limits).19 These eyes were also required to have an optic nerve appearance with features indicative of glaucomatous damage20 or evidence of progressive optic disc changes on the basis of masked grading of stereophotographs.21 In this study, only tests including and subsequent to the first of the three consecutive abnormal visual field tests were used, and only eyes with ≥ 10 eligible tests over ≥ 5 years of follow-up were included.
Visual Field Testing
Visual field tests were performed using the 24–2 strategy with the Swedish Interactive Thresholding Algorithm on the Humphrey Field Analyzer II-i (Carl Zeiss Meditec, Inc., Dublin, CA, USA), and were evaluated for artifacts including eyelid or rim artifacts, inattention, fatigue or learning effects, improper fixation, or evidence that the results were affected by a disease other than glaucoma (e.g. homonymous hemianopia); any tests with such artifacts were excluded from this study. Visual fields were considered unreliable and also excluded if > 33% fixation losses or false negative errors (with the exception for false negative errors when visual field mean deviation [MD] was less than −12 dB), or > 15% false positive errors were recorded.
Computer Simulations of Visual Fields
To evaluate and compare the sample sizes required for a clinical trial using visual field as the outcome measure, computer simulations were used to recreate “real-world” visual field results to enable a comparison of the different methods used to detect a significant treatment effect. The details of these simulations are described in details previously22 and included in Supplement Material. As a brief overview, longitudinal estimates of point-wise sensitivity (or the “true” sensitivity) and variability (or the “noise” component) were obtained from the cohort of glaucoma eyes included in this study that were under routine clinical care. The former was derived using a sigmoid regression model proposed by Otarola and colleagues23 since it can better capture the behavior of sensitivity changes in glaucoma, and the latter was derived using an approach first proposed by Russell and colleagues24 for using longitudinal visual field data to obtain visual field variability estimates. These components were then used to reconstruct “real-world” visual field results in a clinical trial scenario using computer simulations, and also to establish test-retest variability limits for the point-wise event-based progression method.25
Clinical Trial Scenario and Sample Size Calculation
In this study, sample size estimates were obtained for a clinical trial that evaluated the beneficial effect of adding a new treatment for patients being treated with currently available therapy (consistent with routine clinical care, as opposed to being compared to a placebo). This was done to avoid potential concerns of having a placebo control arm, considering the evidence of beneficial effect of IOP-lowering therapy in glaucoma. We therefore compared two arms: i) a treatment arm, where patients would be under conventional therapy and would have the new treatment added, and ii) a control arm, where patients would just be followed under conventional therapy.
These two arms were compared in a clinical trial scenario where patients would undergo 10 visits over a 2-year period, with two visits at baseline and one visit every 3 months subsequently. “Real-world” visual field results were reconstructed for each of these visits. We then assumed that the new treatment applied to the treatment arm would completely halt any further visual field progression in a randomly selected proportion of participants (being “responders” to this new treatment). In effect, halting visual field progression in a randomly selected proportion of participants results in a reduction of the mean rate of visual field change by the same proportion. Note that whilst it is more likely that a new treatment would slow rather than completely halt visual field progression, assumptions that have to be made to simulate slowing at a point-wise level for the non-linear changes in visual sensitivity simulated would be complex. Given the absence of information for the potential mechanisms of a new treatment, the assumption of halting visual field progression provides the simplest scenario for comparing the two outcome measures.
To determine the sample size required for detecting a beneficial treatment effect with 90% power for each outcome measure, we simulated 1000 sequences of clinical trials that included between 10 to 2500 participants in each group. For each simulated trial, eyes with different patterns of visual field damage and change were selected at random from the clinical cohort until the required sample size was reached. The same eyes were then used in the treatment and control arms (in a similar manner to matching the baseline characteristics of the participants in a clinical trial), and a randomly-selected proportion of the eyes in the treatment arm was simulated as being “responders” (thus show no further visual field progression). The sample size required to achieve 90% power in detecting a treatment effect was then determined.
Outcome Measures
Two methods were used for evaluating treatment efficacy: a) the difference in survival rates (survival analysis) based on point-wise event-based progression criterion (see Supplementary Material), which is similar to the commercially-available Guided Progression Analysis (Carl Zeiss Meditec, Inc., Dublin, CA, USA) and b) difference in the rate of visual field MD change (trend analysis) between groups using linear mixed-effects models (LMMs).
For the survival analysis method, an eye was considered to have progressed (i.e., achieved the endpoint) if at least three locations on the visual field tests had pattern deviation (PD) values that fell below the lower 5th percentile of the test-retest limits compared to baseline on three consecutive visits. Baseline measurements were the average of the first two tests, and the test-retest limits were derived using the computer simulation methods described in Supplement 1. The survival analysis method compares the proportion of eyes that reached the endpoint during follow-up in the treatment and control groups.
For the trend-based analysis, LMMs were used to investigate differences in rates of MD change between the treatment and control groups. LMMs take into account the longitudinal (repeated measures) and hierarchical (multiple patients within each treatment group) nature of the data. Note, in contrast to the survival analysis method, LMMs do not require a determination of whether progression occurred in an individual eye of not. Detecting a treatment effect in the LMM is based on finding a statistically significant difference between the mean rate of MD change between the treatment and control groups.
A significance level of 5% for a 2-tailed test was applied for both methods.
RESULTS:
Characteristics of the Clinical Cohort
The clinical cohort included a total of 321 eyes from 240 glaucoma participants seen at 15.6 ± 5.1 visits (range, 10 to 39) over 10.0 ± 2.5 years (range, 5 to 17 years). They were on average 63 ± 11 years old at the first visit (range, 25 to 88 years old). The median (interquartile range) baseline visual field MD and PSD of these eyes were −3.23 dB (−5.53 to −1.85 dB) and 3.58 dB (2.46 to 6.73 dB) respectively. A total of 252 (79%), 59 (18%) and 10 (3%) of the eyes had early (MD ≥ −6 dB), moderate (MD < −6 dB and ≥ −12 dB) and advanced (MD < −12 dB) visual field damage. When simulating the last two years at the end of the follow-up, the participants were on average 71 ± 11 years old (range, 33 to 98) and the median (interquartile range) baseline estimated MD and rate of change over the 2-year period were −4.37 dB (−2.66 to −8.35 dB) and −0.57 dB/year (−0.24 to −0.98 dB/year), respectively. On average, 14.6% of the eyes in the control arm were detected as having progressed based on the point-wise event-based criterion at the end of the simulated two-year follow-up period.
Sample Size Requirements
The sample size required per group to detect a significant treatment effect was substantially smaller when using trend-based analysis of MD change with LMMs versus survival analysis of point-wise event-based change (Table 1). For example, for a 30% treatment effect, a total of 1924 participants would be required per group using survival analysis versus only 277 participants for trend-analysis of rate of MD change using LMMs. The reduction of the required sample size varied between approximately 85 to 90% across the range of new treatment effects.
Table 1:
Sample size required per group to detect a significant treatment effect for different treatment effects and outcome measure (survival analysis versus trend analysis)
| Treatment Effect | Survival Analysis | Trend Analysis |
|---|---|---|
| 20% | 5067 | 601 |
| 30% | 1924 | 277 |
| 40% | 1027 | 153 |
| 50% | 603 | 99 |
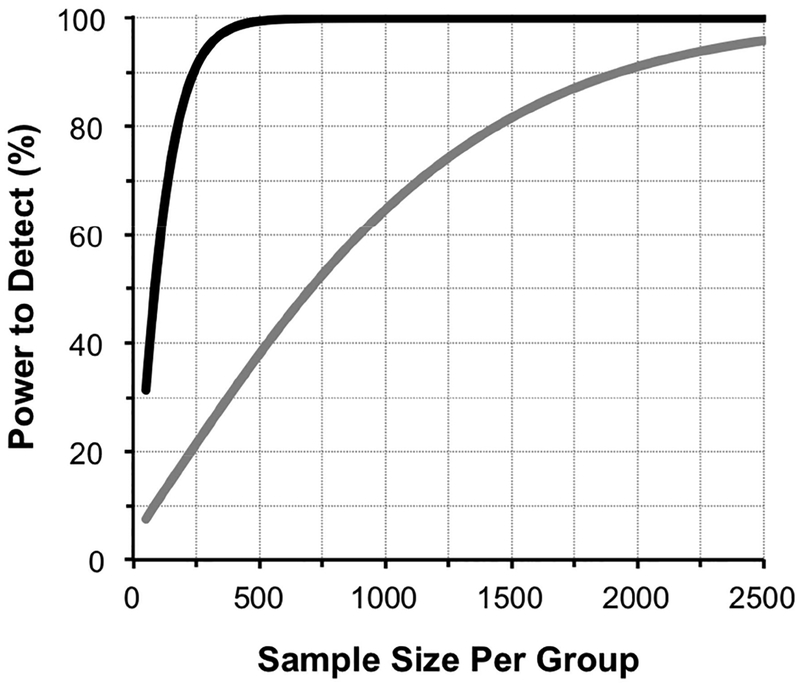
A plot of the power to detect a significant treatment effect against the sample size per group for a 30% treatment effect is illustrated in Figure 1.
Figure 1:
Plot of the power to detect a 30% treatment effect against sample size per group for survival analysis of event-based progression (grey line) versus trend analysis of rates of mean deviation change using linear mixed models (black line).
DISCUSSION
This study demonstrated that a dramatic reduction in the sample size required for short-term clinical trials could be achieved by evaluating differences in the rate of visual field MD change between groups using LMMs, when compared to a more conventional analysis of incident visual field progression using survival analysis. The magnitude of reduction in required sample size for detecting a significant treatment effect was between 85 to 90%, thereby providing a method to improve the feasibility of future glaucoma clinical trials, such as those that seek to evaluate potential neuroprotective treatments.3,26–28
In a previous review, Quigley3 estimated that 495 patients would be required in each group for a 2-year clinical trial where visual field testing was performed every 3 months, assuming that a new treatment slowed the MD rate of change by 30% when compared to an existing treatment. With a similar design, we estimated more optimistically in this study that 277 patients would be required in each group to detect the same treatment effect with 90% power. However, there are several key differences in the methods used by Quigley3 to obtain the previous estimates that prohibit a direct comparison with the estimates in this study. Firstly, their estimates of the MD rates of change (i.e. the mean ± SD) were derived from a study evaluating the natural history of glaucoma,18 assuming that the rates of change in a treated population would simply be a percentage of those natural history rates. However, such estimates may not be as accurate as using the actual distribution of the MD rates of change from a cohort of glaucoma patients under routine clinical care. Secondly, the sample size estimates were obtained by first calculating the MD rate of change for each participant (using ordinary least squares [OLS] linear regressions), and then comparing the difference in the mean MD rate of change between the two groups. This analysis differs from the use of LMMs in our study. With LMMs, the mean MD rate of change is also used as the outcome measure, but the estimates of the rates of change are made more precise since information from every visit is used in a model that takes advantage of the intra- and inter-individual variation to better describe changes over time.29 Indeed, the sample size required to detect a 30% treatment effect with 90% power would be 652 patients per group when comparing the OLS-derived MD rates of change between groups, as compared to 277 patients per group using LMMs.
These characteristics of LMMs account for the substantial reduction in sample size required when compared to survival analyses with visual field point-wise event-based progression as the outcome measure in this study. Whilst survival analyses are beneficial as they provide a relevant evaluation of whether a new treatment reduces the number of individuals that reach a clinical endpoint, this advantage is achieved at a substantial cost to its power to detect true treatment effects.30 In addition, event-based analyses of visual field sensitivity fail to account for the velocity of its change, which has been shown recently to relate to several different aspects of vision-related disability in glaucoma.10–17 These factors provide a compelling rationale for evaluating trend-based differences in visual field sensitivity between groups in future clinical trials, and is supported by the arguments made in a recent review for this approach.9
Although the difference between the sample size required for short-term clinical trials when using survival and trend analysis is clear, we highlight several key considerations when extrapolating the sample size calculations presented in this study for designing future trials. It is important to note that sample size requirements are closely related to the rate of progression in the cohort evaluated and assumptions about how a new treatment would affect this rate. For instance, fewer participants would be needed to detect a significant treatment effect if participants at greater risk for faster rates of visual field progression were included. In our simulations, we evaluated a cohort of participants that were on average 71 years old that had a mild to moderate level of baseline visual field damage (median MD being −4.37 dB) and a moderate median rate of progression (−0.57 dB/year for MD). However, the reports on the rate of visual field progression have varied widely in different clinical populations around the world.31–36 For example, one study reported a median rate of progression of only −0.12 dB/year for MD, despite the subjects having more severe baseline visual field damage than in this study (median MD at baseline being −7.79 dB).33 Another study reported a median rate of −0.62 dB/year for eyes with a median baseline MD of −10.0 dB for a cohort that was on average 71 years old.32 Furthermore, our study assumed that a new treatment would completely halt visual field loss in a proportion of participants (assuming that they were “responders” to this addition of a new treatment). This was required in order to allow us to simulate treatment effect when using point-wise event-based detection of progression and compare it to trend-based analysis. In effect, assuming that a new treatment would halt visual field loss in a proportion of participants is the same as assuming that the new treatment will slow down rate of change by the same proportion. However, it is possible that a new treatment would have varying levels of efficacy for different participants and may slow the point-wise rate of visual sensitivity decline differently for different levels of damage. As such, the assumption about the treatment effect as simulated in this study provides the simplest scenario for comparing the two methods of evaluating treatment efficacy in clinical trials.
Although the sample size benefits of using LMM trend-based analysis of MD change versus conventional event-based survival analysis are clear, future studies are needed to investigate sample size requirements using other promising methods for detecting visual field progression recently proposed in the literature, such as clustering visual field tests at the bookends of the clinical trial, or other approaches incorporating structure and function.37–44
In conclusion, sample size requirements for glaucoma clinical trials can be substantially reduced by evaluating differences in rates of visual field sensitivity change between groups using LMMs, instead of conventional event-based methods for detection of progression.
Supplementary Material
Funding Support:
Supported in part by a National Institutes of Health/National Eye Institute grant EY021818 (FAM); National Health and Medical Research Council Early Career Fellowship (#1104985, ZW)
Financial Disclosure(s):
Zhichao Wu: none; David P. Crabb: Recipient – Allergan (Irvine, CA), Roche (Basel, Switzerland), Santen (Osaka, Japan); Consultant – CenterVue (Padova, Italy); Balwantray C. Chauhan: Financial Support – Heidelberg Engineering (Heidelberg, Germany), Topcon (Livermore, CA); Consultant – Allergan (Irvine, CA), Heidelberg Engineering (Heidelberg, Germany); Jonathan G. Crowston: none; Felipe A. Medeiros: Financial support – Alcon Laboratories (Fort Worth, TX), Bausch & Lomb (Garden City, NY), Carl Zeiss Meditec (Jena, Germany), Heidelberg Engineering (Heidelberg, Germany), Merck (White House Station, NJ), Allergan (Irvine, CA), Topcon (Livermore, CA), Reichert (Dewey, NY), National Eye Institute (Bethesda, MD); Research support – Alcon Laboratories (Fort Worth, TX), Allergan (Irvine, CA), Carl Zeiss Meditec (Jena, Germany), Reichert (Dewey, NY); Consultant – Allergan (Irvine, CA), Carl-Zeiss Meditec, (Jena, Germany), Novartis (Basel, Switzerland).
References
- 1.Weinreb RN, Liebmann JM, Cioffi GA, et al. Oral Memantine for the Treatment of Glaucoma: Design and Results of 2 Randomized, Placebo-Controlled, Phase 3 Studies. Ophthalmology 2018;125(12):1874–85. [DOI] [PubMed] [Google Scholar]
- 2.Garway-Heath D, Crabb D, Bunce C, et al. Latanoprost treatment for open angle glaucoma. The United Kingdom Glaucoma Treatment Study: a multicentre, randomised, placebo-controlled clinical trial. Lancet 2015;385(9975):1295–304. [DOI] [PubMed] [Google Scholar]
- 3.Quigley HA. Clinical trials for glaucoma neuroprotection are not impossible. Curr Opin Ophthalmol 2012;23(2):144–54. [DOI] [PubMed] [Google Scholar]
- 4.Gordon MO, Kass MA. The Ocular Hypertension Treatment Study: design and baseline description of the participants. Arch Ophthalmol 1999;117(5):573–83. [DOI] [PubMed] [Google Scholar]
- 5.Leske MC, Heijl A, Hyman L, et al. Early Manifest Glaucoma Trial: design and baseline data. Ophthalmology 1999;106(11):2144–53. [DOI] [PubMed] [Google Scholar]
- 6.Musch DC, Lichter PR, Guire KE, Standardi CL. The collaborative initial glaucoma treatment study: Study design, methods, and baseline characteristics of enrolled patients. Ophthalmology 1999; 106(4):653–62. [DOI] [PubMed] [Google Scholar]
- 7.AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 1. Study design and methods and baseline characteristics of study patients. Control Clin Trials 1994;15(4):299–325. [DOI] [PubMed] [Google Scholar]
- 8.Krupin T, Liebmann JM, Greenfield DS, et al. The Low-pressure Glaucoma Treatment Study (LoGTS): study design and baseline characteristics of enrolled patients. Ophthalmology 2005;112(3):376–85. [DOI] [PubMed] [Google Scholar]
- 9.De Moraes CG, Liebmann JM, Levin LA. Detection and measurement of clinically meaningful visual field progression in clinical trials for glaucoma. Prog Retin Eye Res 2016;56:107–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lisboa R, Chun YS, Zangwill LM, et al. Association between rates of binocular visual field loss and vision-related quality of life in patients with glaucoma. JAMA Ophthalmol 2013;131(4):486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gracitelli CPB, Abe RY, Tatham AJ, et al. Association Between Progressive Retinal Nerve Fiber Layer Loss and Longitudinal Change in Quality of Life in Glaucoma. JAMA Ophthalmol 2015;133(4): 384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medeiros FA, Gracitelli CP, Boer ER, et al. Longitudinal changes in quality of life and rates of progressive visual field loss in glaucoma patients. Ophthalmology 2015;122(2):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abe RY, Diniz-Filho A, Costa VP, et al. The Impact of Location of Progressive Visual Field Loss on Longitudinal Changes in Quality of Life of Patients with Glaucoma. Ophthalmology 2016;123(3):552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abe RY, Gracitelli CP, Diniz-Filho A, et al. Frequency Doubling Technology Perimetry and Changes in Quality of Life of Glaucoma Patients: A Longitudinal Study. Am J Ophthalmol 2015;160(1):114–22. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diniz-Filho A, Abe RY, Cho HJ, et al. Fast Visual Field Progression Is Associated with Depressive Symptoms in Patients with Glaucoma. Ophthalmology 2016;123(4):754–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baig S, Diniz-Filho A, Wu Z, et al. Association of fast visual field loss with risk of falling in patients with glaucoma. JAMA Ophthalmol 2016;134(8):880–6. [DOI] [PubMed] [Google Scholar]
- 17.Abe RY, Diniz-Filho A, Costa VP, et al. Predicting Vision-Related Disability in Glaucoma. Ophthalmology 2018;125(1):22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heijl A, Bengtsson B, Hyman L, Leske MC. Natural History of Open-Angle Glaucoma. Ophthalmology 2009;116(12):2271–6. [DOI] [PubMed] [Google Scholar]
- 19.Kuang TM, Zhang C, Zangwill LM, et al. Estimating Lead Time Gained by Optical Coherence Tomography in Detecting Glaucoma before Development of Visual Field Defects. Ophthalmology 2015;122(10):2002–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sample PA, Girkin CA, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol 2009;127(9):1136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medeiros FA, Vizzeri G, Zangwill LM, et al. Comparison of retinal nerve fiber layer and optic disc imaging for diagnosing glaucoma in patients suspected of having the disease. Ophthalmology 2008; 115(8):1340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Z, Medeiros FA. Development of a Visual Field Simulation Model of Longitudinal Point-Wise Sensitivity Changes From a Clinical Glaucoma Cohort. Trans Vis Sci Tech 2018;7(3):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otarola F, Chen A, Morales E, et al. Course of Glaucomatous Visual Field Loss Across the Entire Perimetric Range. JAMA Ophthalmol 2016;134(5):496–502. [DOI] [PubMed] [Google Scholar]
- 24.Russell RA, Crabb DP, Malik R, Garway-Heath DF. The relationship between variability and sensitivity in large-scale longitudinal visual field data. Invest Ophthalmol Vis Sci 2012;53(10):5985–90. [DOI] [PubMed] [Google Scholar]
- 25.Wu Z, Medeiros FA. Comparison of Visual Field Point-Wise Event-Based and Global Trend-Based Analysis for Detecting Glaucomatous Progression. Trans Vis Sci Tech 2018;7(4):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang EE, Goldberg JL. Glaucoma 2.0: neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology 2012;119(5):979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sena DF, Lindsley K. Neuroprotection for treatment of glaucoma in adults. Cochrane Database Syst Rev 2013;2(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levin LA, Crowe ME, Quigley HA. Neuroprotection for glaucoma: Requirements for clinical translation. Exp Eye Res 2017;157:34–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982:963–74. [PubMed] [Google Scholar]
- 30.Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med 2006;25(1):127–41. [DOI] [PubMed] [Google Scholar]
- 31.De Moraes CGV, Juthani VJ, Liebmann JM, et al. Risk factors for visual field progression in treated glaucoma. Arch Ophthalmol 2011;129(5):562–8. [DOI] [PubMed] [Google Scholar]
- 32.Heijl A, Buchholz P, Norrgren G, Bengtsson B. Rates of visual field progression in clinical glaucoma care. Acta Ophthalmol 2013;91(5):406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chauhan BC, Malik R, Shuba LM, et al. Rates of Glaucomatous Visual Field Change in a Large Clinical Population. Invest Ophthalmol Vis Sci 2014;55(7):4135–43. [DOI] [PubMed] [Google Scholar]
- 34.Kirwan J, Hustler A, Bobat H, et al. Portsmouth visual field database: an audit of glaucoma progression. Eye 2014;28(8):974–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aptel F, Aryal-Charles N, Giraud JM, et al. Progression of visual field in patients with primary open-angle glaucoma - ProgF study 1. Acta Ophthalmol 2015;93(8):e615–e20. [DOI] [PubMed] [Google Scholar]
- 36.Fujino Y, Asaoka R, Murata H, et al. Evaluation of Glaucoma Progression in Large-Scale Clinical Data: The Japanese Archive of Multicentral Databases in Glaucoma (JAMDIG). Invest Ophthalmol Vis Sci 2016;57(4):2012–20. [DOI] [PubMed] [Google Scholar]
- 37.Crabb DP, Garway-Heath DF. Intervals between visual field tests when monitoring the glaucomatous patient: wait-and-see approach. Invest Ophthalmol Vis Sci 2012;53(6):2770–6. [DOI] [PubMed] [Google Scholar]
- 38.Wu Z, Medeiros FA. Impact of Different Visual Field Testing Paradigms on Sample Size Requirements for Glaucoma Clinical Trials. Scientific Reports 2018;8(1):4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Leary N, Chauhan BC, Artes PH. Visual Field Progression in Glaucoma: Estimating the Overall Significance of Deterioration with Permutation Analyses of Pointwise Linear Regression (PoPLR)Significance of Visual Field Deterioration. Invest Ophthalmol Vis Sci 2012;53(11):6776–84. [DOI] [PubMed] [Google Scholar]
- 40.Zhu H, Russell RA, Saunders LJ, et al. Detecting changes in retinal function: analysis with non-stationary Weibull error regression and spatial enhancement (ANSWERS). PLoS ONE 2014;9(1):e85654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu H, Crabb DP, Ho T, Garway-Heath DF. More Accurate Modeling of Visual Field Progression in Glaucoma: ANSWERS. Invest Ophthalmol Vis Sci 2015;56(10):6077–83. [DOI] [PubMed] [Google Scholar]
- 42.Medeiros FA, Lisboa R, Weinreb RN, et al. A combined index of structure and function for staging glaucomatous damage. Arch Ophthalmol 2012;130(9):1107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Medeiros FA, Leite MT, Zangwill LM, Weinreb RN. Combining structural and functional measurements to improve detection of glaucoma progression using Bayesian hierarchical models. Invest Ophthalmol Vis Sci 2011;52(8):5794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medeiros FA, Zangwill LM, Anderson DR, et al. Estimating the rate of retinal ganglion cell loss in glaucoma. Am J Ophthalmol 2012;154(5):814–24 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.