Abstract
Background
Pneumonia is the leading cause of death among children and young people (CYP) with severe cerebral palsy (CP). Only a few studies used nomogram for assessing risk factors and the probability of pneumonia. Therefore, we aimed to identify risk factors and devise a nomogram for identifying the probability of severe pneumonia in CYP with severe CP.
Methods
This retrospective nationwide population-based cohort study examined CYP with newly diagnosed severe CP before 18 years old between January 1st, 1997 and December 31st, 2013 and followed them up through December 31st, 2013. The primary endpoint was defined as the occurrence of severe pneumonia with ≥ 5 days of hospitalization. Logistic regression analysis was used for determining demographic factors and comorbidities associated with severe pneumonia. These factors were assigned integer points to create a scoring system to identify children at high risk for severe pneumonia.
Results
Among 6,356 CYP with newly diagnosed severe CP, 2,135 (33.59%) had severe pneumonia. Multivariable logistic regression analysis revealed that seven independent predictive factors, namely age <3 years, male sex, and comorbidities of pressure ulcer, gastroesophageal reflux, asthma, seizures, and perinatal complications. A nomogram was devised by employing these seven significant predictive factors. The prediction model presented favorable discrimination performance.
Conclusions
The nomogram revealed that age, male sex, history of pressure ulcer, gastroesophageal reflux, asthma, seizures, and perinatal complications were potential risk factors for severe pneumonia among CYP with severe CP.
Introduction
Cerebral palsy (CP), a crucial global public health concern, is the most common physical disability in early childhood [1–3]. The worldwide prevalence and incidence of CP are approximately 2–2.5 cases per 1,000 live births [2], and approximately 1–4 cases per 1,000 live births, respectively.[4] CP is clinically characterized by nonprogressive motor and cognitive and perceptive impairments secondary to the injury of the immature brain, exerting considerable influence on health outcome, quality of life, and life expectancy [5–7].
Studies have revealed that children with CP frequently develop multisystemic disorders, including respiratory [8], digestive, musculoskeletal, neurologic, and nutritional diseases, which require hospitalization [9]. Children with CP are particularly vulnerable to respiratory infection complications and have a higher risk of mortality [10, 11]. The primary reason for hospitalizations [12] and the leading cause of death among younger individuals with CP is pneumonia [4]. More than half (53%–58.6%) of the deaths among children with CP were attributed to respiratory infection and failure [13, 14].
CYP with CP with gastroesophageal reflex disease (GERD), oromotor dysfunction, seizures, poor nutritional status, or kyphoscoliosis can easily cause respiratory infection and disease during hospitalization [4, 10, 15]. Other risk factors for pneumonia include younger age, underweight, and lower maternal educational status [16]. Although factors associated with pneumonia have been investigated among children with CP, limited studies have focused on the risk factors for severe pneumonia in Asian children with CP using nomogram predictive models.
Nomograms, raphical depictions of predictive statistical models, have been developed and validated to assess various diseases outcomes, mainly cancer outcomes [17, 18]. They have consistently presented more favorable performance characteristics than other available options [19]. Moreover, Kawasaki et al. predicted postoperative pneumonia after major abdominal surgery using a nomogram [20]. This clinical tool has also been validated in a research focused on children with severe pneumonia [21].
Hence, the present study aims to identify factors associated with pneumonia among CYP with severe CP, and to establish a predicting nomogram based on population-based administrative data in Taiwan.
Methods
Data source
Data from the Taiwan’s National Health Insurance Research database (NHIRD) were used, which were released by the National Research Institutes for research purposes. Taiwan’s National Health Insurance (NHI) was established in 1995, which covered 99.6% of the whole population up to 2011. The advantages of using the NHIRD for research purposes have been described in previous literature [22]. The NHIRD registry consists of data of patients’ demographic characteristics, all types of medical visits, the medical costs for reimbursement; codes of diseases diagnosed; laboratory tests and procedures performed; and prescriptions prescribed. This study was approved by the Institutional Review Board of Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan (VGHKS15-CT12-01). Because all personal identifications are replaced with surrogate numbers, no informed consent was required from the study population. NHIRD has a registry for catastrophic illnesses patient database (RCIPD) [23], covering approximately 30 diseases including CP.
Study cohort
This study is a retrospective cohort study analyzing newly diagnosed YCP with severe CP between January 1st,1997 to December 31st, 2013.
Definition of severe CP
Severe CP diagnosis in this study were based on the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes 343.X. and catastrophic illness certificate.
To obtain a catastrophic illness certificate in Taiwan, first, CP diagnosis must be confirmed by the pediatric neurologists or rehabilitation specialists. Their subspecialist licenses were certified by the Ministry of Health and Welfare. Furthermore, they were claimed to train for additional 2–3 years and pass specialty test to become subspecialists. Second, in addition to clinical diagnosis, neonatal magnetic resonance imaging was also used for detecting cerebral palsy when clinical uncertainty. Third, the certification must be verified by the National Health Insurance Administration. Lastly, only those who have proven definite CP diagnosis with moderate to severe physical or mental disability by the designated hospitals can apply for a catastrophic illness certificate.
The disability qualification defined by two or more significant functional impairments according to the International Classification of Functioning (ICF), Disability and Health as follows: 1. Cognition, coherence, and psychological level; 2. Joint mobility (i.e., upper and lower limb joints); 3. Muscle strength loss; 4. Gross Motor Function Classification grades. For example, patients with diagnosis of two or more developmental delays, including mental or cognitive, language, movement and socio-emotional, or have been obtain a medical report on comprehensive developmental delay could acquire disability card [24].
The exclusion criteria for this study were CP diagnosed when patients were older than 18 years old (n = 1519) or incomplete data(n = 108).
Outcomes and predictor variables
The main outcome was the occurrence of severe pneumonia, defined by the inpatient pneumonia code (ICD-9-CM codes: 480–486 and 507.0–507.8) for more than 5 days. 5 days was chosen according to Zhang’s systematic analysis, the mean lengths of stay in hospital for children severe pneumonia is 5.8 days [25]. Patients’ sociodemographic characteristics, including CP diagnosis age (base on RCIPD), sex, residential area (Northern, Central, Southern and others), and hospital level (medical center, regional and others), were obtained from their initial enrollment data. Hospital level was categorized by the Ministry of Health and Welfare in Taiwan based on the staff teaching quantity and quality, physicians’ training capacity, the number of beds, the diversity of specialties, and the rate of emergency department visits. The qualification of the hospital-level will be evaluated periodically. Medical centers in Taiwan have the most physicians’ training implementation and medical care burden.
The comorbidities associated with severe pneumonia were retrieved by ICD-9-CM code from inpatient claims and ambulatory data. The ICD-9-CM codes were as follows: hearing loss (ICD-9-CM code: 389), asthma (ICD-9-CM code: 493), epilepsy (ICD-9-CM code: 345), diabetes mellitus (DM; ICD-9-CM code: 250), perinatal complications (ICD-9-CM codes: 760–764, 766–779, and V137), cerebral vascular accident (CVA; ICD-9-CM codes: 430–438), GERD (ICD-9CM codes: 530.85, 530.11, and 530.81), dysphagia (ICD-9CM code: 787.2), pressure ulcer (ICD-9CM code: 707.0), chronic liver disease (ICD-9CM code: 571), and intellectual disability (ICD-9CM codes: 317, 318, and 319). These comorbidities were included in the analysis if they occurred in an inpatient setting or in ≥3 ambulatory care claims for a chronic disease (or ≥1 ambulatory care claim for an acute disease).
Statistical analysis
The categorical variables for the study groups were compared using the chi-square test, and continuous variables were analyzed using one-way analysis of variance. The multivariable logistic regression model was used to assess variables associated with severe pneumonia, considering the demographic characteristics and comorbidities.
In addition, we constructed the nomogram plot to estimate the severe pneumonia probability. A nomogram was plotted to determine the numerical probability of severe pneumonia based on significant variables (including characteristics and comorbidities) selected from the multivariate logistic regression model. On the basis of the estimated beta coefficients, we ranked the estimated the effects of each variable. Finally, calibration curves were plotted to assess the nomogram calibration, along with the Hosmer–Lemeshow (H–L) test. A significant test statistic implied that the model was not calibrated perfectly.
All statistical analyses were performed using Statistical Analysis Software (SAS; version 9.4; SAS System for Windows) and SPSS (version 20; SPSS Inc., Chicago, IL). A p value of <0.05 was considered statistically significant.
Results
The eligible study participants were 6,356 CYP (59%:male) with newly diagnosed severe CP (median diagnosis age, 3.025 years), including 2,135 CYP (33.59%) with severe pneumonia (CP mean diagnosis age, 3.6 ± 3.9 years); 2,999 CYP (47.18%) without severe pneumonia (CP mean diagnosis age, 5.8 ± 4.6 years); and 1,222 CYP (19.23%) with severe pneumonia and hospitalization for 1–4 days (CP mean diagnosis age, 3.9 ± 3.6 years). The baseline characteristics of patients according to the subgroups of pneumonia are listed in Table 1. Among all relevant variables, CYP with severe pneumonia were significantly younger than those without severe pneumonia (3.6 ± 3.9 years vs. 5.8 ± 4.6 years; p < 0.001). In addition, comparing between the severe pneumonia and non-pneumonia groups revealed that CYP with CP along with severe pneumonia were more likely to be male (62% vs. 38%, p < 0.01) and have more comorbidities, except DM and intellectual disability.
Table 1. Baseline characteristics of children and young people with severe cerebral palsy according to subgroups of pneumonia, n = 6356.
| Variables | None | 1~4 days | ≥ 5 days | P value |
|---|---|---|---|---|
| N = 2999 (%) | N = 1222 (%) | N = 2135 (%) | ||
| Agea (Mean ± SD) | 5.8±4.6 | 3.9±3.6 | 3.6±3.9 | <0.001 |
| Gender | 0.001 | |||
| Male | 1691 (56%) | 745 (61%) | 1314 (62%) | |
| Female | 1308 (44%) | 477 (39%) | 821 (38%) | |
| Hospital characteristics | <0.001 | |||
| Medical center | 1751 (58%) | 705 (58%) | 1371 (64%) | |
| Regional/Others | 1248 (42%) | 517 (42%) | 764 (36%) | |
| Region | <0.001 | |||
| North | 1613 (54%) | 568 (46%) | 1005 (49%) | |
| Middle | 612 (20%) | 316 (26%) | 516 (24%) | |
| South/others | 774 (26%) | 338 (28%) | 564 (26%) | |
| Hearing loss | <0.001 | |||
| Yes | 276 (9%) | 185 (15%) | 315 (15%) | |
| No | 2723 (91%) | 1037 (85%) | 1820 (85%) | |
| Epilepsy | <0.001 | |||
| Yes | 715 (24%) | 540 (44%) | 1086 (51%) | |
| No | 2284 (76%) | 682 (56%) | 1049 (49%) | |
| Asthma | <0.001 | |||
| Yes | 120 (4%) | 174 (14%) | 281 (13%) | |
| No | 2879 (96%) | 1048 (86%) | 1854 (87%) | |
| Perinatal complications | <0.001 | |||
| Yes | 919 (31%) | 586 (48%) | 1094 (51%) | |
| No | 2080 (69%) | 636 (52%) | 1041 (49%) | |
| CVA | <0.001 | |||
| Yes | 266 (9%) | 131 (11%) | 269 (13%) | |
| No | 2733 (91%) | 1091 (89%) | 1866 (87%) | |
| DM | 0.910 | |||
| Yes | 13 (1%) | 6 (1%) | 11 (1%) | |
| No | 2986 (99%) | 1216 (99%) | 2124 (99%) | |
| Intellectual disability | 0.077 | |||
| Yes | 367 (12%) | 177 (15%) | 255 (12%) | |
| No | 2632 (88%) | 1045 (85%) | 1880 (88%) | |
| GERD | <0.001 | |||
| Yes | 54 (2%) | 39 (3%) | 127 (6%) | |
| No | 2945 (98%) | 1183 (97%) | 2009 (94%) | |
| Dysphagia | <0.001 | |||
| Yes | 30 (1%) | 22 (2%) | 64 (3%) | |
| No | 2969 (99%) | 1200 (98%) | 2071 (97%) | |
| Pressure ulcer | 0.006 | |||
| Yes | 9 (1%) | 1 (0.1%) | 16 (1%) | |
| No | 2990 (99%) | 1221 (99.9%) | 2119 (99%) | |
| Chronic liver disease | 0.025 | |||
| Yes | 14 (1%) | 9 (1%) | 24 (1%) | |
| No | 2985 (99%) | 1213 (99%) | 2111 (99%) |
Abbreviations: SD = standard deviation; CVA = Cerebral Vascular Accident; DM = Diabetes Mellitus; GERD = Gastroesophageal Reflux Disease
a Age: The age of newly diagnosed cerebral palsy
The characteristics of the patients and the results of univariate logistic regression analysis are presented in Table 2. We compared with and without severe pneumonia (inpatient care ≥ 5 days and none) among CYP with severe CP. The crude odds ratio (OR) for severe pneumonia in CP children diagnosis aged <3.1 years compared with those aged ≥3.1 years having CP was 2.84 (95% CI: 2.53–2.3.18, p < 0.001). Similarly, CYP with severe CP have comorbidities of hearing loss, DM, dysphagia, pressure ulcer, GERD, asthma, seizures, and perinatal complications were at an increased risk of developing severe pneumonia.
Table 2. Univariate logistic regression analysis for severe pneumonia among children and young people with severe cerebral palsy with ≥5 days inpatient care and none, n = 5134.
| Variables | Beta | OR (95% CI) | P value |
|---|---|---|---|
| Age group (Mean = 3.1) | |||
| 0~3.1 year | 1.042 | 2.84 (2.53–2.3.18) | <0.001 |
| >3.1 year | 1 | ||
| Gender | |||
| Female | 1 | ||
| Male | 0.213 | 1.24 (1.11–1.37) | <0.001 |
| Hearing loss | |||
| Yes | 0.535 | 1.71 (1.44–2.03) | <0.001 |
| No | 1 | ||
| Epilepsy | |||
| Yes | 1.196 | 3.31 (2.94–3.73) | <0.001 |
| No | 1 | ||
| Asthma | |||
| Yes | 1.291 | 3.64 (2.91–4.54) | <0.001 |
| No | 1 | ||
| Perinatal complications | |||
| Yes | 0.866 | 2.38 (2.12–2.67) | <0.001 |
| No | 1 | ||
| CVA | |||
| Yes | 0.393 | 1.48 (1.24–1.77) | <0.001 |
| No | 1 | ||
| DM | |||
| Yes | 0.174 | 1.19 (0.53–2.66) | 0.673 |
| No | 1 | ||
| Intellectual disability | |||
| Yes | 0.028 | 1.03 (0.87–1.22) | 0.751 |
| No | 1 | ||
| GERD | |||
| Yes | 1.230 | 3.42 (2.48–4.73) | <0.001 |
| No | 1 | ||
| Dysphagia | |||
| Yes | 1.118 | 3.06 (1.98–4.74) | <0.001 |
| No | 1 | ||
| Pressure ulcer | |||
| Yes | 0.920 | 2.51 (1.11–5.69) | 0.028 |
| No | 1 | ||
| Chronic liver disease | |||
| Yes | 0.885 | 2.424 (1.25–4.69) | 0.009 |
| No | 1 |
Abbreviation: OR = Odd Ratio; CVA = Cerebral Vascular Accident; DM = Diabetes Mellitus; GERD = Gastroesophageal Reflux Disease.
The results of the stepwise logistic regression analysis are presented in Table 3. After adjustment for age, sex, and comorbidities, CP diagnosis age <3.1 years (OR: 2.49; 95% CI: 2.19–2.85; p < 0.001), male sex (OR: 1.22; 95% CI: 1.08–1.38; p = 0.002), and comorbidities of epilepsy (OR: 3.19; 95% CI: 2.81–3.63; p < 0.001), asthma (OR: 3.60; 95% CI: 2.85–4.58; p < 0.001), perinatal complications (OR: 1.60; 95% CI: 1.40–1.82; p < 0.001), GERD (OR: 1.56; 95% CI: 1.11–2.11; p = 0.012), and pressure ulcer (OR: 3.97; 95% CI: 1.66–9.45; p = 0.002) were identified as independent risk factors for severe pneumonia.
Table 3. Stepwise logistic regression analysis for severe pneumonia among children and young people with ≥ 5 days inpatient care and none, n = 5134.
| Variables | Beta | OR (95% CI) | P value |
|---|---|---|---|
| Age group (Mean = 3.1) | |||
| 0~3.1 year | 0.915 | 2.49 (2.19–2.85) | <0.001 |
| >3.1 year | 1 | ||
| Gender | |||
| Female | 1 | ||
| Male | 0.196 | 1.22 (1.08–1.38) | 0.002 |
| Epilepsy | |||
| Yes | 1.161 | 3.19 (2.81–3.63) | <0.001 |
| No | 1 | ||
| Asthma | |||
| Yes | 1.282 | 3.60 (2.85–4.58) | <0.001 |
| No | 1 | ||
| Perinatal complications | |||
| Yes | 0.468 | 1.60 (1.40–1.82) | <0.001 |
| No | 1 | ||
| GERD | |||
| Yes | 0.447 | 1.56 (1.11–2.21) | 0.012 |
| No | 1 | ||
| Pressure ulcer | |||
| Yes | 1.380 | 3.97 (1.66–9.45) | 0.002 |
| No | 1 |
Prognostic nomogram development and calibration
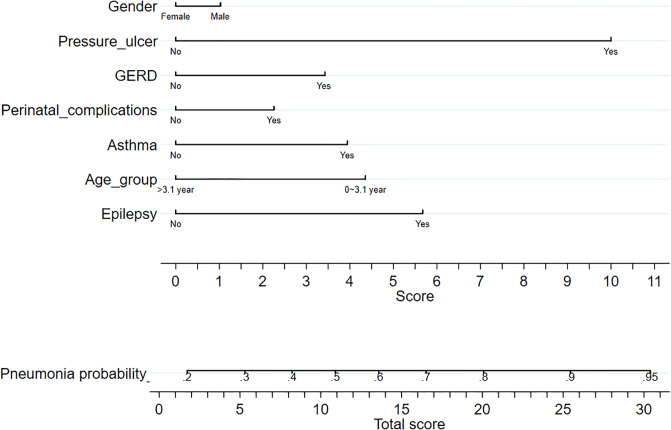
Fig 1 present prognostic nomograms with independent risk factors for severe pneumonia in children with severe CP. Each significant variable listed in Table 3 was assigned a score on the point scale. A straight line could be drawn to estimate the probability of severe pneumonia at each time point by summing up the total score and locating it on the total point scale.
Fig 1. Nomogram plot for predicting severe pneumonia in CP children.
For an individual patient, each variable corresponds to a point in the 8th row (names “score”). The total points were summed up by all points and are indicated in the 10th row (the bottom row). Drawing a vertical line from total point to the 9th row will show the corresponding probability of pneumonia.
For example, if the patient was male and with GERD and pressure ulcer, we located a patient’s gender on the relevant axis first. Next, we draw a straight line downward to the point axis (8th row, named “Score”) to obtain the points based on gender (male was 1 point). Then we repeated this course for age variable (CP diagnosis age is 2 years old:4.5 points), GERD variable (3.5 points) and pressure ulcer variable (10 points). After that, we summed up all the points (19 points) to obtain the “Total score” (the bottom row). Finally, we draw a straight line upward from the 10th row to obtain the probability of developing pneumonia in the 9th row. That is, a male CP who diagnosis age is 2-year-old patient with pressure ulcer and GERD history has a nearly 80% probability of serve pneumonia.
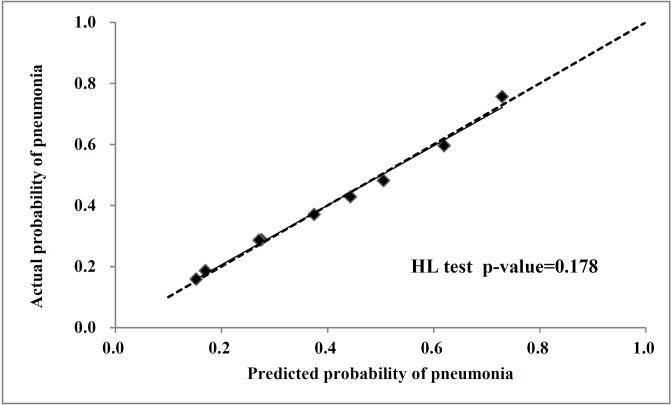
Fig 2 showed a calibration curve that the predicted probabilities of pneumonia (the diagonal square) matched the reference line. The dashed line is an ideal reference line representing the predicted probabilities of pneumonia would match the observed proportions. Additionally, a closer fit to the dashed line represents a better prediction. The H-L test p-value was 0.1783 (p > 0.05), which demonstrated good agreement between prediction and observation. It also indicates the performance of our model is good.
Fig 2. The calibration curve for the radiomics nomogram plot.
Dashed line indicates ideal reference line where predicted probabilities would match the observed proportions (the diagonal square) and, of which a closer fit to the diagonal dotted line represents a better prediction. The Hosmer-Lemeshow test showed the p-value is 0.178.
Discussion
To the best of our knowledge, this is the first cohort study to predict severe pneumonia risk in children with severe CP through nomograms using nationwide population-based data. We classified the risk based on the medical comorbidities and sociodemographic factors. The major finding of this study was that CP diagnosis age < 3 years; male sex; and having comorbidities namely epilepsy, asthma, perinatal complications, GERD, and pressure ulcer were significantly correlated with severe pneumonia in children with severe CP. Second, the nomogram was found to be a suitable and useful tool for predicting the probability of severe pneumonia.
This study has several strengths. This nationwide population-based cohort study included CYP of all ages with newly diagnosed severe CP (<18 years old) between 1997 and 2013, had a longer follow-up period, and the first study to use a prognostic nomogram for CYP CP severe pneumonia prediction.
In past microbiological studies, the most relevant bacterial stains causing children pneumonia included Streptococcus pneumoniae and Haemophilus influenzae type B, followed by Staphylococcus aureus and Mycoplasma pneumoniae [16]. Bacterial pathogens associated with WHO-defined very severe pneumonia (the most advanced form of the disease) were: Streptococcus pneumoniae and Staphylococcus aureus, followed less commonly by Haemophilus influenzae, Escherichia coli, and Pseudomonas aeruginosa [16].
Pressure ulcer was found to be the leading factor for severe pneumonia occurrence. This may be because they share many pathogenic factors and may interact with each other. pressure ulcer has been known to be caused by immobility, sensory loss, and malnutrition [26] and occurred more frequently among children with a deteriorated neurological condition such as CP [27]. According Leonard’s research, malnutrition and higher Gross Motor Function Classification System (GMFCS) level were risk factors for pneumonia [28]. Despite that nutritional status and GMFCS were not accessible in Taiwan NHIRD, diagnosis of pressure ulcer could be an indicator of poorer functional level. Therefore, pressure ulcer is the most dominant factor for severe pneumonia occurrence. The prevalence of pressure ulcer in the current study was low. We presume the reason is that abnormal neuromuscular development among YCP with CP hinders independent movement and withdrawal from pressure [29].
Asthma was found to be the dominant factor for pneumonia in the present study. Some studies have revealed that children with asthma had sustained increase in risk for invasive Pneumococcal pneumonia infections [30–32]. The increased risk was attributed to the structural changes arising around the trachea, bronchi, and bronchioles, leading to chronic inflammation [33]. Another possible reason may due to higher Streptococcus pneumoniae nasopharyngeal carriage and primary immunodeficiencies in children with asthma.[34] Additionally, inhaled corticosteroids were associated with oropharyngeal S. pneumoniae colonization in children with asthma [35].
Younger CP diagnosis age in Children were significantly more risk for getting severe pneumonia, which is consistent with the result of a previous retrospective study.[36] The younger a person is when CP is diagnosed, the more severe the CP is, and there will be more complications, including pneumonia. One study investigating invasive pneumococcal infections in infants and young children in Santiago also found a higher prevalence of pneumonia in younger children (<6 months) [37].
GERD has been considered a factor associated with the long-term risk of pneumonia [38]. Occult micro aspiration has been reported as the key pathological factor connecting GERD and lung disease [39]. Furthermore, spasticity of abdominal muscles causing increased intra-abdominal pressure in patients with severe CP was noted to contribute to pneumonia [8].
Pneumonia has been widely recognized as a complication of seizures. A population-based study on children admitted in intensive care revealed a similar finding that epilepsy was a significant risk factor for pneumonia [40]. The major cause was the aspiration of secretions, when seizure hindered the airway protective reflexes. Second, aspiration occurred frequently in supine position during postictal recovery and increased orotracheal secretions in the postictal state [41].
Perinatal complications were associated with an increased risk of severe pneumonia among CYP CP. The association between perinatal complications and CP have been established [42] and result in younger CP diagnosis. Early diagnosis indicates greater severity of CP with poorer outcomes. As mentioned above, younger CP diagnosis age in Children were significantly more risk for getting severe pneumonia. Moreover, Perinatal complications, such as chorioamnionitis or fetal asphyxia leading to amniotic fluid bacterial infection or colonization of the birth canal were associated with pneumonia [43]. Perinatal anoxia or traumatic brain injury have been demonstrated a linkage to swallowing dysfunction [44]. Without intact swallowing function as integrated epiglottic and cough reflexes, people cannot avoid aspiration and expel infected secretions. Therefore, underlying perinatal complications and CP both predispose children to pneumonia.
Dysphagia has been considered a common symptom in children with CP and tend to present food aspiration, malnutrition, and respiratory infections [45]. Blackmore et al suggested that the strongest modifiable risk factor for respiratory-related hospitalizations in pediatric CP was oropharyngeal dysphagia [10]. However, our study revealed a contradictory result. This may be attributed to the incomparability of the study definition that we used (ICD-9) for diagnosis with low prevalence of dysphagia. Because video-fluoroscopic swallowing study and fiber-optic endoscopic examination were time-efforts consuming and high patient’s cooperation demand in Taiwan. Thus, few patients received this standard evaluation.
Comparing between the severe pneumonia and non-pneumonia groups revealed that CYP with CP along with severe pneumonia were more likely to be male (62% vs. 38%, p < 0.01). According to Nathan’s children prospective cohort study, bacterial pneumonia was seen more in males [46]. We think some factors play the role in it. First, male children usually have poor hygiene and sanitary habits, and that both habits are risk factors for pneumonia. Additionally, male children are more likely to be exposed to outdoor air pollution due to more external activities than female children [47]. Second, the anatomical disparity in the respiratory tract may partially explain the different incidence of pneumonia between men and women. For example, peripheral airways are disproportionately narrowed in male’s early childhood, which can lead to lower respiratory tract infections [48]. Third, human lung development and pulmonary infection susceptibility were affected by altered estrogen and testosterone levels. Evidence have suggested an active role of estrogen in sexual dimorphism by presenting different estrogens levels in lung maturation, preservation, regeneration, alveoli development and surfactant synthesis. Female pulmonary surfactant production was manifested earlier than male [49]. Fourth, estrogen and androgen play the opposite way on immune responses after infection. Androgens in males cause extended susceptibility to infections. Inversely, estrogen makes females less vulnerable to some infectious [50]. Though sex hormone effect is not apparent in young children, it may partially illustrate the sex disparity on infections.
The nomogram was composed of several simple demographic and clinical factors, which may be useful for identifying patients with a high probability of developing severe pneumonia. The nomogram could be used to closely monitor CYP with severe CP and facilitate physicians or healthcare professionals in clinical care. Moreover, the nomogram may provide appropriate information and suggestions to the family of CYP with severe CP, facilitating early alert and transfer for medical management. Considering the public health viewpoint, policymakers are encouraged to enforce severe pneumonia risk screening in CYP with CP and to provide more integrated care such as a combination of medical care and rehabilitation therapy.
Several limitations for interpreting this study results existed. First, the Gross Motor Function Classification System (GMFCS) level was not accessible in Taiwan NHIRD, which is an indicator of poor functional level. GMFCS level V children do worse than those with lower levels of severity. CPY with CP classified at level V GMFCS had higher risk of hospital admissions than other GMFCS levels [10]. However, we used ICF, catastrophic illness certification card and disability cards to make our diagnosis more precise. Second, we could not obtain the birth body weight, nutrition status, parental educational level, breast feeding duration, and household environment, such as presence of cigarette smoke. Third, the prevalence of dysphagia, GERD and pressure ulcer were low result from that we used (ICD-9-CM codes) for diagnosis, and we should carefully interpret the result due to potential dropout bias and underestimation in our database. Presence of enteral feeding (i.e. nasogastric tube, nasoduodenal tube, gastrostomy tube, jejunostomy tube, gastrostomy/jejunostomy tube) were associated with much worse CP outcomes. But in the present study, we did not get the data. Fourth, because we only assessed severe CYP patients and we cannot apply the results to all CP population. Lastly, defining the pathogen of pneumonia can be difficult due to the challenge in collecting samples from young children’s lower respiratory tract and waived from contaminated during the procedure. Therefore, the diagnosis of pneumonia was defined by ICD-9 code and we cannot obtain the pathogens or causes of pneumonia in this database.
Conclusion
Younger age, male sex, and history of pressure ulcer, GERD, asthma, seizures, and perinatal complications were found to be potential risk factors for severe pneumonia in CYP with severe CP, which can be influenced by different interventions in the future. The nomogram was found to be a useful tool for identifying CYP with severe CP having a high risk for severe pneumonia.
Acknowledgments
The authors expressed their appreciation to the Department of Medical Education and Research and Research Center of Medical Informatics in Kaohsiung Veterans General Hospital for inquiries and assistance in data processing.
Abbreviations
- CVA
Cerebral vascular accident
- DM
Diabetes mellitus
- GMFCS
Gross Motor Function Classification System
- ICD-9-CM
International Classification of Disease, Ninth Revision, Clinical Modification
- ICF
International Classification of Functioning
- NHI
National Health Insurance
- NHIRD
National Health Insurance Research database
- OR
Odds ratio
- RCIPD
Registry for catastrophic illnesses patient database
Data Availability
All relevant data are within the paper. The data used in this study were from the Taiwan National Health Insurance Research database (NHIRD), released by the National Research Institutes for research purposes. The application for the dataset may be mailed to the NHRI at nhird@nhri.org.tw or call at +886-037-246166 ext. 33603 for immediate assistance. Office hours: Monday–Friday, 8:00–17:30. The NHIRD, which was open to researchers in Taiwan, was available from the Health and Welfare Data Science Center (HWDC), Ministry of Health and Welfare (MOHW) (http://www.mohw.gov.tw/cht/DOS/). The data underlying this study was obtained from the NHIRD. Applicants interested in obtaining the data are able to propose a formal application to the Ministry of Health and Welfare of Taiwan.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Stavsky M, Mor O, Mastrolia SA, Greenbaum S, Than NG, Erez O. Cerebral Palsy-Trends in Epidemiology and Recent Development in Prenatal Mechanisms of Disease, Treatment, and Prevention. Frontiers in pediatrics. 2017;5:21 Epub 2017/03/01. 10.3389/fped.2017.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oskoui M, Coutinho F, Dykeman J, Jette N, Pringsheim T. An update on the prevalence of cerebral palsy: a systematic review and meta-analysis. Developmental medicine and child neurology. 2013;55(6):509–19. Epub 2013/01/26. 10.1111/dmcn.12080 . [DOI] [PubMed] [Google Scholar]
- 3.Gulati S, Sondhi V. Cerebral Palsy: An Overview. Indian journal of pediatrics. 2018;85(11):1006–16. Epub 2017/11/21. 10.1007/s12098-017-2475-1 . [DOI] [PubMed] [Google Scholar]
- 4.Boel L, Pernet K, Toussaint M, Ides K, Leemans G, Haan J, et al. Respiratory morbidity in children with cerebral palsy: an overview. Developmental medicine and child neurology. 2019;61(6):646–53. Epub 2018/10/16. 10.1111/dmcn.14060 . [DOI] [PubMed] [Google Scholar]
- 5.Strauss D, Brooks J, Rosenbloom L, Shavelle R. Life expectancy in cerebral palsy: an update. Developmental medicine and child neurology. 2008;50(7):487–93. Epub 2008/07/10. 10.1111/j.1469-8749.2008.03000.x . [DOI] [PubMed] [Google Scholar]
- 6.Westbom L, Bergstrand L, Wagner P, Nordmark E. Survival at 19 years of age in a total population of children and young people with cerebral palsy. Developmental medicine and child neurology. 2011;53(9):808–14. Epub 2011/07/13. 10.1111/j.1469-8749.2011.04027.x . [DOI] [PubMed] [Google Scholar]
- 7.Brooks JC, Strauss DJ, Shavelle RM, Tran LM, Rosenbloom L, Wu YW. Recent trends in cerebral palsy survival. Part II: individual survival prognosis. Developmental medicine and child neurology. 2014;56(11):1065–71. Epub 2014/07/22. 10.1111/dmcn.12519 . [DOI] [PubMed] [Google Scholar]
- 8.Seddon PC, Khan Y. Respiratory problems in children with neurological impairment. Archives of disease in childhood. 2003;88(1):75–8. Epub 2002/12/24. 10.1136/adc.88.1.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy NA, Hoff C, Jorgensen T, Norlin C, Young PC. Costs and complications of hospitalizations for children with cerebral palsy. Pediatric rehabilitation. 2006;9(1):47–52. Epub 2005/12/15. 10.1080/13638490500079476 . [DOI] [PubMed] [Google Scholar]
- 10.Blackmore AM, Bear N, Blair E, Langdon K, Moshovis L, Steer K, et al. Predicting respiratory hospital admissions in young people with cerebral palsy. Archives of disease in childhood. 2018;103(12):1119–24. Epub 2018/03/21. 10.1136/archdischild-2017-314346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jahan I, Karim T, Das MC, Muhit M, McIntyre S, Smithers-Sheedy H, et al. Mortality in children with cerebral palsy in rural Bangladesh: a population-based surveillance study. Developmental medicine and child neurology. 2019. Epub 2019/05/14. 10.1111/dmcn.14256 . [DOI] [PubMed] [Google Scholar]
- 12.Young NL, McCormick AM, Gilbert T, Ayling-Campos A, Burke T, Fehlings D, et al. Reasons for hospital admissions among youth and young adults with cerebral palsy. Archives of physical medicine and rehabilitation. 2011;92(1):46–50. Epub 2010/12/29. 10.1016/j.apmr.2010.10.002 . [DOI] [PubMed] [Google Scholar]
- 13.Himmelmann K, Sundh V. Survival with cerebral palsy over five decades in western Sweden. Developmental medicine and child neurology. 2015;57(8):762–7. Epub 2015/02/20. 10.1111/dmcn.12718 . [DOI] [PubMed] [Google Scholar]
- 14.Blair E, Langdon K, McIntyre S, Lawrence D, Watson L. Survival and mortality in cerebral palsy: observations to the sixth decade from a data linkage study of a total population register and National Death Index. BMC Neurol. 2019;19(1):111 Epub 2019/06/06. 10.1186/s12883-019-1343-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veugelers R, Calis EA, Penning C, Verhagen A, Bernsen R, Bouquet J, et al. A population-based nested case control study on recurrent pneumonias in children with severe generalized cerebral palsy: ethical considerations of the design and representativeness of the study sample. BMC Pediatr. 2005;5:25 Epub 2005/07/21. 10.1186/1471-2431-5-252431-5-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonnalagadda S, Rodriguez O, Estrella B, Sabin LL, Sempertegui F, Hamer DH. Etiology of severe pneumonia in Ecuadorian children. PLoS One. 2017;12(2):e0171687 Epub 2017/02/10. 10.1371/journal.pone.0171687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konishi T, Shimada Y, Hsu M, Wei IH, Pappou E, Smith JJ, et al. Contemporary Validation of a Nomogram Predicting Colon Cancer Recurrence, Revealing All-Stage Improved Outcomes. JNCI Cancer Spectr. 2019;3(2):pkz015 Epub 2019/05/24. 10.1093/jncics/pkz015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasegawa W, Yamauchi Y, Yasunaga H, Takeshima H, Sakamoto Y, Jo T, et al. Prognostic nomogram for inpatients with asthma exacerbation. BMC Pulm Med. 2017;17(1):108 Epub 2017/08/06. 10.1186/s12890-017-0450-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–80. Epub 2015/04/08. 10.1016/S1470-2045(14)71116-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawasaki K, Yamamoto M, Suka Y, Kawasaki Y, Ito K, Koike D, et al. Development and validation of a nomogram predicting postoperative pneumonia after major abdominal surgery. Surg Today. 2019;49(9):769–77. Epub 2019/03/29. 10.1007/s00595-019-01796-8 . [DOI] [PubMed] [Google Scholar]
- 21.Mamtani M, Patel A, Hibberd PL, Tuan TA, Jeena P, Chisaka N, et al. A clinical tool to predict failed response to therapy in children with severe pneumonia. Pediatr Pulmonol. 2009;44(4):379–86. Epub 2009/03/31. 10.1002/ppul.21014 . [DOI] [PubMed] [Google Scholar]
- 22.Hsing AW, Ioannidis JP. Nationwide Population Science: Lessons From the Taiwan National Health Insurance Research Database. JAMA Intern Med. 2015;175(9):1527–9. Epub 2015/07/21. 10.1001/jamainternmed.2015.3540 . [DOI] [PubMed] [Google Scholar]
- 23.Administration NHI. Patients with Catastrophic Illnesses or Rare Diseases Taiwan2015 [updated 2016-01-19]. https://www.nhi.gov.tw/english/Content_List.aspx?n=F5B8E49CB4548C60&to%20pn=1D1ECC54F86E9050.
- 24.I. Leslie Rubin JM, Donald E. Greydanus, Dilip R Patel. Health Care for People with Intellectual and Developmental Disabilities across the Lifespan. 3rd ed. Rubin IL, Merrick, J., Greydanus, D.E., Patel, D.R., editor. Switzerland Springer, Cham; 2016 2016/04/25.
- 25.Zhang S, Sammon PM, King I, Andrade AL, Toscano CM, Araujo SN, et al. Cost of management of severe pneumonia in young children: systematic analysis. J Glob Health. 2016;6(1):010408 Epub 2016/05/28. 10.7189/jogh.06.010408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox GN. Management of pressure ulcers. Jama. 2003;289(17):2210; author reply -1. Epub 2003/05/08. 10.1001/jama.289.17.2210-a . [DOI] [PubMed] [Google Scholar]
- 27.Samaniego IA. A sore spot in pediatrics: risk factors for pressure ulcers. Pediatr Nurs. 2003;29(4):278–82. Epub 2003/09/06. . [PubMed] [Google Scholar]
- 28.Leonard M, Dain E, Pelc K, Dan B, De Laet C. Nutritional status of neurologically impaired children: Impact on comorbidity. Arch Pediatr. 2019. Epub 2019/12/04. 10.1016/j.arcped.2019.11.003 . [DOI] [PubMed] [Google Scholar]
- 29.Scheans P. Neonatal Pressure Ulcer Prevention. Neonatal Netw. 2015;34(2):126–32. 10.1891/0730-0832.34.2.126 . [DOI] [PubMed] [Google Scholar]
- 30.Wilson KM, Torok MR, Localio R, McLeod L, Srivastava R, Luan X, et al. Hospitalization for Community-Acquired Pneumonia in Children: Effect of an Asthma Codiagnosis. Hospital pediatrics. 2015;5(8):415–22. Epub 2015/08/02. 10.1542/hpeds.2015-0007 . [DOI] [PubMed] [Google Scholar]
- 31.Boikos C, Quach C. Risk of invasive pneumococcal disease in children and adults with asthma: a systematic review. Vaccine. 2013;31(42):4820–6. Epub 2013/08/24. 10.1016/j.vaccine.2013.07.079 . [DOI] [PubMed] [Google Scholar]
- 32.Hsu K, Pelton S, Karumuri S, Heisey-Grove D, Klein J. Population-based surveillance for childhood invasive pneumococcal disease in the era of conjugate vaccine. Pediatr Infect Dis J. 2005;24(1):17–23. Epub 2005/01/25. 10.1097/01.inf.0000148891.32134.36 . [DOI] [PubMed] [Google Scholar]
- 33.Esposito S, Musio A, Principi N. Paediatric asthma and pneumococcal vaccination. Vaccine. 2013;31(44):5015–9. Epub 2013/09/12. 10.1016/j.vaccine.2013.08.090 . [DOI] [PubMed] [Google Scholar]
- 34.Pekuz S, Soysal A, Akkoc G, Atici S, Yakut N, Gelmez GA, et al. Prevalence of Nasopharyngeal Carriage, Serotype Distribution, and Antimicrobial Resistance of Streptococcus pneumoniae among Children with Chronic Diseases. Jpn J Infect Dis. 2019;72(1):7–13. Epub 2018/09/04. 10.7883/yoken.JJID.2017.410 . [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Prietsch SO, Mendes AP, Von Groll A, Rocha GP, Carrion L, et al. Inhaled corticosteroids increase the risk of oropharyngeal colonization by Streptococcus pneumoniae in children with asthma. Respirology. 2013;18(2):272–7. Epub 2012/10/09. 10.1111/j.1440-1843.2012.02280.x . [DOI] [PubMed] [Google Scholar]
- 36.Taniguchi MH, Moyer RS. Assessment of risk factors for pneumonia in dysphagic children: significance of videofluoroscopic swallowing evaluation. Developmental medicine and child neurology. 1994;36(6):495–502. Epub 1994/06/01. 10.1111/j.1469-8749.1994.tb11879.x . [DOI] [PubMed] [Google Scholar]
- 37.Levine MM, Lagos R, Levine OS, Heitmann I, Enriquez N, Pinto ME, et al. Epidemiology of invasive pneumococcal infections in infants and young children in Metropolitan Santiago, Chile, a newly industrializing country. Pediatr Infect Dis J. 1998;17(4):287–93. Epub 1998/05/12. 10.1097/00006454-199804000-00005 . [DOI] [PubMed] [Google Scholar]
- 38.Hsu WT, Lai CC, Wang YH, Tseng PH, Wang K, Wang CY, et al. Risk of pneumonia in patients with gastroesophageal reflux disease: A population-based cohort study. PLoS One. 2017;12(8):e0183808 Epub 2017/08/25. 10.1371/journal.pone.0183808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morehead RS. Gastro-oesophageal reflux disease and non-asthma lung disease. Eur Respir Rev. 2009;18(114):233–43. Epub 2010/10/20. 10.1183/09059180.00002509 [DOI] [PubMed] [Google Scholar]
- 40.Hsu CL, Lee YS, Chen CJ, Lee ML, Yang CF, Soong WJ, et al. A population-based analysis of children with pneumonia among intensive care units in Taiwan. J Microbiol Immunol Infect. 2015;48(2):153–9. Epub 2013/09/26. 10.1016/j.jmii.2013.07.007 . [DOI] [PubMed] [Google Scholar]
- 41.DeToledo JC, Lowe MR, Gonzalez J, Haddad H. Risk of aspiration pneumonia after an epileptic seizure: a retrospective analysis of 1634 adult patients. Epilepsy Behav. 2004;5(4):593–5. Epub 2004/07/17. 10.1016/j.yebeh.2004.03.009 . [DOI] [PubMed] [Google Scholar]
- 42.Hjern A, Thorngren-Jerneck K. Perinatal complications and socio-economic differences in cerebral palsy in Sweden—a national cohort study. BMC pediatrics. 2008;8:49-. 10.1186/1471-2431-8-49 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duke T. Neonatal pneumonia in developing countries. Arch Dis Child Fetal Neonatal Ed. 2005;90(3):F211–9. Epub 2005/04/23. 10.1136/adc.2003.048108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farneti D, Genovese E. Swallowing Disorders in Newborn and Small Children. Advances in Speech-language Pathology2017.
- 45.Lagos-Guimaraes HN, Teive HA, Celli A, Santos RS, Abdulmassih EM, Hirata GC, et al. Aspiration Pneumonia in Children with Cerebral Palsy after Videofluoroscopic Swallowing Study. Int Arch Otorhinolaryngol. 2016;20(2):132–7. Epub 2016/04/21. 10.1055/s-0035-1566093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nathan AM, Teh CSJ, Jabar KA, Teoh BT, Tangaperumal A, Westerhout C, et al. Bacterial pneumonia and its associated factors in children from a developing country: A prospective cohort study. PloS one. 2020;15(2):e0228056 Epub 2020/02/15. 10.1371/journal.pone.0228056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen TK, Tran TH, Roberts CL, Fox GJ, Graham SM, Marais BJ. Risk factors for child pneumonia—focus on the Western Pacific Region. Paediatr Respir Rev. 2017; 21:95–101. Epub 2016/08/16. 10.1016/j.prrv.2016.07.002 . [DOI] [PubMed] [Google Scholar]
- 48.Gupta R, Helms Pj Fau….Jolliffe IT, Jolliffe It Fau Douglas AS, Douglas AS. Seasonal variation in sudden infant death syndrome and bronchiolitis—a common mechanism? Am J Respir Crit Care Med. 1996;154(2 Pt 1):431–435. 10.1164/ajrccm.154.2.8756818 [DOI] [PubMed] [Google Scholar]
- 49.Silveyra P, Fuentes N, Rivera L. Understanding the Intersection of Environmental Pollution, Pneumonia, and Inflammation: Does Gender Play a Role? Contemporary Topics of Pneumonia. 2017:1. [Google Scholar]
- 50.Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci Biobehav Rev. 2000;24(6):627–38. Epub 2000/08/15. 10.1016/s0149-7634(00)00027-0 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper. The data used in this study were from the Taiwan National Health Insurance Research database (NHIRD), released by the National Research Institutes for research purposes. The application for the dataset may be mailed to the NHRI at nhird@nhri.org.tw or call at +886-037-246166 ext. 33603 for immediate assistance. Office hours: Monday–Friday, 8:00–17:30. The NHIRD, which was open to researchers in Taiwan, was available from the Health and Welfare Data Science Center (HWDC), Ministry of Health and Welfare (MOHW) (http://www.mohw.gov.tw/cht/DOS/). The data underlying this study was obtained from the NHIRD. Applicants interested in obtaining the data are able to propose a formal application to the Ministry of Health and Welfare of Taiwan.