Abstract
Background:
Women who deliver preterm have been reported to have increased future risks of cardiometabolic disorders. However, their long-term risks of ischemic heart disease (IHD) and whether such risks are due to shared familial factors are unclear. A better understanding of these risks may help improve long-term clinical follow-up and interventions to prevent IHD in women.
Objectives:
To determine the long-term risks of IHD in women by pregnancy duration.
Methods:
A national cohort study was conducted of all 2,189,190 women with a singleton delivery in Sweden in 1973–2015, who were followed up for IHD through the end of 2015. Cox regression was used to compute adjusted hazard ratios (aHRs) for IHD associated with pregnancy duration, and co-sibling analyses assessed the influence of shared familial (genetic and/or environmental) factors.
Results:
In 47.5 million person-years of follow-up, 49,955 (2.3%) women were diagnosed with IHD. In the 10 years following delivery, the aHR for IHD associated with preterm delivery (<37 weeks) was 2.47 (95% CI, 2.16–2.82), and further stratified was 4.04 (2.69–6.08) for extremely preterm (22–27 weeks), 2.62 (2.09–3.29) for very preterm (28–33 weeks), 2.30 (1.97–2.70) for late preterm (34–36 weeks), and 1.47 (1.30–1.65) for early term (37–38 weeks), compared with full-term (39–41 weeks). These risks declined but remained significantly elevated after additional follow-up (preterm vs. full-term, 10–19 years: aHR, 1.86; 95% CI, 1.73–1.99; 20–29 years: 1.52; 1.45–1.59; 30–43 years: 1.38; 1.32–1.45). These findings did not appear attributable to shared genetic or environmental factors within families. Additional preterm deliveries were associated with further increases in risk.
Conclusions:
In this large national cohort, preterm delivery was a strong independent risk factor for IHD. This association waned over time but remained substantially elevated up to 40 years later. Preterm delivery should be recognized as a risk factor for IHD in women across the life course.
Keywords: myocardial ischemia, pregnancy, premature delivery, preterm delivery, risk factors, women
Condensed abstract:
Women who deliver preterm have been reported to have increased risks of cardiometabolic disorders, but their long-term risks of ischemic heart disease (IHD) are unclear. In a cohort of >2 million women, preterm and early term delivery were associated with 2.4- and 1.4-fold risks of IHD, respectively, in the next 10 years. These risks declined but remained significantly elevated 30–43 years later, and were not attributable to shared familial factors. Preterm and early term delivery should be recognized as risk factors for IHD across the life course. Women who deliver prematurely need early preventive evaluation and long-term monitoring for IHD.
Introduction
Cardiovascular disease (CVD) is the leading cause of mortality among women in the US and worldwide, but remains understudied and underrecognized in women (1,2). Prior assessments of CVD risk factors have focused primarily on unhealthy lifestyle factors and family history. However, preterm delivery and other pregnancy complications also have been identified as risk factors for CVD in women (3,4). As a consequence, pregnancy has been considered a “natural stress test” that may provide valuable information for understanding future risks (5). A better understanding of pregnancy-related risk factors may further improve risk stratification and targeted interventions to prevent CVD in women.
Women who deliver preterm have been reported to have modestly increased future risks of hypertension, diabetes, and hyperlipidemia, which are major risk factors for ischemic heart disease (IHD) (6). Several studies also have reported associations with IHD, with relative risks in the 1.3–1.6 range (7–11). However, the long-term risks of IHD across the life course and how such risks vary by pregnancy duration remain unclear. Furthermore, previously reported associations may potentially be due to shared familial (genetic and/or environmental) factors that predispose to both preterm delivery and IHD. To our knowledge, this possibility has never been explored. The relative contributions of shared familial factors vs. direct effects of preterm delivery on the development of IHD are still unknown.
To address these knowledge gaps, we conducted a national cohort study of more than 2 million women in Sweden. Our goals were to: (1) provide risk estimates for IHD associated with preterm delivery in the largest population-based cohort to date; (2) examine changes in such risks across the life course with up to 43 years of follow-up; and (3) perform co-sibling analyses to assess the potential influence of shared genetic and/or environmental factors in families. We hypothesized that preterm delivery is associated with long-term increased risks of IHD, and that these risks are partially explained by shared familial factors.
METHODS
Study Population
The Swedish Medical Birth Registry contains prenatal and birth information for nearly all deliveries in Sweden since 1973 (12). Using this registry, we identified 2,194,939 women who had a singleton delivery during 1973–2015. Singleton deliveries were selected to improve internal comparability given the higher prevalence of preterm delivery and its different underlying causes in multiple gestation pregnancies. We excluded 288 (<0.1%) women with a prior diagnosis of IHD and 5,461 (0.2%) others who had missing information for pregnancy duration, leaving 2,189,190 women (99.7% of the original cohort) for inclusion in the study. This study was approved by the ethics committee of Lund University in Sweden. Participant consent was not required as this study used only de-identified registry-based secondary data.
Pregnancy Duration Ascertainment
Pregnancy duration was identified from the Swedish Medical Birth Registry based on maternal report of last menstrual period in the 1970s and ultrasound estimation starting in the 1980s and onward (>70% of the cohort). This was examined alternatively as a continuous variable or categorical variable with 6 groups: extremely preterm (22–27 weeks), very preterm (28–33 weeks), late preterm (34–36 weeks), early term (37–38 weeks), full-term (39–41 weeks, used as the reference group), and post-term (≥42 weeks). In addition, the first 3 groups were combined to provide summary estimates for preterm delivery (<37 weeks).
IHD Ascertainment
The study cohort was followed up for the earliest diagnosis of IHD from delivery through December 31, 2015 (maximum follow-up time, 43 years; median, 22.1; Q1, 9.5; Q3, 33.7). IHD was identified using International Classification of Diseases (ICD) codes in the Swedish Hospital and Outpatient Registries and all deaths attributed to IHD in the Swedish Death Registry (ICD-8/9: 410–414; ICD-10: I20-I25). The Swedish Hospital Registry contains all primary and secondary hospital discharge diagnoses from six populous counties in southern Sweden starting in 1964, and with nationwide coverage starting in 1987; these diagnoses are currently >99% complete and their positive predictive value for IHD has been reported to be 98% (13,14). The Swedish Outpatient Registry contains all diagnoses from specialty clinics nationwide starting in 2001. The Swedish Death Registry includes all deaths and causes of death for all persons registered in Sweden since 1960, with compulsory reporting nationwide.
Other Study Variables
Other perinatal and maternal characteristics that may be associated with pregnancy duration and IHD were identified using the Swedish Medical Birth Registry and national census data, which were linked using an anonymous personal identification number. Maternal age was adjusted for in all analyses as the Cox model time scale (as described below). Covariates included the following maternal factors: calendar year of delivery (continuous and categorical by decade), parity (1, 2, 3, 4, ≥5), education level (≤9, 10–12, >12 years), employment status (yes/no) and income (quartiles) in the year prior to delivery, country or region of origin (Sweden, other Europe/US/Canada, Asia/Oceania, Africa, Latin America, other/unknown), body mass index (BMI; continuous and categorical [<18.5, 18.5–24.9, 25.0–29.9, ≥30.0 kg/m2]) and smoking (0, 1–9, ≥10 cigarettes/day) at the beginning of prenatal care, preeclampsia (ICD-8: 637; ICD-9: 624.4–624.7; ICD-10: O14-O15), other hypertensive disorders (ICD-8: 400–404; ICD-9: 401–405, 642.0–642.3, 642.9; ICD-10: I10-I15, O10-O11, O13, O15-O16), and diabetes mellitus (i.e., pregestational type 1 or type 2 or gestational diabetes; ICD-8: 250; ICD-9: 250, 648.0, 648.8; ICD-10: E10-E14, O24) prior to delivery.
Maternal BMI and smoking were assessed at the beginning of prenatal care starting in 1982 and were available for 56.2% and 67.3% of women, respectively. Data were >99% complete for all other variables. Missing data for each covariate were multiply imputed with 20 imputations using all other covariates and IHD as predictors (15). As alternatives to multiple imputation, sensitivity analyses were performed that (1) restricted to women with complete data (N=1,201,724; 54.9%), or (2) coded missing data for each variable using a missing data indicator.
Statistical Analysis
Cox proportional hazards regression was used to compute hazard ratios (HRs) and 95% confidence intervals (CIs) for associations between pregnancy duration and subsequent risk of IHD. These associations were examined across the maximum possible follow-up (up to 43 years) and in narrower intervals of follow-up (<10, 10–19, 20–29, 30–43 years) among women still living in Sweden without a prior diagnosis of IHD at the beginning of the respective interval. Pregnancy duration was modeled as a time-dependent variable with the exposure category determined by the shortest pregnancy to date. For example, if a woman’s first delivery was full-term and her second was preterm, she entered the preterm exposure category at the date of her second delivery. Maternal age was used as the Cox model time axis with age at delivery as “time zero.” Women were censored at death as identified in the Swedish Death Registry (n=65,468; 3.0%) or emigration as determined by absence of a Swedish residential address in census data (n=93,098; 4.3%). Emigrants and non-emigrants had similar pregnancy durations (median, 40 1/7 weeks for both groups), and thus it was unlikely that emigration introduced any substantial bias. Analyses were conducted both unadjusted and adjusted for covariates (as above). The proportional hazards assumption was assessed by examining log-log plots (16), and no substantial departures were found. Absolute risk differences and 95% CIs, attributable fraction in the exposed (AFe), and population attributable fraction (PAF) were computed for each pregnancy duration group compared with full-term (17). In addition, a sensitivity analysis was performed that was restricted to each woman’s first delivery instead of time-dependent modeling of her shortest pregnancy.
Co-sibling analyses were performed to assess for potential confounding effects of unmeasured shared familial (genetic and/or environmental) factors among all 1,188,730 (54.3%) women with at least one sister who had a singleton delivery (18). Shared environmental factors in families may include lifestyle factors such as diet and physical activity, or ambient exposures such as passive smoking or air pollution. These analyses used stratified Cox regression with a separate stratum for each set of sisters as identified by their mother’s anonymous identification number. In the stratified Cox model, each set of sisters had its own baseline hazard function that reflects their shared genetic and environmental factors, and thus associations between pregnancy duration and IHD were examined within the family, controlling for their shared factors. In addition, these analyses were further adjusted for the same covariates as in the main analyses.
Several other secondary analyses also were performed. First, associations between the number of preterm deliveries (0, 1, ≥2) and IHD risk were examined. Second, IHD risks were assessed after stratifying by spontaneous or medically indicated deliveries that occurred preterm or early term. This information was systematically recorded starting in 1990 (N=1,218,726; maximum 26 years of follow-up). Third, interactions were explored between pregnancy duration and preeclampsia or fetal growth restriction (major causes of medically indicated preterm birth) in relation to IHD risk on both the additive and multiplicative scale (19). In these analyses, preeclampsia was identified using the same ICD codes noted above, and fetal growth restriction was assessed as “small for gestational age” (SGA; birth weight <10th percentile for gestational age) compared with “appropriate for gestational age” (AGA; 10th-90th percentile). All statistical tests were 2-sided and used an α-level of 0.05. All analyses were conducted using Stata version 15.1.
RESULTS
Maternal characteristics by pregnancy duration are reported in Table 1. Women who delivered preterm were more likely than those who delivered full-term to be at the extremes of age, have low education level, be unemployed, have high BMI, smoke, or have preeclampsia, other hypertensive disorders, or diabetes.
Table 1.
Characteristics of study participants by shortest pregnancy duration, Sweden, 1973–2015.
| Extremely preterm | Very preterm | Late preterm | Early term | Full-term | Post-term | |
|---|---|---|---|---|---|---|
| (22–27 wks) | (28–33 wks) | (34–36 wks) | (37–38 wks) | (39–41 wks) | (≥42 wks) | |
| N=9,100 | N=45,438 | N=140,213 | N=551,741 | N=1,353,180 | N=89,518 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Age at first delivery (yrs) | ||||||
| <20 | 806 (8.9) | 4,123 (9.1) | 11,400 (8.1) | 35,948 (6.5) | 64,181 (4.7) | 2,811 (3.1) |
| 20–24 | 2,584 (28.4) | 13,332 (29.3) | 42,385 (30.2) | 162,323 (29.4) | 364,039 (26.9) | 17,758 (19.8) |
| 25–29 | 2,774 (30.5) | 14,585 (32.1) | 47,275 (33.7) | 194,606 (35.3) | 498,601 (36.9) | 31,064 (34.7) |
| 30–34 | 1,873 (20.6) | 9,048 (19.9) | 27,458 (19.6) | 112,822 (20.4) | 304,770 (22.5) | 24,746 (27.6) |
| 35–39 | 855 (9.4) | 3,502 (7.7) | 9,652 (6.9) | 37,791 (6.9) | 101,781 (7.5) | 11,045 (12.3) |
| ≥40 | 208 (2.3) | 848 (1.9) | 2,043 (1.5) | 8,251 (1.5) | 19,808 (1.5) | 2,094 (2.3) |
| Year of delivery | ||||||
| 1973–1979 | 1,445 (15.9) | 10,134 (22.3) | 31,067 (22.2) | 112,447 (20.4) | 338,107 (25.0) | 33,920 (37.9) |
| 1980–1989 | 1,772 (19.5) | 10,759 (23.7) | 33,784 (24.1) | 126,372 (22.9) | 258,777 (19.1) | 11,880 (13.3) |
| 1990–1999 | 1,992 (21.9) | 10,003 (22.0) | 30,431 (21.7) | 121,692 (22.1) | 267,620 (19.8) | 12,206 (13.6) |
| 2000–2009 | 2,240 (24.6) | 9,329 (20.5) | 29,148 (20.8) | 126,479 (22.9) | 279,231 (20.6) | 12,955 (14.5) |
| 2010–2015 | 1,651 (18.1) | 5,213 (11.5) | 15,783 (11.3) | 64,751 (11.7) | 209,445 (15.5) | 18,557 (20.7) |
| Final parity | ||||||
| 1 | 1,726 (19.0) | 8,353 (18.4) | 23,567 (16.8) | 86,307 (15.6) | 326,647 (24.1) | 46,394 (51.8) |
| 2 | 3,069 (33.7) | 18,074 (39.8) | 62,524 (44.6) | 263,457 (47.8) | 669,663 (49.5) | 29,684 (33.2) |
| 3 | 2,500 (27.5) | 11,456 (25.2) | 34,755 (24.8) | 140,344 (25.4) | 269,088 (19.9) | 9,983 (11.2) |
| 4 | 1,126 (12.4) | 4,746 (10.4) | 12,656 (9.0) | 42,722 (7.7) | 64,300 (4.8) | 2,432 (2.7) |
| ≥5 | 678 (7.4) | 2,809 (6.2) | 6,711 (4.8) | 18,991 (3.4) | 23,482 (1.7) | 1,025 (1.1) |
| Education (years) | ||||||
| ≤9 | 1,509 (16.6) | 7,494 (16.5) | 21,398 (15.3) | 75,831 (13.7) | 181,799 (13.4) | 16,092 (18.0) |
| 10–12 | 4,215 (46.3) | 21,517 (47.4) | 65,445 (46.7) | 247,684 (44.9) | 591,354 (43.7) | 38,871 (43.3) |
| >12 | 3,376 (37.1) | 16,427 (36.1) | 53,370 (38.1) | 228,226 (41.4) | 580,027 (42.9) | 34,645 (38.7) |
| Employment Income (quartile) | 7,524 (82.7) | 38,283 (84.3) | 120,050 (85.6) | 474,773 (86.0) | 1,173,672 (86.7) | 75,523 (84.4) |
| 1st (highest) | 2,497 (27.4) | 10,391 (22.9) | 31,246 (22.3) | 131,154 (23.8) | 348,887 (25.8) | 24,442 (27.3) |
| 2nd | 2,224 (24.4) | 10,724 (23.6) | 33,572 (23.9) | 132,303 (24.0) | 342,011 (25.3) | 25,361 (28.3) |
| 3rd | 1,910 (21.0) | 11,604 (25.5) | 35,897 (25.6) | 138,540 (25.1) | 338,997 (25.1) | 22,460 (25.1) |
| 4th (lowest) | 2,469 (27.1) | 12,719 (28.0) | 39,498 (28.2) | 149,744 (27.1) | 323,285 (23.9) | 17,255 (19.3) |
| Birth country or region | ||||||
| Sweden | 6,953 (76.4) | 37,435 (82.4) | 116,268 (82.9) | 449,450 (81.5) | 1,114,560 (82.4) | 72,314 (80.8) |
| Other Europe/US/Canada | 963 (10.6) | 3,975 (8.8) | 12,124 (8.7) | 48,847 (8.9) | 128,982 (9.5) | 10,412 (11.6) |
| Asia/Oceania | 741 (8.1) | 2,670 (5.9) | 8,358 (6.0) | 38,257 (6.9) | 73,884 (5.5) | 3,702 (4.1) |
| Africa | 326 (3.6) | 901 (2.0) | 1,932 (1.4) | 8,161 (1.5) | 21,729 (1.6) | 2,211 (2.5) |
| Latin America | 108 (1.2) | 398 (0.9) | 1,392 (1.0) | 6,484 (1.2) | 12,656 (0.9) | 759 (0.9) |
| Other/unknown | 9 (0.1) | 59 (0.1) | 139 (1.0) | 542 (0.1) | 1,369 (0.1) | 120 (0.1) |
| Body mass index (kg/m2) | ||||||
| <18.5 | 223 (2.5) | 1,292 (2.8) | 4,215 (3.0) | 16,244 (2.9) | 29,739 (2.2) | 1,048 (1.2) |
| 18.5–24.9 | 6,921 (76.0) | 36,692 (80.8) | 114,028 (81.3) | 450,446 (81.6) | 1,110,017 (82.0) | 72,130 (80.6) |
| 25.0–29.9 | 1,350 (14.8) | 5,171 (11.4) | 15,665 (11.2) | 62,517 (11.3) | 158,259 (11.7) | 11,213 (12.5) |
| ≥30.0 | 606 (6.7) | 2,283 (5.0) | 6,305 (4.5) | 22,534 (4.1) | 55,165 (4.1) | 5,127 (5.7) |
| Smoking (cigarettes/day) | ||||||
| 0 | 6,597 (72.5) | 31,241 (68.8) | 99,871 (71.2) | 410,313 (74.4) | 992,881 (73.4) | 57,914 (64.7) |
| 1–9 | 2,061 (22.6) | 12,036 (26.5) | 34,519 (24.6) | 121,529 (22.0) | 312,931 (23.8) | 29,576 (33.0) |
| ≥10 | 442 (4.9) | 2,161 (4.8) | 5,823 (4.2) | 19,899 (3.6) | 38,368 (2.8) | 2,028 (2.3) |
| Preeclampsia | 1,028 (11.3) | 6,995 (15.4) | 14,159 (10.1) | 35,697 (6.5) | 61,307 (4.5) | 4,100 (4.6) |
| Other hypertensive disorders | 102 (1.1) | 681 (1.5) | 1,755 (1.3) | 6,224 (1.1) | 12,614 (0.9) | 738 (0.8) |
| Diabetes mellitus | 213 (2.3) | 1,386 (3.1) | 4,312 (3.1) | 9,981 (1.8) | 11,097 (0.8) | 476 (0.5) |
In 47.5 million person-years of follow-up, 49,955 (2.3%) women were diagnosed with IHD. The median age at first delivery was 27.3 (mean 27.6 ± 5.2), at IHD diagnosis was 57.4 (mean 57.1 ± 9.3), and at end of follow-up was 50.5 (mean 50.6 ± 13.3) years. Absolute incidence rates for IHD by pregnancy duration and follow-up time are reported in Table 2. Most (55.3%) IHD diagnoses were acute myocardial infarction, and the remainder were other types of acute or chronic IHD (Supplemental Table 1). The prevalence of hypertension (based on outpatient and inpatient diagnoses) at the end of follow-up was 20.3% among women who ever delivered preterm, and further stratified was 20.0% for extremely preterm, 22.4% for very preterm, 19.6% for late preterm, and 17.5% for early term, compared with 16.6% among those with only full-term deliveries.
Table 2.
Associations between women’s pregnancy duration and subsequent risk of IHD.
| IHD cases | Ratea | Age-adjusted HR (95% CI) | Full modelb | Risk differencec (95% CI) | AFed | PAFe | ||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | |||||||
| 0–43 years after delivery | ||||||||
| Preterm (<37 wks) | 6,078 | 152.6 | 1.70 (1.65–1.75) | 1.53 (1.49–1.58) | <0.001 | 58.2 (54.2, 62.8) | 38.1% | 6.7% |
| Extremely preterm (22–27 wks) | 255 | 165.8 | 1.99 (1.76–2.25) | 1.72 (1.52–1.94) | <0.001 | 71.4 (51.0, 91.8) | 43.1% | 0.4% |
| Very preterm (28–33 wks) | 1,578 | 170.5 | 1.89 (1.79–1.98) | 1.68 (1.60–1.77) | <0.001 | 76.1 (67.6, 84.6) | 44.6% | 2.3% |
| Late preterm (34–36 wks) | 4,245 | 146.2 | 1.62 (1.57–1.68) | 1.48 (1.43–1.52) | <0.001 | 51.8 (47.3, 56.3) | 35.4% | 4.6% |
| Early term (37–38 wks) | 12,621 | 113.1 | 1.26 (1.23–1.28) | 1.24 (1.21–1.27) | <0.001 | 18.6 (16.4, 20.9) | 16.5% | 5.1% |
| Full-term (39–41 wks) | 28,530 | 94.4 | Reference | Reference | Reference | |||
| Post-term (≥42 wks) | 3,022 | 120.3 | 1.13 (1.09–1.17) | 1.04 (1.01–1.08) | 0.03 | 25.9 (21.5, 30.3) | 21.5% | 2.1% |
| Per additional week (trend) | 0.93 (0.93–0.94) | 0.94 (0.94–0.95) | <0.001 | |||||
| <10 years after delivery | ||||||||
| Preterm (<37 wks) | 312 | 22.0 | 3.14 (2.75, 3.58) | 2.47 (2.16, 2.82) | <0.001 | 15.7 (13.2, 18.2) | 71.4% | 20.4% |
| Extremely preterm (22–27 wks) | 24 | 41.3 | 5.21 (3.47, 7.83) | 4.04 (2.69, 6.08) | <0.001 | 35.0 (18.5, 51.5) | 84.8% | 2.5% |
| Very preterm (28–33 wks) | 86 | 26.4 | 3.56 (2.85, 4.45) | 2.62 (2.09, 3.29) | <0.001 | 20.1 (14.5, 25.7) | 76.1% | 7.6% |
| Late preterm (34–36 wks) | 202 | 19.5 | 2.86 (2.45, 3.34) | 2.30 (1.97, 2.70) | <0.001 | 13.2 (10.5, 16.0) | 67.7% | 14.0% |
| Early term (37–38 wks) | 442 | 10.8 | 1.56 (1.39, 1.76) | 1.47 (1.30, 1.65) | <0.001 | 4.5 (3.4, 5.6) | 41.7% | 15.1% |
| Full-term (39–41 wks) | 777 | 6.3 | Reference | Reference | Reference | |||
| Post-term (≥42 wks) | 61 | 5.1 | 0.81 (0.62, 1.05) | 0.77 (0.59, 0.99) | 0.04 | −1.2 (−2.5, 0.2) | (18.8%)f | (1.7%)g |
| Per additional week (trend) | 0.87 (0.85, 0.88) | 0.89 (0.87, 0.90) | <0.001 | |||||
| 10–19 years after delivery | ||||||||
| Preterm (<37 wks) | 1,075 | 84.5 | 2.15 (2.01, 2.31) | 1.86 (1.73, 1.99) | <0.001 | 43.1 (37.9, 48.3) | 51.0% | 11.8% |
| Extremely preterm (22–27 wks) | 51 | 103.3 | 2.52 (1.92, 3.33) | 2.13 (1.61, 2.81) | <0.001 | 61.9 (33.5, 90.3) | 59.9% | 0.8% |
| Very preterm (28–33 wks) | 322 | 108.4 | 2.70 (2.41, 3.02) | 2.24 (2.00, 2.52) | <0.001 | 66.9 (55.0, 78.9) | 61.8% | 5.1% |
| Late preterm (34–36 wks) | 702 | 75.8 | 1.95 (1.80, 2.12) | 1.71 (1.57, 1.85) | <0.001 | 34.4 (28.7, 40.2) | 45.4% | 7.4% |
| Early term (37–38 wks) | 2,012 | 56.6 | 1.44 (1.36, 1.52) | 1.41 (1.33, 1.49) | <0.001 | 15.2 (12.3, 18.0) | 26.8% | 9.7% |
| Full-term (39–41 wks) | 3,576 | 41.4 | Reference | Reference | Reference | |||
| Post-term (≥42 wks) | 324 | 56.5 | 1.08 (0.97, 1.21) | 0.97 (0.86, 1.08) | 0.55 | 15.1 (8.8, 21.4) | 26.7% | 2.2% |
| Per additional week (trend) | 0.90 (0.90, 0.91) | 0.92 (0.91, 0.93) | <0.001 | |||||
| 20–29 years after delivery | ||||||||
| Preterm (<37 wks) | 2,330 | 268.9 | 1.64 (1.57, 1.71) | 1.52 (1.45, 1.59) | <0.001 | 91.6 (80.2, 103.1) | 34.1% | 6.2% |
| Extremely preterm (22–27 wks) | 93 | 291.5 | 1.81 (1.48, 2.22) | 1.63 (1.33, 2.00) | <0.001 | 114.2 (54.8, 173.5) | 39.2% | 0.3% |
| Very preterm (28–33 wks) | 583 | 287.7 | 1.75 (1.61, 1.90) | 1.61 (1.48, 1.75) | <0.001 | 110.4 (86.8, 134.0) | 38.4% | 2.0% |
| Late preterm (34–36 wks) | 1,654 | 261.8 | 1.59 (1.51, 1.68) | 1.48 (1.41, 1.56) | <0.001 | 84.5 (71.4, 97.5) | 32.3% | 4.4% |
| Early term (37–38 wks) | 4,954 | 209.6 | 1.25 (1.21, 1.29) | 1.26 (1.22, 1.31) | <0.001 | 32.3 (25.5, 39.1) | 15.4% | 5.0% |
| Full-term (39–41 wks) | 10,393 | 177.3 | Reference | Reference | Reference | |||
| Post-term (≥42 wks) | 1,049 | 244.9 | 1.19 (1.12, 1.27) | 1.05 (0.99, 1.12) | 0.11 | 67.5 (52.4, 82.8) | 27.6% | 2.5% |
| Per additional week (trend) | 0.94 (0.93, 0.95) | 0.94 (0.94, 0.95) | <0.001 | |||||
| 30–43 years after delivery | ||||||||
| Preterm (<37 wks) | 2,361 | 555.3 | 1.46 (1.40, 1.52) | 1.38 (1.32, 1.45) | <0.001 | 147.5 (124.1, 170.9) | 26.6% | 3.9% |
| Extremely preterm (22–27 wks) | 87 | 603.4 | 1.61 (1.31, 1.99) | 1.45 (1.17, 1.79) | 0.001 | 195.6 (68.6, 322.5) | 32.4% | 0.2% |
| Very preterm (28–33 wks) | 587 | 590.3 | 1.56 (1.44, 1.69) | 1.47 (1.36, 1.60) | <0.001 | 182.5 (134.2, 230.7) | 30.9% | 1.3% |
| Late preterm (34–36 wks) | 1,687 | 541.9 | 1.42 (1.35, 1.49) | 1.35 (1.28, 1.42) | <0.001 | 134.1 (107.3, 160.8) | 24.7% | 2.7% |
| Early term (37–38 wks) | 5,213 | 451.7 | 1.16 (1.13, 1.20) | 1.17 (1.13, 1.21) | <0.001 | 43.9 (29.9, 58.0) | 9.7% | 2.7% |
| Full-term (39–41 wks) | 13,784 | 407.8 | Reference | Reference | Reference | |||
| Post-term (≥42 wks) | 1,588 | 500.5 | 1.14 (1.08, 1.20) | 1.07 (1.01, 1.13) | 0.01 | 92.7 (67.2, 118.2) | 18.5% | 1.9% |
| Per additional week (trend) | 0.96 (0.95, 0.96) | 0.96 (0.95, 0.96) | <0001 | |||||
IHD incidence rate per 100,000 person-years.
Adjusted for maternal age, year of delivery, parity, education, employment, income, region of origin, BMI, smoking, preeclampsia, other hypertensive disorders, and diabetes prior to delivery.
Incidence rate difference per 100,000 person-years.
Attributable fraction among the exposed.
Population attributable fraction.
Prevented fraction among the exposed.
Population prevented fraction.
Pregnancy Duration and Risk of IHD
Across the full duration of follow-up (0–43 years after delivery), each additional week of pregnancy was associated with a 6% lower risk of IHD (full model: adjusted HR, 0.94; 95% CI, 0.94–0.95; P<0.001; Table 2). The adjusted HRs for IHD associated with preterm, extremely preterm, or early term delivery were 1.53 (95% CI, 1.49–1.58), 1.72 (1.52–1.94), and 1.24 (1.21–1.27), respectively, compared with full-term delivery. The fully adjusted HRs (as above) were only moderately lower than those adjusted only for age (Table 2).
HRs were highest in the first 10 years after delivery then subsequently declined, whereas the absolute risk differences associated with preterm delivery increased with additional follow-up (Table 2, Central Illustration). During the first 10 years, each additional week of pregnancy was associated with an 11% lower risk of IHD (full model: adjusted HR, 0.89; 95% CI, 0.87–0.90; P<0.001; Table 2). The adjusted HR for IHD associated with preterm delivery was 2.47 (95% CI, 2.16–2.82), and further stratified was 4.04 (2.69–6.08) for extremely preterm, 2.62 (2.09–3.29) for very preterm, 2.30 (1.97–2.70) for late preterm, and 1.47 (1.30–1.65) for early term, compared with full-term.
Central Illustration. Hazard ratios and absolute risk differences for IHD in women by pregnancy duration compared with full-term delivery, Sweden, 1973–2015.
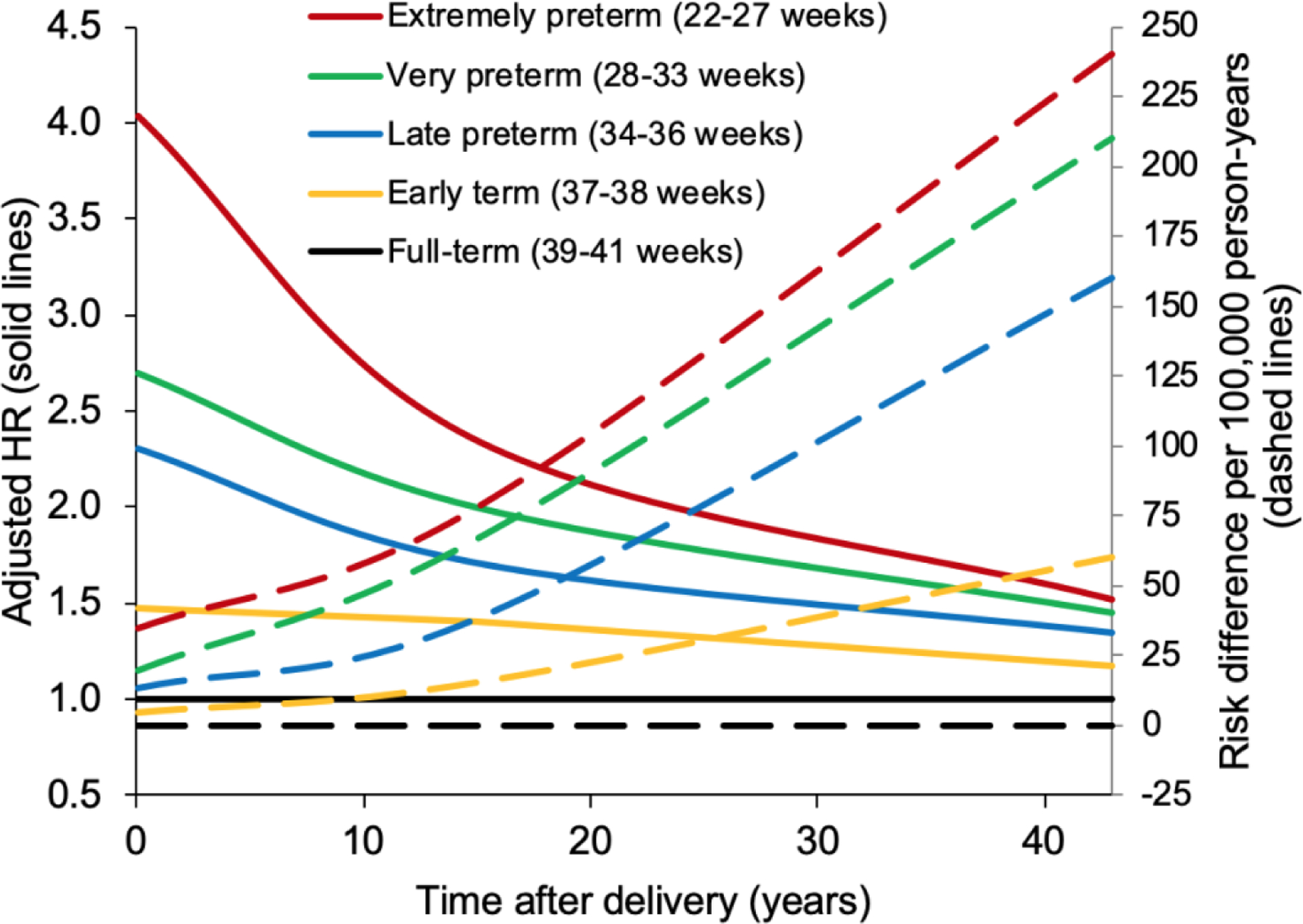
Adjusted HRs and absolute risk differences for IHD are shown for different pregnancy durations compared with women who delivered at full-term. The adjusted HRs for IHD associated with preterm or early term delivery (indicated by solid lines) were highest in the first 10 years after delivery, and subsequently declined but remained significantly elevated even 30–43 years later. In contrast, absolute risk differences for IHD associated with preterm or early term delivery (indicated by dashed lines) increased with additional follow-up. HR = hazard ratio, IHD = ischemic heart disease.
After additional follow-up, the association between preterm delivery and IHD declined but remained significantly elevated (e.g., full model, adjusted HR, 10–19 years: 1.86; 95% CI, 1.73–1.99; 20–29 years: 1.52; 95% CI, 1.45–1.59; 30–43 years: 1.38; 95% CI, 1.32–1.45). Early term delivery was associated with a modestly increased risk of IHD even 30–43 years later, compared with full-term (adjusted HR, 1.17; 95% CI, 1.13–1.21; P<0.001).
Preterm delivery was associated with 15.7 (95% CI, 13.2–18.2) excess IHD cases per 100,000 person-years at <10 years, 43.1 (37.9–48.3) cases at 10–19 years, 91.6 (80.2–103.1) cases at 20–29 years, and 147.5 (124.1–170.9) cases at 30–43 years of follow-up, compared with full-term delivery (Table 2, risk differences). At <10, 10–19, 20–29, and 30–43 years of follow-up, respectively, an estimated 71.4%, 51.0%, 34.1%, and 26.6% of IHD cases among women who delivered preterm, and 20.4%, 11.8%, 6.2%, and 3.9% of all IHD cases in the population, were related to preterm delivery (Table 2, AFe and PAF). The Central Illustration shows HRs and absolute risk differences for IHD by time since delivery for different pregnancy durations.
In sensitivity analyses, all results were similar to the main results and the conclusions were unchanged. For example, exploring alternatives to multiple imputation, the fully adjusted HR for IHD at 0–43 years after delivery comparing preterm with full-term was 1.59 (95% CI, 1.51–1.68; P<0.001) when restricting to women with complete data, and 1.51 (1.47–1.55; P<0.001) when coding missing data using a missing data indicator. In addition, the corresponding HR when repeating the main analyses while focusing on each woman’s first delivery, instead of her shortest pregnancy adjusted for parity, was 1.50 (95% CI, 1.43–1.57; P<0.001).
Co-Sibling Analyses
Co-sibling analyses to control for unmeasured shared familial factors resulted in modest attenuation of certain risk estimates but increases in others (Table 3). For example, comparing preterm with full-term delivery, the adjusted HRs for IHD at ages <10 years after delivery were 2.47 (95% CI, 2.16–2.82) in the primary analysis vs. 2.84 (1.20–6.73) in the co-sibling analysis, at 10–19 years were 1.86 (1.73–1.99) vs. 1.81 (1.40–2.36), at 20–29 years were 1.52 (1.45–1.59) vs. 1.30 (1.12–1.51), and at 30–43 years were 1.38 (1.32–1.45) vs. 1.31 (1.11–1.54). Overall, these findings suggest that the associations observed in the primary analyses were not mainly attributable to shared genetic or environmental factors in families.
Table 3.
Co-sibling analyses of women’s pregnancy duration and subsequent risk of IHD.
| Cases | HR (95% CI)a | P | |
|---|---|---|---|
| 0–43 years after delivery | |||
| Preterm (<37 wks) | 3,912 | 1.40 (1.29–1.51) | <0.001 |
| Early term (37–38 wks) | 7,825 | 1.18 (1.11–1.25) | <0.001 |
| Full-term (39–41 wks) | 12,937 | Reference | |
| Per additional week (trend) | 0.96 (0.94–0.97) | <0.001 | |
| <10 years after delivery | |||
| Preterm (<37 wks) | 126 | 2.84 (1.20, 6.73) | 0.02 |
| Early term (37–38 wks) | 219 | 1.02 (0.61, 1.72) | 0.94 |
| Full-term (39–41 wks) | 338 | Reference | |
| Per additional week (trend) | 0.88 (0.79, 0.99) | 0.03 | |
| 10–19 years after delivery | |||
| Preterm (<37 wks) | 651 | 1.81 (1.40, 2.36) | <0.001 |
| Early term (37–38 wks) | 1,133 | 1.59 (1.29, 1.94) | <0.001 |
| Full-term (39–41 wks) | 1,544 | Reference | |
| Per additional week (trend) | 0.93 (0.89, 0.96) | <0.001 | |
| 20–29 years after delivery | |||
| Preterm (<37 wks) | 1,533 | 1.30 (1.12, 1.51) | 0.001 |
| Early term (37–38 wks) | 3,166 | 1.15 (1.03, 1.29) | 0.01 |
| Full-term (39–41 wks) | 4,879 | Reference | |
| Per additional week (trend) | 0.96 (0.94, 0.98) | <0.001 | |
| 30–43 years after delivery | |||
| Preterm (<37 wks) | 1,602 | 1.31 (1.11, 1.54) | 0.002 |
| Early term (37–38 wks) | 3,307 | 1.13 (1.00, 1.27) | 0.05 |
| Full-term (39–41 wks) | 6,176 | Reference | |
| Per additional week (trend) | 0.97 (0.94, 0.99) | 0.004 | |
Adjusted for shared familial (genetic and/or environmental) factors, and additionally for maternal age, year of delivery, parity, education, employment, income, BMI, smoking, preeclampsia, other hypertensive disorders, and diabetes prior to delivery.
Secondary Analyses
Associations between the number of preterm deliveries and IHD risk are reported in Table 4. Women with more than one preterm delivery had further increases in IHD risk. Among women with at least 2 singleton deliveries, the adjusted HRs for IHD per each additional preterm delivery were 1.53 (95% CI, 1.35–1.75) at <10 years of follow-up, 1.54 (1.46–1.63) at 10–19 years, 1.35 (1.30–1.40) at 20–29 years, and 1.27 (1.23–1.32) at 30–43 years.
Table 4.
Associations between number of preterm deliveries and subsequent risk of IHD among women with ≥2 singleton deliveries.
| Cases | Ratea | HR (95% CI)b | P | |
|---|---|---|---|---|
| 0–43 years after first delivery | ||||
| 0 preterm | 20,593 | 69.6 | Reference | |
| 1 preterm | 3,071 | 105.3 | 1.42 (1.36–1.47) | <0.001 |
| ≥2 preterm | 863 | 137.4 | 1.92 (1.79–2.05) | <0.001 |
| Per additional preterm delivery | 1.36 (1.33–1.39) | <0.001 | ||
| <10 years after first delivery | ||||
| 0 preterm | 531 | 4.4 | Reference | |
| 1 preterm | 110 | 9.4 | 1.86 (1.51, 2.29) | <0.001 |
| ≥2 preterm | 29 | 11.6 | 2.23 (1.53, 3.25) | <0.001 |
| Per additional preterm delivery | 1.53 (1.35, 1.75) | <0.001 | ||
| 10–19 years after first delivery | ||||
| 0 preterm | 2,599 | 29.3 | Reference | |
| 1 preterm | 490 | 55.3 | 1.69 (1.53, 1.86) | <0.001 |
| ≥2 preterm | 157 | 81.3 | 2.47 (2.10, 2.91) | <0.001 |
| Per additional preterm delivery | 1.54 (1.46, 1.63) | <0.001 | ||
| 20–29 years after first delivery | ||||
| 0 preterm | 7,979 | 137.9 | Reference | |
| 1 preterm | 1,194 | 204.6 | 1.39 (1.30–1.47) | <0.001 |
| ≥2 preterm | 349 | 273.2 | 1.88 (1.69–2.10) | <0.001 |
| Per additional preterm delivery | 1.35 (1.30, 1.40) | <0.001 | ||
| 30–43 years after first delivery | ||||
| 0 preterm | 9,484 | 332.5 | Reference | |
| 1 preterm | 1,277 | 461.2 | 1.29 (1.22–1.37) | <0.001 |
| ≥2 preterm | 328 | 580.7 | 1.70 (1.52–1.89) | <0.001 |
| Per additional preterm delivery | 1.27 (1.23, 1.32) | <0.001 | ||
IHD incidence rate per 100,000 person-years.
Adjusted for maternal age, year of delivery, parity, education, employment, income, region of origin, BMI, smoking, preeclampsia, other hypertensive disorders, and diabetes prior to delivery.
Both spontaneous and medically indicated deliveries at preterm or early term were associated with increased risk of IHD compared with full-term (e.g., preterm vs. full-term, adjusted HRs, 1.43; 95% CI, 1.24–1.65; P<0.001; and 2.20; 1.95–2.48; P<0.001, respectively; Table 5). However, medically indicated delivery was the stronger risk factor (P<0.001 for difference in HRs among either preterm or early term).
Table 5.
Spontaneous or medically indicated delivery and subsequent risk of IHD, Sweden, 1990–2015.
| Cases | Ratea | HR (95% CI)b | P | |
|---|---|---|---|---|
| Preterm | ||||
| Spontaneous | 204 | 92.5 | 1.43 (1.24, 1.65) | <0.001 |
| Medically indicated | 352 | 185.3 | 2.20 (1.95, 2.48) | <0.001 |
| Early term | ||||
| Spontaneous | 690 | 81.1 | 1.28 (1.17, 1.39) | <0.001 |
| Medically indicated | 634 | 130.5 | 1.69 (1.55, 1.84) | <0.001 |
| Full-term | 2,902 | 60.1 | Reference | |
IHD incidence rate per 100,000 person-years.
Adjusted for maternal age, year of delivery, parity, education, employment, income, region of origin, BMI, smoking, preeclampsia, other hypertensive disorders, and diabetes prior to delivery.
Interactions between pregnancy duration and preelampsia are reported in Supplemental Table 2. Preterm delivery was associated with an increased risk of IHD regardless of whether preeclampsia was present (adjusted HR, 1.63; 95% CI, 1.50–1.76; P<0.001) or not (1.52; 1.47–1.57; P<0.001) (Supplemental Table 2, right-most column). A positive additive (but not multiplicative) interaction was found between preterm delivery and preeclampsia (i.e., their combined effect on IHD risk was greater than the sum of their separate effects, P<0.001). The positive additive interaction suggests that preterm delivery accounted for more IHD cases among women who also had preeclampsia compared with those who did not.
Both preterm delivery and SGA were independently associated with an increased risk of IHD (e.g., adjusted HRs: preterm vs. full-term, 1.52; 95% CI, 1.48–1.56; P<0.001; SGA vs. AGA, 1.37; 1.33–1.40; P<0.001), but preterm delivery was the stronger risk factor (P<0.001 for difference in HRs). Women who delivered preterm had an increased risk of IHD regardless of whether their infant was SGA (adjusted HR, 1.39; 95% CI, 1.28–1.51; P<0.001) or AGA (1.53; 1.48–1.58; P<0.001) (Supplemental Table 3, right-most column). A negative multiplicative (but not additive) interaction was found between preterm delivery and SGA (i.e., their combined effect on IHD risk was less than the product of their separate effects, P=0.03). The absence of additive interaction suggests that preterm delivery accounted for a similar number of IHD cases among women who had SGA vs. AGA infants.
DISCUSSION
In this large national cohort study, shorter pregnancy duration was associated with higher subsequent risk of IHD. Women who delivered preterm or extremely preterm had ~2.5- and 4-fold risks of IHD in the next 10 years compared with those who delivered full-term, after adjusting for other maternal factors including preeclampsia, diabetes, high BMI, and smoking. These risks subsequently declined but remained significantly elevated (~1.4-fold) even 30–43 years after delivery. Early term delivery (37–38 weeks) also was associated with a modestly increased risk of IHD that persisted 30–43 years afterward. Co-sibling analyses suggested that these findings were not explained by shared genetic or environmental factors in families.
To our knowledge, this is the largest study to date of preterm delivery in relation to IHD risk in women, and the first to include a co-sibling analysis to assess the potential influence of shared familial factors. Most prior studies have assessed IHD based on hospital diagnoses (7–10) or self-report (11). The largest of these included an Australian cohort study of 797,056 women that reported more than a 1.6-fold risk of IHD among those who delivered preterm (adjusted HR, 1.65; 95% CI, 1.32–2.07) (7). A Scottish cohort study of 750,350 women also reported nearly a 1.6-fold risk of IHD (adjusted HR, 1.58; 95% CI, 1.47–1.71) (8). A Danish cohort study of 427,765 women and a Swedish cohort study of 403,550 women reported 1.4- and 1.3-fold risks of IHD, respectively, associated with preterm delivery (adjusted HR, 1.42; 95% CI, 1.34–1.52; and 1.30; 95% CI, 1.11–1.52) (9,10). In addition, a US cohort study of 70,182 women found a 1.4-fold risk of self-reported CVD (myocardial infarction or stroke) among those who delivered preterm (adjusted HR, 1.42; 95% CI, 1.16–1.72) (11). A meta-analysis of these 5 studies with a total of 2,411,083 women reported a pooled relative risk of 1.49 (95% CI, 1.38–1.60) (3).
The present study extends this evidence by providing risk estimates for IHD in a larger population-based cohort, affording the statistical power needed to examine narrowly defined pregnancy durations. The findings suggest that women who delivered either preterm or early term have substantially increased risks of IHD that may persist at least 40 years. Our risk estimates were slightly larger in magnitude than those of prior studies. This may have been due to more complete IHD ascertainment, as well as differences in the reference group. In contrast with prior studies, the present study ascertained IHD using both inpatient and outpatient diagnoses, rather than inpatient only (7–10,20) or self-report (11). In addition, the present study’s reference group (full-term, 39–41 weeks) did not include early term deliveries (37–38 weeks).
We found that women who delivered at early term had approximately 1.4- and 1.2-fold risks of IHD in the next 20 or 20–43 years, respectively, compared with full-term. Both spontaneous and medically indicated deliveries at early term were associated with increased IHD risks. Prior evidence has suggested that spontaneous labor at early term is associated with pathological processes including placental ischemia, infection, or inflammation (21) that may have long-term implications for infant and maternal health. Offspring born at early term have been reported to have increased risks of IHD as well as hypertension, diabetes, lipid disorders, and premature mortality in adulthood (18,22–26). However, long-term maternal outcomes of early term delivery have rarely been studied. Additional large epidemiologic and clinical studies with information on specific causes of early term delivery are needed to confirm our findings and further elucidate the underlying mechanisms.
The higher risk of IHD we observed after medically indicated compared with spontaneous preterm delivery (P<0.001) is consistent with findings from two prior studies (7,8) (pooled risk ratios, medically indicated: 1.80; 95% CI, 1.62–2.00; spontaneous: 1.48; 95% CI, 1.36–1.60) (3). In addition, we found that preterm delivery either with or without preeclampsia or fetal growth restriction was associated with an increased risk of IHD. However, preterm delivery accounted for more IHD cases among women who also had preeclampsia. These findings are broadly consistent with those previously reported for preterm delivery and preeclampsia in relation to CVD mortality (27), or preterm delivery and SGA in relation to CVD incidence (20).
To our knowledge, the present study is the first to use a co-sibling design to assess the potential influence of unmeasured familial factors on associations between preterm delivery and future maternal risk of IHD. Common genetic factors have previously been proposed (28). However, contrary to expectation, our co-sibling analyses suggested that these associations were largely independent of shared familial factors, whether genetic or environmental, that might predispose to both preterm delivery and IHD. Instead, they suggest that individual-specific processes involved with premature delivery may have direct effects on the future development of IHD.
The underlying mechanisms are not established but may involve inflammatory pathways that are linked with preterm delivery and the development of atherosclerosis. Increased production of proinflammatory cytokines, specifically IL-1, IL-6, and TNF, have been associated with uterine activation and the timing and initiation of preterm delivery (29–31). Recent evidence suggests that preterm delivery might be a key event that triggers endothelial-specific inflammation that was undetectable prior to pregnancy (32). Atherosclerosis often originates with inflammatory changes in the endothelium and expression of the adhesion molecule VCAM-1, triggering an inflammatory cell response, release of proinflammatory cytokines, and development of the fatty streak and atherosclerotic plaque, which are precursors of IHD (33–35). Further elucidation of these mechanisms is an intensive area of ongoing research and may potentially reveal new targets for prevention of preterm delivery and IHD.
The long-term increased risks of IHD that we found among women who delivered preterm may have important clinical implications. Preterm delivery should now be recognized as an independent risk factor for IHD across the life course. Cardiovascular risk assessment in women should routinely include reproductive history that covers preterm delivery and other pregnancy complications. Women with a history of preterm delivery may warrant early preventive actions to reduce other IHD risk factors, including obesity, physical inactivity, and smoking, and long-term monitoring for timely detection and treatment of IHD. Better access to high-quality preconception and prenatal care is also critically needed to help reduce preterm delivery (36).
Strengths and Limitations
A key strength of the present study is its large national cohort design with up to 43 years of follow-up. The availability of highly complete nationwide birth and medical registry data, both inpatient and outpatient, helped minimize potential selection and ascertainment biases. The large sample size enabled higher statistical power than prior studies and assessment of more narrowly defined pregnancy durations. The results were controlled for multiple other maternal factors, as well as unmeasured shared familial factors using co-sibling analyses.
This study also had several limitations. First, detailed clinical data were unavailable to verify IHD diagnoses, although a positive predictive value of 98% has been reported (14). Second, outpatient diagnoses were available starting in 2001, resulting in underreporting of IHD especially in earlier years. Consequently, the absolute risks of IHD associated with preterm delivery may potentially be higher than those reported in this study. Underreporting or other misclassification of IHD is expected to be non-differential with respect to women’s pregnancy duration, and therefore to influence results conservatively (i.e., toward the null hypothesis). Third, residual confounding by maternal smoking, BMI, or other risk factors during pregnancy cannot be excluded. Other pregnancy-related information such as placental site, infections, and antepartum hemorrhage was unavailable for most years of this cohort and would be useful to examine in future studies. Lastly, this study was limited to Sweden and will need replication in other countries, including racially diverse populations to explore for potential heterogeneity of findings. Additional follow-up to older ages will also be needed in this or other large cohorts.
CONCLUSIONS
In summary, this is the largest population-based study to date of preterm delivery in relation to the long-term risk of IHD in women, and the first to assess the influence of shared familial factors. The findings suggest that preterm and early term delivery are important independent risk factors for the development of IHD, and that the associated risks may persist up to 40 years later. Women who deliver prematurely need early preventive evaluation and long-term monitoring for IHD.
Supplementary Material
PERSPECTIVES.
Competency in Medical Knowledge:
Preterm (<37 weeks) and early term (37–38 weeks) delivery are independent risk factors for later development of ischemic heart disease in women.
Translational Outlook:
Additional investigations may help elucidate the mechanisms responsible for later development of ischemic heart disease in women after preterm delivery.
Funding:
This work was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health [R01 HL139536 to C.C. and K.S.]; the Swedish Research Council; the Swedish Heart-Lung Foundation; and ALF project grant, Region Skåne/Lund University, Sweden. The funding agencies had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Abbreviations:
- AFe
attributable fraction in the exposed
- AGA
appropriate for gestational age
- BMI
body mass index
- CI
confidence interval
- CVD
cardiovascular disease
- HR
hazard ratio
- ICD
International Classification of Diseases
- IHD
ischemic heart disease
- PAF
population attributable fraction
- SGA
small for gestational age
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: There are no conflicts of interest.
REFERENCES
- 1.Benjamin EJ, Muntner P, Alonso A et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 2.Mehta LS, Beckie TM, DeVon HA et al. Acute Myocardial Infarction in Women: A Scientific Statement From the American Heart Association. Circulation 2016;133:916–47. [DOI] [PubMed] [Google Scholar]
- 3.Wu P, Gulati M, Kwok CS et al. Preterm Delivery and Future Risk of Maternal Cardiovascular Disease: A Systematic Review and Meta-Analysis. J Am Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu P, Haththotuwa R, Kwok CS et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ Cardiovasc Qual Outcomes 2017;10. [DOI] [PubMed] [Google Scholar]
- 5.Williams D Pregnancy: a stress test for life. Curr Opin Obstet Gynecol 2003;15:465–71. [DOI] [PubMed] [Google Scholar]
- 6.Tanz LJ, Stuart JJ, Williams PL et al. Preterm Delivery and Maternal Cardiovascular Disease Risk Factors: The Nurses’ Health Study II. J Womens Health (Larchmt) 2019;28:677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ngo AD, Chen JS, Figtree G, Morris JM, Roberts CL. Preterm birth and future risk of maternal cardiovascular disease - is the association independent of smoking during pregnancy? BMC Pregnancy Childbirth 2015;15:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hastie CE, Smith GC, Mackay DF, Pell JP. Maternal risk of ischaemic heart disease following elective and spontaneous pre-term delivery: retrospective cohort study of 750 350 singleton pregnancies. Int J Epidemiol 2011;40:914–9. [DOI] [PubMed] [Google Scholar]
- 9.Catov JM, Wu CS, Olsen J, Sutton-Tyrrell K, Li J, Nohr EA. Early or recurrent preterm birth and maternal cardiovascular disease risk. Ann Epidemiol 2010;20:604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wikstrom AK, Haglund B, Olovsson M, Lindeberg SN. The risk of maternal ischaemic heart disease after gestational hypertensive disease. BJOG 2005;112:1486–91. [DOI] [PubMed] [Google Scholar]
- 11.Tanz LJ, Stuart JJ, Williams PL et al. Preterm Delivery and Maternal Cardiovascular Disease in Young and Middle-Aged Adult Women. Circulation 2017;135:578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Statistics Sweden. The Swedish Medical Birth Register.
- 13.Ludvigsson JF, Andersson E, Ekbom A et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linnersjo A, Hammar N, Gustavsson A, Reuterwall C. Recent time trends in acute myocardial infarction in Stockholm, Sweden. Int J Cardiol 2000;76:17–21. [DOI] [PubMed] [Google Scholar]
- 15.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: Wiley, 1987. [Google Scholar]
- 16.Grambsch PM. Goodness-of-fit and diagnostics for proportional hazards regression models. Cancer Treat Res 1995;75:95–112. [DOI] [PubMed] [Google Scholar]
- 17.Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions (3rd ed.). New York: Wiley, 2003. [Google Scholar]
- 18.Crump C, Sundquist J, Winkleby MA, Sundquist K. Gestational age at birth and mortality from infancy into mid-adulthood: a national cohort study. Lancet Child Adolesc Health 2019;3:408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.VanderWeele TJ. Causal interactions in the proportional hazards model. Epidemiology 2011;22:713–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonamy AK, Parikh NI, Cnattingius S, Ludvigsson JF, Ingelsson E. Birth characteristics and subsequent risks of maternal cardiovascular disease: effects of gestational age and fetal growth. Circulation 2011;124:2839–46. [DOI] [PubMed] [Google Scholar]
- 21.Brown HK, Speechley KN, Macnab J, Natale R, Campbell MK. Biological determinants of spontaneous late preterm and early term birth: a retrospective cohort study. BJOG 2015;122:491–9. [DOI] [PubMed] [Google Scholar]
- 22.Crump C, Sundquist K, Winkleby MA, Sundquist J. Early-term birth (37–38 weeks) and mortality in young adulthood. Epidemiology 2013;24:270–6. [DOI] [PubMed] [Google Scholar]
- 23.Crump C, Howell EA, Stroustrup A, McLaughlin MA, Sundquist J, Sundquist K. Association of Preterm Birth With Risk of Ischemic Heart Disease in Adulthood. JAMA Pediatr 2019; doi: 10.1001/jamapediatrics.2019.1327. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crump C, Sundquist J, Sundquist K. Risk of hypertension into adulthood in persons born prematurely: a national cohort study. Eur Heart J 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crump C, Sundquist J, Sundquist K. Preterm birth and risk of type 1 and type 2 diabetes: a national cohort study. Diabetologia 2020;63:508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crump C, Sundquist J, Sundquist K. Association of preterm birth with lipid disorders in early adulthood: A Swedish cohort study. PLoS Med 2019;16:e1002947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irgens HU, Reisaeter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study. BMJ 2001;323:1213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeFranco E, Teramo K, Muglia L. Genetic influences on preterm birth. Semin Reprod Med 2007;25:40–51. [DOI] [PubMed] [Google Scholar]
- 29.Thomson AJ, Telfer JF, Young A et al. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod 1999;14:229–36. [PubMed] [Google Scholar]
- 30.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci 2009;16:206–15. [DOI] [PubMed] [Google Scholar]
- 31.Cappelletti M, Della Bella S, Ferrazzi E, Mavilio D, Divanovic S. Inflammation and preterm birth. J Leukoc Biol 2016;99:67–78. [DOI] [PubMed] [Google Scholar]
- 32.Lane-Cordova AD, Gunderson EP, Carnethon MR et al. Pre-pregnancy endothelial dysfunction and birth outcomes: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Hypertens Res 2018;41:282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blake GJ, Ridker PM. Novel clinical markers of vascular wall inflammation. Circ Res 2001;89:763–71. [DOI] [PubMed] [Google Scholar]
- 34.Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr 2006;83:456S–460S. [DOI] [PubMed] [Google Scholar]
- 35.Libby P, Ridker PM, Hansson GK, Leducq Transatlantic Network on A. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol 2009;54:2129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shapiro-Mendoza CK, Barfield WD, Henderson Z et al. CDC Grand Rounds: Public Health Strategies to Prevent Preterm Birth. MMWR Morb Mortal Wkly Rep 2016;65:826–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


