Abstract
Objective
Gastrointestinal (GI) bleeding in patients who had a stroke is strongly associated with a higher risk of death and loss of independence. However, it is unknown whether GI bleeding increases risk for recurrence of stroke. In this study, we assess the potential relationship between GI bleeding and stroke recurrence in patients within 12 months of an acute ischaemic stroke (AIS), using the China National Stroke Registry (CNSR).
Methods
This study included 22 216 patients who had an ischaemic stroke included in the CNSR from 2007 to 2008. We analysed baseline patient characteristics, GI bleeding and outcomes of patients who had an AIS, specifically stroke recurrence at 3, 6 and 12 months. We used multivariable logistic regression to evaluate a possible association between GI bleeding and stroke recurrence.
Results
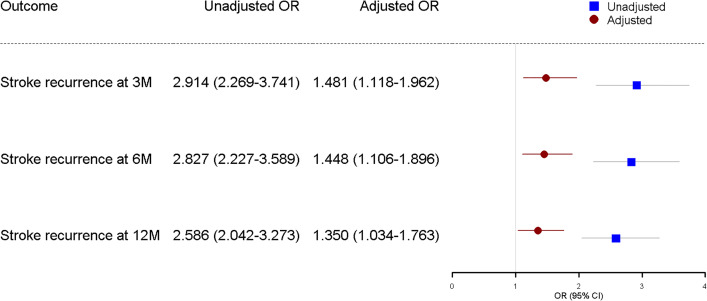
Of the 12 415 patients included in our study, 12.3%, 15.5% and 17.7% had a stroke recurrence at 3, 6 and 12 months, respectively. GI bleeding was an independent stroke recurrence risk factor in patients after ischaemic stroke at 3 months (adjusted OR 1.481, 95% CI 1.118 to 1.962), 6 months (adjusted OR 1.448, 95% CI 1.106 to 1.896) and 12 months (adjusted OR 1.350; 95% CI 1.034 to 1.763).
Conclusion
GI bleeding was associated with the increased risk of stroke recurrence after an AIS.
Keywords: stroke, complication
Introduction
Patients hospitalised with an acute ischaemic stroke are susceptible to multiple medical complications, which may increase rates of mortality and disability.1–7 One of the major medical complications of stroke is gastrointestinal (GI) bleeding.
GI bleeding during stroke hospitalisation is associated with poor outcomes in patients who had an acute ischaemic stroke (AIS).6–10 An association between GI bleeding and a higher risk of death or loss of independence in patients with stroke has been shown previously using data from the China National Stroke Registry (CNSR).11 12 Aside from one study suggesting the GI bleeding is not related to stroke recurrence,6 little data exist regarding the impact of GI bleeding on stroke recurrence. Therefore, we assessed the association between GI bleeding during hospitalisation and recurrent stroke within 12 months of AIS onset.
Methods
Data collection and study population
The CNSR was a prospective, national cohort study that has been detailed extensively in prior literature.13 Patients who had an acute stroke were included in the CSNR if they were: (1) male or female adults, (2) diagnosed with acute stroke using WHO International Classification of Diseases, 10th Revision criteria and confirmed by brain CT or MRI, (3) within 14 days after stroke onset and (4) either by themselves or via their surrogate, consented to participate in the study. We only included patients who had an AIS in this study and excluded patients with other stroke aetiologies such as transient ischaemic attack (TIA), undetermined stroke, intracerebral haemorrhage or subarachnoid haemorrhage. The CNSR study recruited patients between September 2007 and August 2008 and followed up with patients in August 2009. We collected data for our study at baseline (stoke onset) and at 3, 6 and 12 months after the stroke onset. National Institute of Health Stroke Scale (NIHSS) score was used to determine the severity of neurological impairment.14 The interviews conducted at baseline were performed for all patients by a trained investigator, and patients’ clinical characteristics including demographic information, medical history, stroke family history and baseline NIHSS scores were recorded. GI bleeding and other relevant clinical patient data were recorded during hospitalisation, typically directly from the patient or from their caregivers or surrogates. Our study divided patients into two groups based on the presence or absence of in-hospital GI bleeding.
Definition of GI bleeding
We defined GI bleeding as the presence of coffee-ground emesis, haematemesis, blood in the nasogastric tube, melena or blood in the rectum that occurred during hospitalisation and required blood transfusion or palliative intervention. All participating clinical centres used these criteria for GI bleeding diagnosis.11
Stroke recurrence assessment
The recurrence of stroke was the primary clinical outcome in this study, defined as an aggravated primary neurological deficit, a new neurological deficit or rehospitalisation with a diagnosis of ischaemic or haemorrhagic stroke15 that occurred any time from the patient’s hospital discharge until the end of the 12-month follow-up period. Once a recurrent stroke was reported by the patients, their caregivers or surrogates during the follow-up period, stroke certificates were faxed to Beijing Tiantan Hospital. Clinical coordinators and the principal investigator of the research assessed and verified suspected recurrent stroke.
Statistical analysis
Categorical variables are expressed as percentages, while continuous variables are shown as shown as mean±SD or median with IQR. We used t-tests for continuous variables and the χ2 test for categorical variables to assess differences between patients with and without GI bleeding. Univariate logistic regression analyses were used to identify the relationship between patient characteristics and recurrent stroke as well as GI bleeding and recurrent stroke, and data were expressed using unadjusted ORs with corresponding 95% CIs. Adjusted potential covariates included sex, age, baseline NIHSS, hyperlipidaemia, hypertension, atrial fibrillation, coronary heart disease, diabetes mellitus, history of TIA or stroke, family history of stroke, history of heavy drinking, history of smoking and antithrombotic treatment. We analysed data using SAS statistical software, V.9.4. A two-tailed p value <0.05 was deemed statistically significant.
Study protocol approval and patient informed consent
Before screening subjects for the CNSR, the study design and procedures were approved in each participating facility following institutional review. All participants or their legal guardians gave written informed consent to participate in the study.
Results
A total of 22 216 patients who had a stroke hospitalised in 132 participating centres were included in the original CNSR study. A total of 18 580 of these patients agreed to participate in follow-up studies after their first visit. Of this group, 12 415 patients were diagnosed with AIS (figure 1) and 322 (2.59%) had in-hospital GI bleeding.
Figure 1.
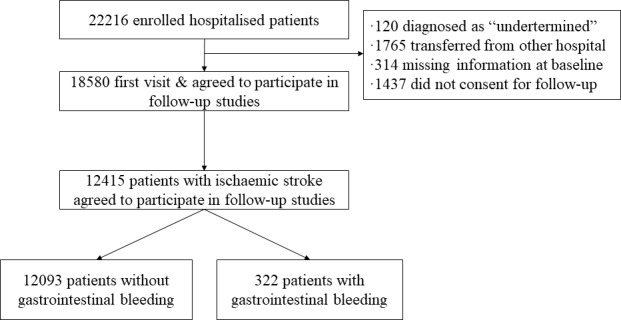
Selection of study population from CNSR database. CNSR, China National Stroke Registry.
The baseline characteristics of AIS patients with or without in-hospital GI bleeding is shown in table 1. The median age of the patients was 65 years old, 61.7% of which were male and 38.3% were female. Patients with GI bleeding were significantly older, had higher NIHSS scores, suffered from hypertension, coronary heart disease, atrial fibrillation, had a history of stroke and suffered from in-hospital pneumonia or urinary tract infection, compared with those who did not have GI bleeding. Whereas gender, hyperlipidaemia, diabetes mellitus, history of TIA, family history of stroke, history of smoking, history of heavy alcohol use and antiplatelet therapy did not significantly impact incidence of GI bleeding.
Table 1.
Baseline characteristics of patients with initial ischaemic stroke (n=12 415)
| Variable | Total (n=12 415) | Without GIB (n=12 093) | With GIB (n=322) | P value |
| Age, mean±SD (years) | 65.46±12.32 | 65.35±12.32 | 69.31±11.80 | <0.05 |
| Age category (years), n (%) | ||||
| <65 | 5500 (44.3) | 5403 (44.7) | 97 (30.1) | <0.05 |
| ≥65 | 6915 (55.7) | 6690 (55.3) | 225 (69.9) | <0.05 |
| Male, n (%) | 7658 (61.7) | 7453 (61.6) | 205 (63.5) | 0.459 |
| Baseline NIHSS, median (IQR) | 4 (2–9) | 4 (2–9) | 14 (6–22) | <0.05 |
| Hypertension, n (%) | 7909 (63.7) | 7687 (63.6) | 222 (68.9) | <0.05 |
| Hyperlipidaemia, n (%) | 1390 (11.2) | 1355 (11.2) | 35 (10.9) | 0.85 |
| Diabetes mellitus, n (%) | 2677 (21.6) | 2604 (21.5) | 73 (22.7) | 0.62 |
| Coronary heart disease, n (%) | 1792 (14.4) | 1729 (14.3) | 63 (19.6) | <0.05 |
| Atrial fibrillation, n (%) | 918 (7.4) | 881 (7.3) | 37 (11.5) | <0.05 |
| History of TIA, n (%) | 474 (3.8) | 464 (3.8) | 10 (3.1) | 0.50 |
| History of stroke, n (%) | 4234 (34.1) | 4085 (33.8) | 149 (46.3) | <0.05 |
| Family history of stroke, n (%) | 1519 (13.1) | 1468 (13.0) | 51 (15.8) | 0.05 |
| History of smoking, n (%) | 4934 (39.8) | 4808 (39.8) | 126 (39.1) | 0.82 |
| History of heavy alcohol use, n (%) | 1171 (9.4) | 1148 (9.5) | 23 (7.1) | 0.15 |
| Antithrombotic therapy, n (%) | 2067 (16.6) | 2003 (16.6) | 64 (19.9) | 0.12 |
| Pneumonia, n (%) | 1439 (11.6) | 1244 (10.3) | 195 (60.6) | <0.05 |
| Urinary tract infection, n (%) | 474 (3.8) | 431 (3.6) | 43 (13.4) | <0.05 |
GIB, gastrointestinal bleeding; NIHSS, National Institute of Health Stroke Scale; TIA, transient ischaemic attack.
Incidence of stroke recurrence at 3, 6 and 12 months post-stroke are shown in figure 2. Overall stroke recurrence for patients at 3, 6 and 12 months after onset was 12.3%, 15.5% and 17.7%, respectively. While the stroke recurrence for patients with GI bleeding was always much higher during follow-up period (28.1%, 33.3% and 35% at 3, 6 and 12 months, respectively)
Figure 2.
Stroke recurrence at 3, 6 and 12 months after stroke onset. GIB, gastrointestinal bleeding; M, months.
Univariate logistic regression analysis revealed that age, male gender, baseline NIHSS, high blood pressure, diabetes mellitus, coronary heart disease, atrial fibrillation, history of TIA or stroke, antithrombotic therapy, and in-hospital pneumonia or urinary tract infection were all associated with stroke recurrence at 3, 6 and 12 months following an initial AIS (table 2). GI bleeding during hospitalisation was associated with a higher risk of stroke recurrence in analyses adjusted for age, gender, baseline NIHSS, hypertension, hyperlipidaemia, diabetes mellitus, coronary heart disease, history of TIA or stroke, family history of stroke, atrial fibrillation, history of smoking, history of heavy alcohol use and antithrombotic therapy (figure 3). The adjusted ORs of recurrent stroke were approximately 1.5, 1.5 and 1.4-fold higher in patients with GI bleeding at 3, 6 and 12 months, respectively.
Table 2.
Univariate logistic regression analysing the effect of confounders on stroke recurrence at 3, 6 and 12 months after initial ischaemic stroke
| Variables | Stroke recurrence OR (95% CI) | ||
| 3 months | 6 months | 12 months | |
| Age | 1.588 (1.418 to 1.778) | 1.759 (1.584 to 1.953) | 1.771 (1.602 to 1.958) |
| Male gender | 1.245 (1.115 to 1.389) | 1.291 (1.168 to 1.428) | 1.265 (1.148 to 1.394) |
| Baseline NIHSS | 1.057 (1.050 to 1.063) | 1.060 (1.054 to 1.066) | 1.058 (1.051 to 1.064) |
| Hypertension | 1.044 (0.932 to 1.169) | 1.078 (0.971 to 1.196) | 1.141 (1.031 to 1.262) |
| Hyperlipidaemia | 0.983 (0.828 to 1.168) | 0.997 (0.852 to 1.166) | 1.008 (0.867 to 1.171) |
| Diabetes mellitus | 1.175 (1.034 to 1.335) | 1.230 (1.095 to 1.382) | 1.257 (1125 to 1.406) |
| Coronary heart disease | 1.540 (1.340 to 1769) | 1.554 (1.367 to 1.766) | 1.634 (1.445 to 1.848) |
| Atrial fibrillation | 2.361 (2.002 to 2.785) | 2.358 (2.020 to 2.753) | 2.370 (2.038 to 2.756) |
| History of TIA or stroke | 1.683 (1.509 to 1.877) | 1.708 (1.545 to 1.888) | 1.783 (1.619 to 1.964) |
| Family history of stroke | 0.930 (0.784 to 1.103) | 0.853 (0.728 to 1.000) | 0.866 (0.744 to 1.007) |
| History of smoking | 0.787 (0.703 to 0.881) | 0.788 (0.711 to 0.874) | 0.799 (0.723 to 0.882) |
| History of heavy alcohol use | 0.743 (0.606 to 0.911) | 0.712 (0.590 to 0.859) | 0.691 (0.576 to 0.829) |
| Antithrombotic therapy | 1.411 (1.233 to 1.614) | 1.424 (1.258 to 1.611) | 1.399 (1.240 to 1.577) |
| Pneumonia | 3.157 (2.764 to 3.605) | 3.059 (2.698 to 3.468) | 2.915 (2.577 to 3.298) |
| Urinary tract infection | 2.306 (1.848 to 2.876) | 2.426 (1.975 to 2.980) | 2.196 (1.791 to 2.693) |
NIHSS, National Institute of Health Stroke Scale; TIA, transient ischaemic attack.
Figure 3.
Unadjusted and adjusted ORs of stroke recurrence in patients with gastrointestinal bleeding following acute ischaemic stroke. *Adjusted for age, sex, baseline NIHSS, hypertension, diabetes mellitus, hyperlipidaemia, history of coronary heart disease, history of TIA, family history of stroke, atrial fibrillation, history of smoking, history of heavy alcohol use and antithrombotic therapy. M, months; NIHSS, National Institute of Health Stroke Scale; TIA, transient ischaemic attack.
We also assessed the impact of antithrombotic therapy on stroke recurrence in patients with and without GI bleeding (table 3). Patients with in-hospital GI bleeding were less likely to have received antithrombotic therapy compared with those without in-hospital GI bleeding, during the 12 months after initial AIS hospitalisation.
Table 3.
Antithrombotic therapy usage within 12 months of initial stroke
| Antithrombotic therapy | Total (n=12 415) |
Without GIB (n=12 093) | With GIB (n=322) |
P values |
| At discharge (n) | 8517 | 8433 | 84 | |
| Usage at 3 months (%) | 73.55 | 73.67 | 60.71 | 0.007 |
| Usage at 6 months (%) | 67.49 | 67.65 | 51.19 | 0.001 |
| Usage at 12 months (%) | 43.85 | 44.04 | 25.00 | <0.001 |
GIB, gastrointestinal bleeding.
Discussion
This study examined the impact of in-hospital GI bleeding on stroke recurrence in patients who had an AIS from the CNSR. The CNSR is the most extensive stroke registry in China and includes patients who had an acute stroke from all over the country. The prevalence of in-hospital GI bleeding in patients who had an AIS form this national prospective cohort was about 2.6%. We identified an association between in-hospital GI bleeding and increased risk of stroke recurrence in patients who had an AIS in the initial 12 months after hospitalisation.
The prevalence of GI bleeding in patients who had an AIS varies between 1.24% and 8.1% in prior studies,6–11 16–18 which may be due to geographically distinct study populations and the different AIS treatment approaches in these settings.
Limited data exist regarding risk factors for poststroke GI bleeding. Using data from the CNSR, Ji et al 19 identified advanced age, male gender, history of peptic ulcer or previous GI bleeding, history of hypertension, prestroke lack of independence, admission stroke severity, impaired consciousness and middle cerebral artery territory ischaemia as risk factors for GI bleeding after AIS. In their study, hepatic cirrhosis and posterior circulation stroke were associated with poststroke GI bleeding.19 Rumalla and Mittal identified a few previously undescribed predictors of poststroke GI bleeding, such as fluid and electrolyte imbalance, paralysis, alcohol abuse and deficiency anaemia.7
The different antithrombotic therapy may have different impacts on GI bleeding, including antiplatelet therapy and anticoagulant therapy.
Many studies suggest that patients who had a stroke are susceptible to GI bleeding, which may be associated with poor patient outcomes.6–10 The influence of GI bleeding on stroke outcomes has been discussed in prior studies, but the focus of prior work has primarily been on the impact of GI bleeding on mortality and disability.6–8 10–12 16 17 The aforementioned study from Rumalla and Mittal found that the death rate of patients who had an ischaemic stroke was 1.8-fold higher in patients with a GI bleed compared with those without.7 Earlier work from our group corroborated this finding, as we found similar increases in risk of death in stroke patients with a GI bleed.11 Other studies have shown that stroke patients with a GI bleed had a greater risk of losing functional independence poststroke versus those without GI bleeding.6–8 10 12 Our data help further quantify the impact of in-hospital GI bleeding in stroke patients by showing that this subset of patients has approximately a 1.5-fold higher risk of stroke recurrence at 3, 6 and 12 months after initial stroke hospitalisation.
The exact mechanism of how in-hospital GI bleeding increases the risk of recurrent stroke remains unclear. The presence of GI bleeding usually causes clinicians to stop the use of antithrombotic therapy, which may leave the patient in a prothrombotic state. This could cause platelet and coagulation cascade activation, thereby increasing the risk of thrombotic events.20 To reduce the risk of stroke recurrence, antithrombotic medication is recommended as a secondary prevention therapy after an ischaemic stroke of presumed arterial origin.21–23 Because antithrombotic therapy in essential in patients who had an ischaemic stroke, in-hospital GI bleeding that leads to a cessation of antithrombotic therapy could be a candidate mechanism explaining our findings. This hypothesis is supported by observed differences in antithrombotic therapy compliance of patients with and without in-hospital GI bleeding. A significant GI bleeding also influences haemodynamic stability, which is likely to increase the risk of developing an ischaemic stroke. Another mechanism may be related with the severity of stroke. Patients with severe stroke would have more complications including GI bleeding and increased risk of stroke recurrence. We did not directly assess this possible mechanism as a cause of stroke recurrence in our study, and further investigation is necessary to develop appropriate therapeutic approaches aimed at reducing stroke recurrence in patients with GI bleeding.
Our study has several limitations. First, we included patients in this study solely based on a diagnosis of GI bleeding during hospitalisation but did not extensively evaluate the therapies they were given to reduce GI bleeding, which may impact stroke recurrence. Second, patients with new GI bleeds after initial hospitalisation were not included in our study. This limited our ability to evaluate the relationship between recurrent stroke and a new GI bleed that occurred during the follow-up period. Third, the time of GI bleeding after index stroke onset, the detailed antithrombotic therapy including antiplatelet and anticoagulation medication and how long antithrombotic therapy not used were missing in the data collection. This information might have some impact on stroke recurrence. Fourth, we also did not extensively evaluate the psychological and cognitive functions of patients after AIS, which may also affect their antithrombotic medication usage.
Conclusion
We found that in patients who had an AIS, in-hospital GI bleeding significantly increased the chance of stroke recurrence. This may be indirectly caused by decreased antithrombotic therapy usage in patients having GI bleeding. Thus, approaches used to prevent and treat GI bleeding after stroke should be carefully considered to help avoid increased risk of stroke recurrence.
Footnotes
PW and YW contributed equally.
Contributors: YoW and PW are co-corresponding author. Study concept and design: YoW, PW and WD. Provision of study materials or patients: all authors. Drafting of the manuscript: YoW, PW and WD. Critical revision of the manuscript for important intellectual content: GL and RJ. Statistical analysis: AW, YP and HG. Study supervision and organization of the project: YoW, XZ, YiW, LL and KD.
Funding: This study was supported by grants from the National Key Research and Development Program from the Ministry of Science and Technology of China (2018YFC1312400), the Ministry of Science and Technology of the People’s Republic of China (2016YFC0901002, 2016YFC0901001, 2017YFC1310901, 2017YFC1307905 and 2018YFC1312903), grants from Beijing Municipal Administration of Hospitals’ Mission Plan (SML20150502), grants from National Natural Science Foundation of China (81600999), grants from Beijing Municipal Science & Technology Commission (D171100003017002 and D151100002015003) and grants from National Science and Technology Major Project (2017ZX09304018).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study was approved by ethics committee at Beijing Tiantan Hospital.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request.
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke Statistics-2016 update: a report from the American heart association. Circulation 2016;133:e38–60. 10.1161/CIR.0000000000000350 [DOI] [PubMed] [Google Scholar]
- 2. Liu M, Wu B, Wang W-Z, et al. Stroke in China: epidemiology, prevention, and management strategies. Lancet Neurol 2007;6:456–64. 10.1016/S1474-4422(07)70004-2 [DOI] [PubMed] [Google Scholar]
- 3. Johnston SC, Mendis S, Mathers CD. Global variation in stroke burden and mortality: estimates from monitoring, surveillance, and modelling. Lancet Neurol 2009;8:345–54. 10.1016/S1474-4422(09)70023-7 [DOI] [PubMed] [Google Scholar]
- 4. Towfighi A, Saver JL. Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead. Stroke 2011;42:2351–5. 10.1161/STROKEAHA.111.621904 [DOI] [PubMed] [Google Scholar]
- 5. Mozaffarian D, Benjamin EJ, Go AS, et al. Executive Summary: Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation 2016;133:447–54. 10.1161/CIR.0000000000000366 [DOI] [PubMed] [Google Scholar]
- 6. Chou Y-F, Weng W-C, Huang W-Y. Association between gastrointestinal bleeding and 3-year mortality in patients with acute, first-ever ischemic stroke. J Clin Neurosci 2017;44:289–93. 10.1016/j.jocn.2017.06.068 [DOI] [PubMed] [Google Scholar]
- 7. Rumalla K, Mittal MK. Gastrointestinal bleeding in acute ischemic stroke: a population-based analysis of hospitalizations in the United States. J Stroke Cerebrovasc Dis 2016;25:1728–35. 10.1016/j.jstrokecerebrovasdis.2016.03.044 [DOI] [PubMed] [Google Scholar]
- 8. O'Donnell MJ, Kapral MK, Fang J, et al. Gastrointestinal bleeding after acute ischemic stroke. Neurology 2008;71:650–5. 10.1212/01.wnl.0000319689.48946.25 [DOI] [PubMed] [Google Scholar]
- 9. Ogata T, Kamouchi M, Matsuo R, et al. Gastrointestinal bleeding in acute ischemic stroke: recent trends from the Fukuoka stroke Registry. Cerebrovasc Dis Extra 2014;4:156–64. 10.1159/000365245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shi G-M, Zhang Y-D, Geng C, et al. Profile and 1-year outcome of ischemic stroke in East China: Nanjing first Hospital stroke Registry. J Stroke Cerebrovasc Dis 2016;25:49–56. 10.1016/j.jstrokecerebrovasdis.2015.08.032 [DOI] [PubMed] [Google Scholar]
- 11. Wang P-L, Zhao X-Q, Yang Z-H, et al. Effect of in-hospital medical complications on case fatality post-acute ischemic stroke: data from the China national stroke Registry. Chin Med J 2012;125:2449–54. [PubMed] [Google Scholar]
- 12. Wang P-L, Zhao X-Q, DU W-L, et al. In-hospital medical complications associated with patient dependency after acute ischemic stroke: data from the China national stroke Registry. Chin Med J 2013;126:1236–41. [PubMed] [Google Scholar]
- 13. Wang Y, Cui L, Ji X, et al. The China National Stroke Registry for patients with acute cerebrovascular events: design, rationale, and baseline patient characteristics. Int J Stroke 2011;6:355–61. 10.1111/j.1747-4949.2011.00584.x [DOI] [PubMed] [Google Scholar]
- 14. Brott T, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864–70. 10.1161/01.STR.20.7.864 [DOI] [PubMed] [Google Scholar]
- 15. Pan Y, Jing J, Chen W, et al. Post-Glucose load measures of insulin resistance and prognosis of nondiabetic patients with ischemic stroke. J Am Heart Assoc 2017;6:1 10.1161/JAHA.116.004990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davenport RJ, Dennis MS, Warlow CP. Gastrointestinal hemorrhage after acute stroke. Stroke 1996;27:421–4. 10.1161/01.STR.27.3.421 [DOI] [PubMed] [Google Scholar]
- 17. Hamidon BB, Raymond AA. The risk factors of gastrointestinal bleeding in acute ischaemic stroke. Med J Malaysia 2006;61:288–91. [PubMed] [Google Scholar]
- 18. Hsu H-L, Lin Y-H, Huang Y-C, et al. Gastrointestinal hemorrhage after acute ischemic stroke and its risk factors in Asians. Eur Neurol 2009;62:212–8. 10.1159/000229018 [DOI] [PubMed] [Google Scholar]
- 19. Ji R, Shen H, Pan Y, et al. Risk score to predict gastrointestinal bleeding after acute ischemic stroke. BMC Gastroenterol 2014;14:130 10.1186/1471-230X-14-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eikelboom JW, Mehta SR, Anand SS, et al. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 2006;114:774–82. 10.1161/CIRCULATIONAHA.106.612812 [DOI] [PubMed] [Google Scholar]
- 21. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med 2013;369:11–19. 10.1056/NEJMoa1215340 [DOI] [PubMed] [Google Scholar]
- 22. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2160–236. 10.1161/STR.0000000000000024 [DOI] [PubMed] [Google Scholar]
- 23. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart Association/American stroke association. Stroke 2018;49:e46–110. 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]