Abstract
Introduction:
Glucocorticoids (GCs), especially low-dose GCs, are commonly prescribed for rheumatoid arthritis (RA), although the risk/benefit ratio is controversial. A randomized, double-blind clinical trial was performed to evaluate the efficacy and safety of low-dose oral GCs combined with methotrexate (MTX) and hydroxychloroquine (HCQ) in early RA (ERA).
Methods:
Eighty untreated ERA patients were randomized into the trial (GCs + MTX + HCQ) and control (placebo + MTX + HCQ) groups, for 1-year treatment. Therapeutic evaluation indices were American College of Rheumatology (ACR) 20 of ACR, disease activity score (DAS) 28- erythrocyte sedimentation rate (ESR), visual analog scale scores, joint function, health assessment questionnaire-disability index score, morning stiffness duration, C-reaction protein and ESR. The clinical indicators were evaluated pre-treatment and at 1st, 3th, 6th and 12th month of treatment. The MRI data of single joint (ie, the most swollen joint) for each patient were acquired with a revised OMERACT RAMRIS Scoring System before and after treatment. The correlation analysis was adopted to confirm whether the efficacy of GC treatment is related to the time of RA onset. The side effects (eg, gastrointestinal reactions, liver dysfunction, upper respiratory tract infection, leukocyte reduction) were also monitored.
Results:
At 1st month, 55% and 20% cases in the experimental and control groups achieved ACR20 response, respectively, indicating a significant difference (χ2 = 16.157, P < .001). This trend continued until 6th month. At 12th month, the number of patients achieved ACR20 response was similar in both groups. At 1st to 6th month, DAS28- ESR scores in the experimental group were significantly lower than control values (all p < .05). The experimental group showed improved inflammation, quality of life and radiological symptoms. Bone erosion remained unchanged in the experimental group, while worsening in control group. Correlation coefficients between RA duration and DAS28-ESR score were 0.496, 0.464, 0.509, and 0.550 at 1st, 3th, 6th, and 12th month, respectively. No differences were found in adverse events between the 2 groups.
Conclusions:
Low-dose GCs combined with MTX and HCQ significantly achieves disease remission indexed by ACR20 and DAS28-ESR, and improves clinical and radiological outcomes in ERA patients at the early stage, with superiority over placebo + MTX + HCQ, without enhancing adverse reactions.
Keywords: early rheumatoid arthritis, efficacy, hydroxychloroquine, low-dose glucocorticoids, methotrexate, safety
1. Introduction
Rheumatoid arthritis (RA) is a chronic, progressive, and aggressive autoimmune disease characterized by symmetrical polyarthritis, with a prevalence rate of about 0.4% in China.[1] The diagnosis can be difficult, especially if not all the diagnostic criteria or typical clinical features are seen.[2,3] Moreover, Joint erosion can be observed within 6 months of disease onset in the majority of patients, and occurs more rapidly in the first year compared with late disease stage.[4,5] Early intervention that prevents irreversible damage would offer the best opportunities for achieving favorable outcomes in patients with early, aggressive RA.[6–9] Therefore, early diagnosis and treatment to achieve disease remission are of great significance.[10,11]
Glucocorticoids (GCs) have been commonly used in RA since the 1950 s, initially as symptomatic treatment, but in the last few years as disease-modifying therapy.[12] The risk/benefit ratio of GCs remains controversial.[13] However, it has been reported that low-dose GCs combined with disease-modifying antirheumatic drugs (DMARDs) could improve clinical and radiological outcomes in RA patients.[14–17] Currently, the European League Against Rheumatism (EULAR) guidelines recommend considering short-term GCs when initiating or changing conventional synthetic DMARDs for patients with RA; besides, these guidelines recommend tapering GCs as rapidly as clinically feasible.[18] The 2015 American College of Rheumatology (ACR) guidelines state that GCs should be used at the lowest possible dose and for the shortest possible duration based on a benefit-risk analysis.[19]
Considering the importance of early treatment in RA,[11] in a previous double-blind clinical trial, we evaluated the efficacy and safety of low-dose prednisone combined with methotrexate (MTX) versus MTX alone in the treatment of early RA (ERA).[20] The results showed that low-dose GCs combined with MTX could significantly improve symptoms, signs, and laboratory inflammatory indexes in ERA patients, with enhanced curative effects compared with MTX alone. The combination therapy was found to be safe and well-tolerated, not increasing the incidence rates of adverse reactions compared with MTX alone. However, the treatment duration of this study lasted only 3 months, and only GCs combined with MTX was considered as a treatment regimen. In recent years, with further studies on hydroxychloroquine (HCQ), it has been shown that HCQ promotes synovial cell apoptosis in RA,[21] reduces serum lipid levels,[22] blood glucose levels and diabetes risk,[23] and helps prevent osteoporosis[24] and severe infection.[21] Therefore, its clinical application is increasingly widespread. To our knowledge, no study explored the efficacy of low-dose GCs combined with multiple DMARDs (MTX + HCQ).
Therefore, this study aimed to compare low-dose GCs combined with multiple DMARDs (MTX + HCQ) and placebo combined with MTX + HCQ in patients with ERA for efficacy after 1-year of treatment. We hypothesized that Low-dose GCs combined with MTX and HCQ could significantly improve symptoms, signs, and inflammation in ERA, especially in the early stage.
2. Materials and methods
2.1. Study design
This study was approved by the clinical research ethics committees of the Jinhua Central Hospital (2014[8]). We did a single-center, randomized, double-blind clinical trial at the Department of Rheumatology in Jinhua Central Hospital, Jinhua Zhejiang, China. Magnetic resonance imaging (MRI) with high sensitivity was used to assess changes in patients before and after treatment (including early lesions such as synovitis and bone marrow edema). Based on several clinical indicators (eg, quality of life, functional assessment, disease activity, side effects), the efficacy and safety of the standard regimen of low-dose GCs combined with DMARDs (MTX + HCQ) and placebo combined with MTX + HCQ were explored. This study followed the Good Clinical Practice guidelines and the guidelines of the Helsinki Declaration. The study protocol was registered at the Chinese Clinical Trial Registry and the registration number is ChiCTR1900026116.
2.2. Patients
A total of 110 RA patients were screened between January 2014 and May 2015. RA patients were classified using the 2010 ACR/EULAR criteria.[25] None had received RA medication before this study, and all had a disease onset of less than 12 months. Exclusion criteria were: diabetes and osteoporosis prone to brittle fracture; severe infections (such as hepatitis, pneumonia and pyelonephritis) in the last 2 months; pregnancy or lactation in women; tuberculosis; tumors, multiple sclerosis, central nervous demyelination or congestive heart failure; other serious diseases affecting vital visceral organs such as the heart, liver, or kidney; blood or endocrine system disease. Besides, all of the patients were required to sign a written informed consent before the enrollment. The sample size was calculated using G∗Power 3.1 based on 1) the study design (ie, mixed factorial design); 2) a type I error rate of 5% (α=0.05); 3) a statistical power of 95% (1-β=0.95); and 4) a moderate effect size of 0.34 based on our previous study.[20] The total estimated sample included 70 patients.
2.3. Randomization and blinding
An independent doctor from our department generated random numbers (in a 1:1 ratio). During the study period, continuously recruited patients were randomly assigned to experimental or control groups by their primary nurse according to the randomization sequence. Study personnel, health-care team members, and patients were blinded to the group assignment throughout the study period. In an emergency, unblinding of the treatment allocation could be requested, and the patient would be quit from the study.
2.4. Procedures
MTX + HCQ + GCs tablets were administrated to the experimental group. MTX was started at 7.5 mg per week orally for 2 weeks, and gradually increased by 2.5 mg per week every 2 weeks, with a maximum of 20 mg per week. The dosage of HCQ was 0.4 g daily. Oral GCs was administered at 10 mg daily in the first 3 months, then reduced to 5 mg daily from the 4th month and stopped at 6th month. The control group was treated with MTX + HCQ + placebo tablets, following the same regimen for MTX and HCQ as in the experimental group. In patients not achieving clinical remission or showing no noticeable improvement after 3 months, sulfasalazine tablets were added as a rescue. Non-steroidal anti-inflammatory analgesics were allowed in both groups before the effects of slow-acting drugs. At the beginning of treatment, patients in both groups were treated with proton pump inhibitors prevent gastrointestinal reactions; meanwhile calcium tablets (Calcium D), as well as active vitamin D, were administered to prevent osteoporosis.
2.5. Outcomes
2.5.1. Clinical indicators
The following indicators were evaluated pre-treatment and at 1st, 3th, 6th, and 12th month of treatment: morning stiffness, joint swelling index, joint tenderness index, visual analog scale (VAS)[26] score for pain, VAS score for disease activity assessed by physicians (a total of 4 doctors with the Associate Chief Physician title or above participated in case enrollment, and have been trained in randomized trials with participation in related projects), VAS score for disease activity assessed by the patient, and health assessment questionnaire-disability index (HAQ-DI) score.[27] Meanwhile, adverse events occurring during the treatment process, including infection, bone marrow suppression, and liver and kidney dysfunction, were collected.
2.5.2. Laboratory indicators
Blood and urine routine examinations, blood biochemistry, and erythrocyte sedimentation rate (ESR) and C-reaction protein (CRP) were assessed before treatment and at 1st, 3th, 6th, and 12th month of treatment, respectively. Lung computed tomography and electrocardiography (before enrollment), and joint Magnetic Resonance Imaging (MRI; before and after treatment) were performed following previously described procedures.[28] The MRI data of single joint (ie, the most swollen joint) for each patient were acquired with a revised OMERACT RAMRIS Scoring System (including synovium, bone erosion, bone marrow edema, as well as tendon sheath and tendon).[29–31] The score range of synovium, bone marrow edema and tendon is from 0 to 3 points. Bone erosion is graded by percentage volume (0–10, by 10% volume increments) of the assessed bone. All of the images were scored twice by 2 experienced readers blinded to patient details.
2.5.3. Efficacy evaluation
The standard ACR20 and disease activity score (DAS) 28-ESR[27] were used for efficacy evaluation. ACR20 response was defined as a 20% improvement in the amounts of tender and swollen joints, as well as ≥20% improvement in at least 3 of the following 5 items: pain VAS score, physician VAS score for disease activity, patient VAS score for disease activity, HAQ-DI score, ESR or CRP. DAS28-ESR score < 2.6 was considered to indicate remission. ACR20 response is primary and DAS28-ESR is the secondary outcomes.
2.6. Statistical analysis
An intention-to-treat, a per-protocol (PP) and a safety analysis were performed. The safety analysis used all available data. The PP analysis included only patients following the protocol until 12 months. The intention-to-treat analysis imputed data when missing and was applied to analyze the remission assessment results of ACR20 response and DAS28-ESR.
The SPSS22.0 statistical software was used for data analysis. Measurement data are mean ± standard deviation, and were analyzed by independent samples t-test. Chi-square test was adopted to analyze the remission assessment results of ACR20 response and DAS28-ESR for efficacy evaluation. Repeated measures ANOVA was used to assess differences of clinical and radiological symptoms within and between groups at different time points. The Spearman correlation test was used to analyze the association of RA duration and the efficacy of GC treatment in order to confirm whether the efficacy of GC treatment is related to the time of RA onset. Two-sided P < .05 was considered statistically significant.
3. Results
3.1. Comparison of demographic and clinical data in patients before study
As shown in Figure 1, 80 patients were enrolled and randomly allocated into experimental group (n = 40) and control group (n = 40). Patients with high, moderate and low disease activity in the experimental group were 21, 18, and 1, respectively, and 22, 16, and 2, respectively, in the control group. During the study, a total of 3 (refusal, 1; time-point skipping, 2) and 8 (refusal, 2; time-point skipping, 3; adverse events, 3) were excluded from the experimental and control groups, respectively. Therefore, 37 patients in the experimental group and 32 patients in the control group were included in the final data analysis.
Figure 1.
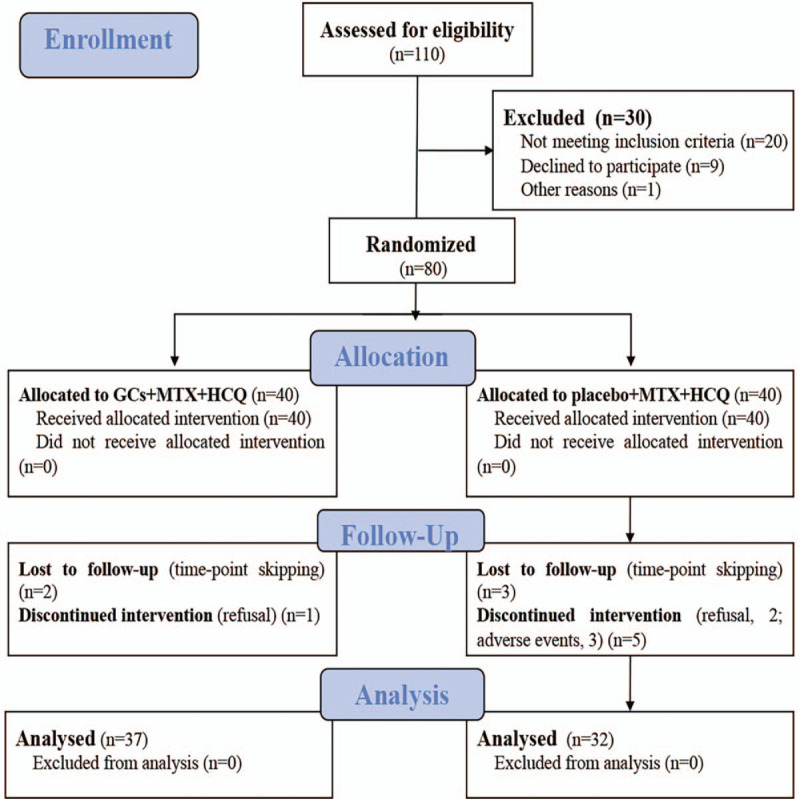
Trial profile. Data analysis included all patients in the groups to which they were randomly assigned.
There was no significant difference in demographic data including age, gender, RA duration, CRP, ESR, morning stiff time, numbers of joint tenderness, numbers of joint swelling, pain VAS scores, HAQ-DI, or DAS28-ESR between the experimental and control group (all P > .05, Table 1).
Table 1.
Baseline demographic and RA related clinical indicators of patients in the 2 groups.

3.2. ACR20 response
The percentages of patients in the experimental and control groups who achieved an ACR20 response at various time points during the study were shown in Figure 2. Statistical analysis showed that 55% of patients (22 cases) in the experimental group met ACR20 criteria at 1st month, versus 20% (8 cases) in the control group, indicating a significantly higher rate in the experimental group compared with control patients (χ2 = 16.157, P < .001). At 3th, 6th, and 12th month, significantly more patients in the experimental group achieved an ACR20 response compared with control group (85%, 87.5%, and 90% respectively, versus 47.5%, 60%, and 72.5% respectively; all P < .05). These results showed that low dose GC combined with DMARDs could significantly relieve symptoms in ERA patients.
Figure 2.

Percentages of patients achieving ACR20 response at different time points. An intention-to-treat (ITT) analysis was performed. Abscissas are assessment times, and ordinates are the percentages of patients achieving ACR20 response. ACR = American College of Rheumatology.
3.3. DAS28-ESR scores
Figure 3 shows average DAS28-ESR scores in the experimental and control groups at various time points. Average DAS28-ESR scores in the experimental group decreased rapidly and became flat from the 1st month. Statistical analysis showed that the main effects of both group and time were significant (group: F(1, 67) = 6.81, P = .011, η2 = .09; time: F(4, 268) = 321.36, P < .001, η2 = .83). The interaction effect between group and time was also significant (F(4,268) = 18.04, P < .001, η2 = .21). Further simple effect test showed that average DAS28-ESR scores in the experimental group were significantly lower than those of the control group at 1st to 6th month (all P < .01). These results suggested that low dose GCs combined with DMARDs therapy was effective at the early stage of treatment.
Figure 3.

Average DAS28-ESR scores in the 2 groups at different time points. Abscissas are assessment times, and ordinates are average DAS28-ESR scores. DAS = disease activity score.
With DAS28-ESR < 2.6 as the remission standard, the remission compliance rate was calculated at 12th month. Data analysis showed that although remission rate in the experimental group (62.5%) was higher than that of the control group (55%), there was no statistical significance (χ2 = .464, P = .496).
3.4. Clinical features and laboratory findings
Changes of various clinical parameters and laboratory indicators (from baseline values) in the experimental and control groups were calculated (see Table 2). Data analysis showed better improvement in the experimental group compared with control patients from the 1st month of treatment. During the first 3 months of treatment specifically, GCs combined therapy was effective. The advantage of this treatment lasted for 6 months. At 12 months of treatment, there were no significant differences in indicators between the 2 groups (all P > .05).
Table 2.
Changes of clinical and laboratory indicators compared with baseline values.

3.5. MRI scores
Only single joint (ie, the most swollen joint) for each patient was scanned. Specifically, in experimental group, 22 hand joints, 10 wrist joints and 5 knee joints were analyzed, and in control group, 18 hand joints, 10 wrist joints and 4 knee joints were analyzed. Figure 4 shows the MRI scores for 4 items, including synovium, bone erosion, bone marrow edema, as well as tendon sheath and tendon in both groups. The results revealed that the main effects of both group and MRI evaluation content were significant (group: F(1, 67) = 7.57, P = .008, η2 = .10; MRI evaluation content: F(3, 201) = 136.04, P < .001, η2 = 0.67). In addition, the interaction between time and group as well as time and MRI evaluation content were also significant (time X group: F(1, 67) = 14.17, P < .001, η2 = 0.18; time X MRI evaluation content: F(3, 201) = 10.76, P < .001, η2 = 0.14). Considering our study aim, a simple effect test was performed on the interaction between time and group. The results showed that radiologic findings were improved significantly after treatment in the experimental group (F(1, 36) = 26.78, P < .001, η2 = .43); however, no significant differences in radiology findings were found between pre- and post-treatment values (P = .186) in the control group. To further assess the changes of bone erosion after treatment, bone erosion MRI scores were reanalyzed with the MRI score of bone erosion as a dependent variable. We found that the main effects of both time and group were significant (time: F(1,67) = 15.23, P < .001, η2 = .19; group: F(1, 67) = 4.78, P = .032, η2 = 0.067), and the interaction between time and group was also significant (F(1, 67) = 10.12, P = .002, η2 = .13). Furthermore, simple effect test showed that there was no significant change of bone erosion after treatment in the experimental group (P = .422), while significant progress of bone erosion was observed in the control group (F(1, 31) = 14.02, P < .001, η2 = 0.31).
Figure 4.

MRI scores of each item in the experimental and control groups before and after treatment. MRI = magnetic resonance imaging.
3.6. Correlation between RA duration and GC treatment efficacy
Correlation coefficients between RA duration and DAS28-ESR score at 1, 3, 6, and 12 months were 0.496, 0.464, 0.509, and 0.550, respectively (all P < .01), indicating that RA duration was closely associated with GC treatment efficacy. The shorter the RA duration before treatment, the better the efficacy of GC treatment, suggesting the importance of the early diagnosis and treatment.
3.7. Drug safety
In the experimental group, gastrointestinal reactions were observed in 5 patients; liver dysfunction was found in 4 patients with slightly elevated liver enzymes; upper respiratory tract infection was found in 2 patients. In the control group, gastrointestinal reactions, liver dysfunction and leukocyte reduction were found in 3, 4, and 3 cases, respectively. Both groups showed no side effects such as fracture, hypertension, hyperglycemia and severe respiratory tract infection.
4. Discussion
The present study assessed the efficacy and safety of low-dose GCs combined with MTX + HCQ in untreated patients with ERA using a randomized, double-blind design. The results showed that compared with MTX + HCQ, low-dose GCs combined with MTX + HCQ improved symptoms, signs, and laboratory inflammatory activity index in ERA patients more effectively, and increased the somatic-motor-function-based quality of life (HAQ-DI score). More importantly, the combined treatment regimen helped control the radiological progression of the joint, especially bone erosion, with a certain overall improvement in radiological signs. This effect was mainly manifested by early remission of inflammation. At 1st month of treatment, all indicators in the experimental group were significantly improved compared with control group. Indeed, the proportion of subjects who achieved ACR20 response was significantly higher while DAS28-ESR scores were remarkably lower in the experimental group compared with control patients. Throughout the early stage of the trial (first 6 months), the experimental group maintained superior therapeutic effects. At 12th month of treatment, there were no significant differences in the assessed indicators between the 2 groups. Adverse events during the trial were similar in both groups, suggesting that low-dose GCs does not cause more side effects while providing protective measures.
These results corroborated other reports.[16,32,33] For instance, Bakker et al[14] performed a randomized study of low-dose GCs combined with MTX and MTX monotherapy in 236 ERA patients with disease onset below 1 year, and found that after 2 years of treatment, low dose GCs combined with MTX is more effective than MTX monotherapy in reducing disease activity and body disability; in addition, imaging scores of joint damage in the combined treatment group were found to be significantly lower than those of the monotherapy group. The combined treatment could achieve long-term remission and reduce the use of biological agents. The rates of adverse events were similar in both groups, and some adverse events occurred even less often in the GCs combined treatment group.
Unlike previous studies that did not strictly distinguish between newly diagnosed and previously treated ERA patients, this work identified newly diagnosed ERA patients as subjects, providing additional and more profound evidence for current researches. The current results also suggested that the efficacy of low-dose GCs combined with MTX + HCQ was characterized by rapid onset and remission at the early stage of treatment. Importantly, the combined regimen inhibited radiological progression of the joint, effectively improved synovitis and inhibited bone erosion. More importantly, this study found a significant correlation between RA duration and the efficacy of GC treatment. The shorter the duration of RA, the better the efficacy of GC treatment. These findings conferred great significance to early treatment in RA, especially getting the benefit of inhibition of radiological progress. The highlighting was consistent with the “inverted pyramid” theory (step-down concept)[10] and the “opportunity window”[11] proposed by Wilske and Healey. The earlier the RA stage, the less the lesion cells and the better the response to timely treatment. Seizing the “opportunity window” and taking effective treatment measures could achieve twice the results with half the effort.
GCs has been used in the treatment of RA for several decades. They inhibit disease activity and relieve RA.[34,35] However, long-term use of GCs might cause side effects,[36] which limit their clinical application for RA treatment.[37] It was shown that the earlier and stronger the disease activity, the better the prognosis in RA.[38] The therapeutic effect of DMARDs in clinical application is unsatisfactory and unstable, with slow onset. Indeed, it was pointed out that a large proportion of RA patients still fail to achieve clinical remission or low disease activity after treatment with DMARDs.[39] Therefore, low-dose GCs combined with DMARDs for the treatment of ERA has attracted widespread attention in recent years, and has been included in current guidelines for RA treatment by many professional organizations. For example, in 2013, the EULAR further suggested that usage of GCs should be 1 of the most critical strategies in the early stage of RA treatment.[40,41] Multiple meta-analyses have shown that clinical remission rate, bone erosion score, and joint stenosis score are all significantly improved in ERA patients treated with low-dose GCs compared with the conventional treatment group, with no significant increase in adverse reactions.[13,14,42] In a recent cohort study, 7-year data of 602 ERA patients revealed that low-dose GCs is extremely safe for the early treatment of ERA patients.[43]
Another exciting and essential question is whether different treatment regimens (ie, GCs combined with multiple DMARDs versus GCs combined with MTX alone) have different effects. To our knowledge, this is the first time to add HCQ to the treatment regimen of low-dose GCs plus multiple DMARDs. Compared with our previous report,[20] the findings of the current study showed that this new treatment regimen was better than GCs combined with MTX in a 3-month observation. These findings highlight the role of HCQ in improving the efficacy of GCs in relieving symptoms and reducing side effects. However, Verschueren et al compared different regimens of GCs (especially moderate-dose GCs) combined with DMARDs for ERA treatment for efficacy and safety, but could not draw a definite conclusion that GCs combined with multiple DMARDs (MTX+ sulfasalazine or MTX+LEF) is superior to GCs combined with MTX.[44] Further studies are required to compare GCs combined with different DMARDs for effectiveness.
There are several limitations. First, considering the single-center design, even though we had adopted the randomization and blinding procedure, it is hard to guarantee the “real” blinding. Second, the MRI data were based on only single joint (ie, the most swollen joint), which limited the generalization of the results. Third, we cannot assess the independent role of HCQ in the treatment because we did not compare the treatment effect of multiple DMARDs (HCQ + MTX) with MTX monotherapy directly.
In conclusion, we propose a new treatment regimen (ie, low-dose GCs combined with MTX, and HCQ), yielding excellent results in the patients with ERA. Low-dose GCs combined with MTX and HCQ significantly improves symptoms, signs, and inflammation in ERA, with superiority over placebo + MTX + HCQ, without enhancing adverse reactions.
Author contributions
Conceptualization: Li Hua.
Formal analysis: Mingliang Ying.
Funding acquisition: Li Hua.
Investigation: Mingliang Ying, Honghua Wu, Jia Fan.
Methodology: Li Hua, Honghua Wu.
Project administration: Li Hua, Hongwei Du.
Writing – original draft: Li Hua, Hongwei Du.
Writing – review & editing: Xiaowei Shi.
Footnotes
Abbreviations: ACR = American College of Rheumatology, CRP = C-reaction protein, DAS = disease activity score, ERA = early rheumatoid arthritis, ESR = erythrocyte sedimentation rate, GCs = Glucocorticoids, HAQ-DI = health assessment questionnaire-disability index, HCQ = hydroxychloroquine, MTX = methotrexate, RA = rheumatoid arthritis, VAS = visual analog scale.
How to cite this article: Hua L, Du H, Ying M, Wu H, Fan J, Shi X. Efficacy and safety of low-dose glucocorticoids combined with methotrexate and hydroxychloroquine in the treatment of early rheumatoid arthritis: a single-center, randomized, double-blind clinical trial. Medicine. 2020;99:27(e20824).
This work was supported by the 2 projects from Jinhua Science and Technology Bureau (grant agreement number is 2014-3-054/2019-4-025).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Li R, Sun J, Ren LM, et al. Epidemiology of eight common rheumatic diseases in China: a large-scale cross-sectional survey in Beijing. Rheumatology 2012;51:721–9. [DOI] [PubMed] [Google Scholar]
- [2].Devaraj NK. The difficult rheumatology diagnosis. Ethiop J Health Sci 2018;28:101–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Devaraj NK. The Atypical presentation of rheumatoid arthritis in an elderly woman: a case report. Ethiop J Health Sci 2019;29:957–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].van der Heijde DM, van Leeuwen MA, van Riel PL, et al. Radiographic progression on radiographs of hands and feet during the first 3 years of rheumatoid arthritis measured according to Sharp's method (van der Heijde modification). J Rheumatol 1995;22:1792–6. [PubMed] [Google Scholar]
- [5].Lindqvist E, Jonsson K, Saxne T, et al. Course of radiographic damage over 10 years in a cohort with early rheumatoid arthritis. Ann Rheum Dis 2003;62:611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Landewe RB, Boers M, Verhoeven AC, et al. COBRA combination therapy in patients with early rheumatoid arthritis: long-term structural benefits of a brief intervention. Arthritis Rheum 2002;46:347–56. [DOI] [PubMed] [Google Scholar]
- [7].Mottonen T, Hannonen P, Korpela M, et al. Delay to institution of therapy and induction of remission using single-drug or combination-disease-modifying antirheumatic drug therapy in early rheumatoid arthritis. Arthritis Rheum 2002;46:894–8. [DOI] [PubMed] [Google Scholar]
- [8].Tsakonas E, Fitzgerald AA, Fitzcharles MA, et al. Consequences of delayed therapy with second-line agents in rheumatoid arthritis: a 3 year followup on the hydroxychloroquine in early rheumatoid arthritis (HERA) study. J Rheumatol 2000;27:623–9. [PubMed] [Google Scholar]
- [9].O’Dell JR. Treating rheumatoid arthritis early: a window of opportunity? Arthritis Rheum 2002;46:283–5. [DOI] [PubMed] [Google Scholar]
- [10].Wilske KR, Healey LA. Remodeling the pyramid--a concept whose time has come. J Rheumatol 1989;16:565–7. [PubMed] [Google Scholar]
- [11].Boers M. Understanding the window of opportunity concept in early rheumatoid arthritis. Arthritis Rheum 2003;48:1771–4. [DOI] [PubMed] [Google Scholar]
- [12].Gorter SL, Bijlsma JW, Cutolo M, et al. Current evidence for the management of rheumatoid arthritis with glucocorticoids: a systematic literature review informing the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 2010;69:1010–4. [DOI] [PubMed] [Google Scholar]
- [13].Kavanaugh A, Wells AF. Benefits and risks of low-dose glucocorticoid treatment in the patient with rheumatoid arthritis. Rheumatology 2014;53:1742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kirwan JR, Bijlsma JWJ, Boers M, et al. Effects of glucocorticoids on radiological progression in rheumatoid arthritis. Cochrane Database Syst Rev 2007;CD006356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kirwan JR. Combination therapy including glucocorticoids: the new gold standard for early treatment in rheumatoid arthritis? Ann Intern Med 2012;156:390–1. [DOI] [PubMed] [Google Scholar]
- [16].Bakker MF, Jacobs JW, Welsing PM, et al. Low-dose prednisone inclusion in a methotrexate-based, tight control strategy for early rheumatoid arthritis: a randomized trial. Ann Intern Med 2012;156:329–39. [DOI] [PubMed] [Google Scholar]
- [17].Svensson B, Boonen A, Albertsson K, et al. Low-dose prednisolone in addition to the initial disease-modifying antirheumatic drug in patients with early active rheumatoid arthritis reduces joint destruction and increases the remission rate: a two-year randomized trial. Arthritis Rheum 2005;52:3360–70. [DOI] [PubMed] [Google Scholar]
- [18].Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. [DOI] [PubMed] [Google Scholar]
- [19].Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016;68:1–26. [DOI] [PubMed] [Google Scholar]
- [20].Hua L, Wu H, Du H. Clinical observation on the curative effect of low-dose corticosteroids combined with methotrexate in the treatment of early rheumatoid arthritis. Zhejiang J Integr Tradit Chin West Med 2014;24:383–6. [Google Scholar]
- [21].Smolen JS, Landewe R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis 2010;69:964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Morris SJ, Wasko MC, Antohe JL, et al. Hydroxychloroquine use associated with improvement in lipid profiles in rheumatoid arthritis patients. Arthritis Care Res 2011;63:530–4. [DOI] [PubMed] [Google Scholar]
- [23].Penn SK, Kao AH, Schott LL, et al. Hydroxychloroquine and glycemia in women with rheumatoid arthritis and systemic lupus erythematosus. J Rheumatol 2010;37:1136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lakshminarayanan S, Walsh S, Mohanraj M, et al. Factors associated with low bone mineral density in female patients with systemic lupus erythematosus. J Rheumatol 2001;28:102–8. [PubMed] [Google Scholar]
- [25].Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- [26].Felson DT, Anderson JJ, Boers M. The American College of Rheumatology preliminary core set of disease measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum 1993;36:729–40. [DOI] [PubMed] [Google Scholar]
- [27].Siegert CE, Vleming LJ, Vandenbroucke JP, et al. Measurement of disability in Dutch rheumatoid arthritis patients. Clin Rheumatol 1984;3:305–9. [DOI] [PubMed] [Google Scholar]
- [28].Liu X, Li X, Hong N. Clinical value of modified MRI scoring system in evaluating the activity of rheumatoid arthritis. Chinese Journal of Magnetic Resonance Imaging 2010;1:110–4. [Google Scholar]
- [29].Østergaard M, Edmonds J, McQueen F, et al. An introduction to the EULAR–OMERACT rheumatoid arthritis MRI reference image atlas. Ann Rheum Dis 2005;64: Suppl 1: i3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Conaghan P, Bird P, Ejbjerg B, et al. The EULAR-OMERACT rheumatoid arthritis MRI reference image atlas: the metacarpophalangeal joints. Ann Rheum Dis 2005;64: Suppl 1: i11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ejbjerg B, McQueen F, Lassere M, et al. The EULAR-OMERACT rheumatoid arthritis MRI reference image atlas: the wrist joint. Ann Rheum Dis 2005;64: Suppl 1: i23–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wassenberg S, Rau R, Steinfeld P, et al. Very low-dose prednisolone in early rheumatoid arthritis retards radiographic progression over two years: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum 2005;52:3371–80. [DOI] [PubMed] [Google Scholar]
- [33].Montecucco C, Todoerti M, Sakellariou G, et al. Low-dose oral prednisone improves clinical and ultrasonographic remission rates in early rheumatoid arthritis: results of a 12-month open-label randomised study. Arthritis Res Ther 2012;14:R112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kirwan JR. The effect of glucocorticoids on joint destruction in rheumatoid arthritis. The arthritis and rheumatism council low-dose glucocorticoid study group. N Engl J Med 1995;333:142–6. [DOI] [PubMed] [Google Scholar]
- [35].van Everdingen AA, Jacobs JW, Siewertsz Van Reesema DR, et al. Low-dose prednisone therapy for patients with early active rheumatoid arthritis: clinical efficacy, disease-modifying properties, and side effects: a randomized, double-blind, placebo-controlled clinical trial. Ann Intern Med 2002;136:1–2. [DOI] [PubMed] [Google Scholar]
- [36].Hoes JN, Jacobs JW, Verstappen SM, et al. Adverse events of low- to medium-dose oral glucocorticoids in inflammatory diseases: a meta-analysis. Ann Rheum Dis 2009;68:1833–8. [DOI] [PubMed] [Google Scholar]
- [37].van der Goes MC, Jacobs JW, Boers M, et al. Patient and rheumatologist perspectives on glucocorticoids: an exercise to improve the implementation of the European League Against Rheumatism (EULAR) recommendations on the management of systemic glucocorticoid therapy in rheumatic diseases. Ann Rheum Dis 2010;69:1015–21. [DOI] [PubMed] [Google Scholar]
- [38].Emery P, Breedveld FC, Dougados M, et al. Early referral recommendation for newly diagnosed rheumatoid arthritis: evidence based development of a clinical guide. Ann Rheum Dis 2002;61:290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cash JM, Klippel JH. Second-line drug therapy for rheumatoid arthritis. N Engl J Med 1994;330:1368–75. [DOI] [PubMed] [Google Scholar]
- [40].Gaujoux-Viala C, Nam J, Ramiro S, et al. Efficacy of conventional synthetic disease-modifying antirheumatic drugs, glucocorticoids and tofacitinib: a systematic literature review informing the 2013 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis 2014;73:510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Smolen JS, Landewe R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014;73:492–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Da Silva JA, Jacobs JW, Kirwan JR, et al. Safety of low dose glucocorticoid treatment in rheumatoid arthritis: published evidence and prospective trial data. Ann Rheum Dis 2006;65:285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Roubille C, Rincheval N, Dougados M, et al. Seven-year tolerability profile of glucocorticoids use in early rheumatoid arthritis: data from the ESPOIR cohort. Ann Rheum Dis 2017;76:1797–802. [DOI] [PubMed] [Google Scholar]
- [44].Verschueren P, De Cock D, Corluy L, et al. Methotrexate in combination with other DMARDs is not superior to methotrexate alone for remission induction with moderate-to-high-dose glucocorticoid bridging in early rheumatoid arthritis after 16 weeks of treatment: the CareRA trial. Ann Rheum Dis 2015;74:27–34. [DOI] [PubMed] [Google Scholar]


