Abstract
Previously, sex-dependent alterations in energy homeostasis were reported in adult mice fed a standard chow attributed to exposure to a mixture of organophosphate flame retardants (OPFRs) via estrogen receptors (ERα). In this study, adult male and female mice (C57BL/6J; Taconic) were treated with the same mixture of OPFRs (1 mg/kg each of tricresyl phosphate (TCP), triphenyl phosphate (TPP), and tris(1–3-dichloro-2propyl)phosphate (TDCPP) for 7 weeks on a low-fat diet (LFD, 10% kcal fat) or a high fat (HFD, 45% kcal fat) in a diet-induced obesity model. Consistent with our previous observations, OPFRs altered weight gain in males, differentially with diet, while females remained unaffected. OPFR treatment also revealed sex-dependent perturbations in metabolic activity. During the night (approximately 0100–0400 hr), males exhibited elevated activity and oxygen consumption, while in females these parameters were decreased, irrespective of diet. OPFR disrupted feeding behavior and abolished diurnal water intake patterns in females, while increasing nighttime fluid consumption in males. Despite no marked effect of OPFRs on glucose or insulin tolerance, OPFR treatment altered circulating insulin and leptin in females and ghrelin in males. Data indicate that adult OPFR exposure might influence, and perhaps exacerbate, the effects of diet-induced obesity in adult mice by altering activity, ingestive behavior, and metabolism.
Keywords: flame retardants, metabolism, ingestive behavior, locomotor activity, diet-induced obesity
INTRODUCTION
Metabolic syndrome, a constellation of conditions including obesity, hypertension, dyslipidemia, and pre-diabetes, has emerged as a national health crisis affecting over 90 million adults and costing over $100 billion each year (Boudreau et al. 2009). A major factor underlying the alarming rise in metabolic syndrome is the consumption of western diets high in fat and sugar (Drake et al. 2018; Rodriguez-Monforte et al. 2017; Moreno-Fernandez et al. 2018). However, it is also clear that diet is not the only factor. Other factors include environmental exposure to endocrine-disrupting compounds (EDCs) that perturb nutrient and hormone metabolism through central and peripheral actions (Decherf and Demeneix 2013). Indeed, investigators demonstrated that exposure to EDCs or metabolic disruptors increase sensitivity to the western diet (Brulport et al. 2017; Strakovsky et al. 2015; Grun and Blumberg 2009; Mackay et al. 2013). One group of ubiquitous EDCs are flame retardants used in the production of electronics, furniture, toys, and foodstuffs (Yang et al. 2019; Peng et al. 2020; Young et al. 2018; Li et al. 2019). The flame retardant market used to be dominated by polybrominated diphenyl ethers (PBDE), before being phased out of American and European production due to neurological and metabolic health concerns in 2004 (Zota et al. 2011; Herbstman et al. 2010; Gilbert et al. 2012; Shaw et al. 2010; Dorman et al 2018).
Recently, an alternative class of retardants, organophosphate flame retardants (OPFRs), has emerged as the leading replacement for PBDEs. Despite already existing toxicity data on these flame retardants at high concentrations, OPFRs quickly became widely used among home furnishing manufacturers resulting in widespread human exposure. OPFRs are embedded in household products and released into household and workplace dust through which humans are exposed primarily via inhalation and ingestion, resulting in biologically relevant levels in human serum (680–709 ng/g lipid), urine (1–10 ng/ml), and breast milk samples (1–10 ng/ml) (Ma et al. 2017; 2019; Butt et al. 2014; Meeker et al. 2013; Hoffman et al. 2017). While OPFRs are not yet reported to accumulate within adipose tissue to the same degree as do PBDEs, aryl OPFRs are hydrophobic (Yang et al. 2019) and demonstrate accumulation in other biological tissues (Hou et al. 2017; Ma et al. 2013). Further, several investigators demonstrated OPFRs’ ability to interact with nuclear receptors important in the pathogenesis of metabolic syndrome (Gray et al. 2005; Pap et al. 2016; Belcher et al. 2014; Pillai et al. 2014), leading to concern over potential long-term adverse health effects.
Homeostatic regulation of feeding behaviors and energy balance is a complex system but predominantly controlled via neuroendocrine pathways originating in the hypothalamus (Waye and Trudeau 2011). Briefly, the hypothalamus consists of multiple nuclei in which discrete neuronal subgroups communicate with each other to integrate peripheral indicators of energy state (Williams et al. 2001). With emotional and reward inputs from the limbic forebrain, the hypothalamus synthesizes feeding drive and communicates with the hindbrain for execution (Grill and Hayes 2012; Berthoud 2002). Within the hypothalamus lies the arcuate nucleus (ARC) which sits adjacent to a leaky portion of the blood-brain-barrier, and thus its neurons are in a unique position to directly sense energy state through peripheral signals such as glucose, insulin, leptin, and ghrelin (Schwartz et al. 2000; Saper et al. 2002). ARC neurons express receptors for these molecules, and their combined inputs to the paraventricular nucleus (PVN) and lateral hypothalamus (LH) help dictate food intake (Arora and Anubhuti 2006; Nahon 2006). Because hypothalamic control of energy homeostasis is highly regulated through hormone signaling pathways including estrogen receptors (ER) α and peroxisome proliferator-activated receptor (PPAR) γ (Sarruf et al. 2009; Garretson et al. 2015; Roepke et al. 2011; Mauvais-Jarvis et al. 2013), any EDC, such as OPFRs, that interact with these receptors may disrupt the complex balance of these pathways, sensitizing the system to metabolic disorders such as obesity and diabetes.
Despite the increasing focus of epidemiological and toxicological data surrounding OPFR exposure, physiological influences of OPFRs on energy homeostasis in adult mammals is underexplored. While most endocrine disruption studies are focused on developmental exposure, it is important to understand the mechanistic role of EDC exposures throughout the lifespan, including adulthood. Little is yet known on how adult exposure to OPFR may interact with neuroendocrine control over energy homeostasis; however, in our previously published exploratory study, adult, sub-chronic OPFR exposure decreased body weight gain and energy intake in intact male mice (Krumm et al. 2018). Further, expression of genes central to hypothalamic control of energy homeostasis were markedly altered by OPFR exposure, differentially in intact males and ovariectomized female mice (Krumm et al. 2018). These data indicate a crucial need for further investigation before OPFR may be considered any safer than their PBDE predecessors.
Since ERα and PPARγ receptors are highly expressed in the ARC and hypothalamus as a whole, and because OPFRs are known to interact with these receptors, OPFRs may be disrupting energy homeostasis as a consequence of these interactions. Previously, Krumm et al (2018) used triphenyl phosphate (TPP), tricresyl phosphate (TCP), and tris(1–3-dichloro-2propyl)phosphate (TDCPP) in a mixture of 1 mg/kg of each OPFR. This mixture was selected because of the prevalence of these compounds in human environments and since the parent compounds or their metabolites interact with ERα and PPARγ, which may play a role in pathogenesis of these disorders. Since Krumm et al (2018) reported that exposure of adults to OPFRs elicited sex-specific changes in body weight, peripheral peptide hormone expression, and gene expression, it was of interest to determine whether this may translate to an increased sensitivity to diet-induced obesity, attributed to effects on feeding behavior, fat accumulation, metabolism, and activity patterns. Thus, the aim of this study was to investigate these parameters in intact adult male and female mice with or without a high-fat diet (HFD) challenge for 7 weeks with continuous daily oral dosing of the same 1 mg/kg OPFR mixture. This dose was selected to be consistent with previous observations (Krumm et al. 2018; Patisaul et al. 2013; Wang et al. 2019b), and because Krumm et al (2018) reported murine serum concentrations of TPP, TCP, and TDCPP similar to those detected within human serum samples (Ma et al. 2017).
MATERIALS AND METHODS
Animals
All animal experiments were approved by the Rutgers University Institutional Animal Care and Use Committee and followed guidelines based upon National Institutions of Health standards. Female and male wild-type (WT C57BL/6J; Taconic) mice bred in-house and provided food and water ad libitum under controlled temperature (23 °C) and light cycle (12/12 hr light/dark cycle). At weaning, animals were ear-tagged for identification and fed a standard low-phytoestrogen chow diet (Lab Diets 5V75) until the start of the experiment.
To examine the effects of OPFR exposure on an adult mouse model of diet-induced obesity, mice were fed either a low-fat diet (LFD, 3.85 kcal/g, 10% fat, 20% protein, 70% carbohydrate; D12450H) or high-fat diet (HFD, 4.73 kcal/g, 45% fat, 20% protein, 35% carbohydrate; D12451; Research Diets). Mice were fed LFD or HFD concurrently with OPFR treatment starting at 10 weeks of age and separated into weight-matched groups (male: OIL – 27.4 ± 0.6 g, OPFR – 27.1 ± 0.4 g; female: OIL – 20.6 ± 0.3 g, OPFR – 20.9 ± 0.2 g) and dosed for the duration of the study.
Organophosphate flame retardants (OPFR) Dosing
The OPFR mixture consisted of 100 mg each of tricresyl phosphate (TCP, CAS #1330–78-5; purity = 99%) which was purchased from AccuStandard (New Haven, CT), and triphenyl phosphate (TPP, CAS #115–86-6; purity 99%) and tris(1,3-dichloro-2-propyl)phosphate (TDCPP, CAS #13674–87-8; purity 95.6%) purchased from Sigma-Aldrich (St. Louis, MO). One hundred (100) mg of each OPFR were dissolved together in the same 1 ml of acetone (Sigma-Aldrich) for long term storage. One hundred (100) μl of acetone stock was transferred to 10 ml sesame oil (Sigma-Aldrich) to create a 1 mg/ml mixture of OPFR-oil. The mixture was stirred for 48–72 hr to completely evaporate the acetone from the mixture. For dosing, the OPFR mixture or vehicle (oil:acetone) was mixed with powdered peanut butter (approximately 50 mg) for oral dosing of mice on per body weight basis for a total exposure of 1 mg/kg of each OPFR/day of OPFR-oil or vehicle control-oil. Weekly body weights were recorded and used for dosage calculation. Starting at 10 weeks of age, all mice were dosed at 900–1100 hr daily for an approximate 7 total weeks in a subchronic paradigm.
Experimental Design
Adult male and female mice (n = 16 males, n = 14 females) were pair-housed and weight-matched per group, fed LFD or HFD, and dosed with either vehicle-oil or OPFR-oil for 4 weeks in two sequential batches of mice (8 males/batch; 6–8 females/batch) to ensure sufficient sample size for metabolic and feeding behavior investigations. Body composition (fat and lean mass) was assessed by EchoMRI™ Body Composition (Houston, TX) on the day of first dose. Body weight and food intake were measured weekly. After 4 weeks exposure, body composition was determined followed by Comprehensive Lab Animal Monitoring System (CLAMS, Columbus Instruments, Columbus, OH) to measure oxygen consumption (V.O2), carbon-dioxide production (V.CO2), respiratory exchange ratio (RER), energy expenditure, and general locomotor activity in 72 hr trial under constant 25 °C and 12:12 hr light/dark cycle. Mice were single-housed for the duration. The respiratory exchange ratio (RER) is a measurement of substrate utilization (ratio of carbohydrates vs. lipids). General metabolic rate is also determined through mouse heat expenditure. Food and water intake and activity (X, Y, and Z plane and running wheel) were also recorded. After CLAMS, mice were transferred to the Biological Data Acquisition (BioDAQ, Research Diets, New Brunswick, NJ) chambers for 1 week with 72 hr habituation and 96 hr measurement of feeding behaviors (meal size, frequency, duration). LFD or HFD chow were contained in a touch-sensitive hopper and food consumption was measured by decreases in hopper food weight. Whenever the mouse touched the hopper for food, the system denoted that as a “bout.” When the interval between bouts was greater than 300 sec, the food ingested was determined to be a “meal.” A meal could consist of any number of bouts, until the inter-bout interval exceeded 300 sec. Subsequently, all mice were tested for glucose and insulin tolerance. For the glucose tolerance test (GTT), mice were fasted for 5 hr and then intraperitoneally (IP) injected with a bolus of 2 g/kg glucose. Blood-glucose was measured from tail bleeds using an AlphaTrak glucometer (Zoetis, Parsippany, NJ). Glucose measurements were taken at 0, 15, 30, 60, 90, and 120 min post-injection. Four days later, insulin tolerance tests (ITT) were performed using an IP injection of 0.75 U/kg insulin after a 4 hr fast. After insulin injection, glucose was measured in tail-blood at 0, 15, 30, 60, 90, and 120 min. With 1-week recovery from ITT, mice were dosed at 0900 hr, fasted at 1000 hr, and euthanized at 1100 hr by decapitation after sedation with ketamine (100 mg/ml). Female mice were euthanized during diestrus to control for cycling steroid hormone levels. Trunk blood was collected in K+-EDTA coated tubes with the addition of proteinase inhibitor 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (1 mg/ml, Sigma-Aldrich) to protect against peptide degradation. Samples were maintained on ice until centrifugation at 1,000g for 15 min at 4 °C. Plasma supernatant was collected and stored at −80 °C until analysis for insulin, leptin, and ghrelin levels, using a multiplex assay (MMHMAG-44 K, EMD Millipore, Billerica, MA).
Data Analysis
All data are presented as mean ± SEM. Data were analyzed using GraphPad Prisim software (GraphPad Software, LA Jolla, CA) by a two-way ANOVA (OPFR and Diet) with a post-hoc Newman-Keuls multiple comparisons test, or with Statistica 7.1 software (StatSoft, Tulsa, OK, USA) by multi-factorial ANOVA or with repeated-measures, three-way ANOVA (Diet, OPFR, Time), followed with post-hoc Newman-Keuls multiple comparisons test. Effects were considered significant at p ≤ 0.05.
RESULTS
Physiological Parameters
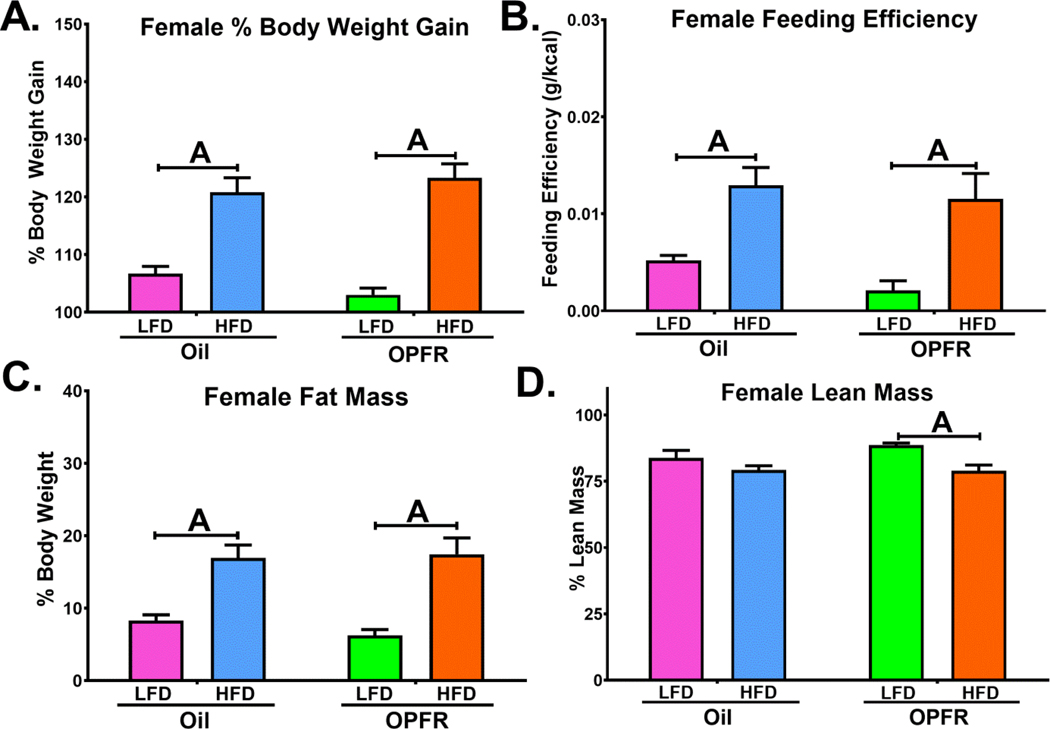
Body weight gain and crude food intake of mice fed control-oil and OPFR-oil mixture (1 mg/kg each of TCP, TPP, and TDCPP) were taken over the course of 4 weeks and followed by body composition assessment by EchoMRI™ (Figures 1 and 2). Feeding efficiency was calculated using the ratio of body weight gain to crude food intake and depicted as grams (g) gained to kcal consumed. As expected, bodyweight gain and feeding efficiency were increased in all HFD fed compared to LFD animals. However, OPFR-treated male mice fed HFD exhibited significantly greater weight gain (Figure 1A) after 4 weeks over their oil-treated counterparts. HFD-fed male mice also displayed elevated fat mass and decreased lean mass following OPFR exposure (Figure 1C). While no marked main effect of OPFRs or interactions were observed in female mice, HFD-fed females gained more weight and more fat mass than LFD-fed counterparts. OPFR-treated mice administered HFD resulted in reduced lean mass (Figure 2D), whereas in oil-treated mice no marked difference in diet was noted, indicating a potential influence of OPFR exposure on HFD effects.
Figure 1.
Physiological parameters in WT males orally dosed with an OPFR mixture (1 mg/kg) for ~7 weeks. (A) Percent Body Weight Gain over 4 weeks; (B) Feeding Efficiency; (C) Percent Fat Mass; (D) Percent Lean Mass. Data were analyzed by a two-way ANOVA with post-hoc Newman-Keuls multiple comparisons test. Uppercase letters denote diet effects within treatment group. Lowercase letters denote treatment effects within diet (A/a=P<.05). Data (A, C, D: n=16; B: n=8 (per cage)) are presented as mean ± SEM.
Figure 2.
Physiological parameters in WT females orally dosed with an OPFR mixture (1 mg/kg) for ~7 weeks. (A) % Body Weight Gain over 4 weeks; (B) Feeding Efficiency; (C) Percent Fat Mass; (D) Percent Lean Mass. Data were analyzed by a two-way ANOVA with post-hoc Newman-Keuls multiple comparisons test. Uppercase letters denote diet effects within treatment group. Lowercase letters denote treatment effects within diet (A/a=P<.05). Data (A, C, D: n=14; B: n=7 (per cage)) are presented as mean ± SEM.
Metabolic Parameters
Metabolic parameters such as V.O2, V.CO2, RER, and heat were measured in a 72 hr run in the CLAMS system (Figures 3 and 4). While diet-induced patterns were distinct in both males and females, the OPFR mixture effect appeared only in LFD groups and during peak feeding time around 200–300 hr for both sexes. In LFD-fed males, OPFR significantly augmented V.O2 and V.CO2 during 200–300 hr (Figure 3A), while in contrast LFD-fed females responded with diminished V.O2 during 200–300 hr (Figure 3A) and decreased V.CO2 during 200–400 hr (Figure 3B). It is noteworthy that females fed LFD also exhibited significantly elevated RER from 300–500 hr (Figure. 4C), as well as reduced RER from 1700–1800 hr, right before lights off (Figure 4C). There were no marked main effects in RER for males. Finally, OPFR decreased energy expenditure in females fed both LFD (Figure 4D, 200–400 hr), and females fed HFD (Figure 4D 100–200 hr). Interestingly, a significant rise was found in male mice fed LFD in energy expenditure during 200–300 hr (Figure 3D) indicating contrasting differences in activity dependent upon sex. Diet did not exert a marked effect on energy expenditure, save for an elevated energy expenditure in LFD-fed males following OPFR treatment at a single time point: 200–300 hr (Figure 3D).
Figure 3.
Analysis of metabolism in WT male mice orally dosed with an OPFR mixture (1 mg/kg) for ~5 weeks. (A) V.O2; (B) V.CO2; (C) Respiratory Exchange Ratio; (D) Energy Expenditure. Dark line above X-axis represents dark/light hours. Data were analyzed with a repeated-measures three-way ANOVA with post-hoc Newman-Keuls multiple comparisons test. Lowercase letters denote treatment effect within diet group. Uppercase letters denote diet effects within treatment group, or when barred, denote comparisons between day and night (A/a=P<.05). Data (n=16) are presented as mean ± SEM.
Figure 4.
Analysis of metabolism in WT female mice orally dosed with an OPFR mixture (1 mg/kg) for ~5 weeks. (A) V.O2; (B) V.CO2; (C) Respiratory Exchange Ratio; (D) Energy Expenditure. Dark line above X-axis represents dark/light hours. Data were analyzed with a repeated measures three-way ANOVA with post-hoc Newman-Keuls multiple comparisons test. Lowercase letters denote treatment effect within diet group; uppercase letters denote diet effects within treatment group, or when above a capped line, denote comparisons between day and night (A/a=P<.05). Data (n=14) are presented as mean ± SEM.
Activity
In the same CLAMS system, activity levels were also measured over 72 hr (Figure 5). As mice are a nocturnal species, movement and use of the exercise wheel were increased during nighttime in all groups. However, in female mice, OPFR significantly decreased nighttime activity and wheel use in both LFD (Figure 5E and 5F) and HFD groups (Figure 5E and 5F). There was a significant main effect of OPFR on both activity and wheel use (Figure 5E and 5F), as well as an interaction between OPFR and time (Figure 5E and 5F). Male mice did not exhibit this OPFR-induced pattern but did respond to HFD with a reduction in nighttime activity (Figure 5C).
Figure 5.
Analysis of daytime vs. nighttime activity in WT male and female mice orally dosed with an OPFR mixture (1 mg/kg) for ~5 weeks. (A & D) Water Intake; (B & E) Locomotor Activity; and (C & F) Wheel Running. Data were analyzed by a three-way ANOVA with post-hoc Newman-Keuls multiple comparisons test. Lowercase letters denote treatment effect within diet group. Uppercase letters denote diet effects within treatment group, or when above a capped line, denote comparisons between day and night (A/a=P<.05). Data (males: n=16; female: n=14) are presented as mean ± SEM.
Perhaps more interestingly, water intake was augmented in males during the nighttime in both LFD- and HFD-fed groups (Figure 5A). OPFR induced an overall significant effect as well as OPFR interactions with both time and diet (Figure 5A). Further, OPFR exposure induced marked differences between diets and time (Figure 5A), while differences were not observed in oil-treated male mice. Females exhibited the opposite effect, wherein oil-treated groups exhibited typical elevated water intake during the night (Figure 5D), but within OPFR treatment, the differences between daytime and nighttime drinking were abrogated, indicating potential dysregulation of diurnal fluid intake behaviors.
Feeding Behaviors
The BioDAQ apparatus was utilized for reliable analysis of total and hourly food intake, as well as meal size, duration, and frequency over a 96 hr trial period (Figure 6). Overall, OPFR-treated female mice on a HFD ate less food (Figure 6A) and consumed fewer meals per day (Figure 6B) than oil-treated counterparts. When hourly feeding patterns were analyzed, the difference between HFD-fed groups was also evident with OPFR-treated females who consumed less HFD than oil-treated during two periods during the dark cycle. Oil-treated HFD mice displayed a spike in food intake at 0300 hr, whereas OPFR-treated HFD animals food intake was significantly less during this time (Figure 6E). In addition, OPFR exposure decreased consumption of HFD at 2000 hr, respective of either treatment or diet (Figure 6E). These time-specific perturbations suggest OPFR may be dysregulating diurnal feeding patterns in female mice. Surprisingly, no significant effects of OPFRs were observed on meal patterns in males; however, in the analysis of hourly consumption, OPFR treatment significantly altered HFD consumption at two time points 0400 hr and 2100 hr similar to females (data not shown).
Figure 6.
Feeding behaviors in WT females orally dosed with an OPFR mixture (1 mg/kg) for ~5 weeks. (A) Total Food Ingested; (B) Meals Frequency; (C) Meal Duration; (D) Meal Size; and (E) Average Hourly Food Intake. Data were analyzed by a two-way ANOVA (A-D) and a repeated-measures, three-way ANOVA (E) with post-hoc Newman-Keuls multiple comparisons test. Lowercase letters denote treatment effects within diets. Uppercase letters above a capped line denote diet effects within treatment group (A/a=P<.05). Data (n=14 for LFD groups and n=8–10 for HFD groups) are presented as mean ± SEM.
Glucose and Insulin Tolerance
Glucose and insulin tolerance tests (Figures 7 and 8) were performed to determine whether endocrine disruption by OPFRs might compromise the body’s ability to tolerate sudden changes in glucose homeostasis. Overall, there were minimal to no alterations in glucose or insulin tolerance attributed to OPFR exposure. As expected, HFD elevated circulating glucose levels and increased the latency time to return to baseline after glucose or insulin injection, but did not display significant differences between treatments.
Figure 7.
Glucose tolerance tests in WT male and female mice orally dosed with an OPFR mixture (1 mg/kg) for ~6 weeks. (A & D) Fasting Glucose, (B & E) GTT, and (C & F) Area under the curve (AUC) of GTT. Data were analyzed by a two-way ANOVA (A, C, D, F) or a repeated-measures, three-way ANOVA (B, E) with post-hoc Newman-Keuls multiple comparisons test. Uppercase letters above a capped line denote diet effects within treatment group and lowercase letters denote treatment effect within diet (A/a=P<.05). Data was collected only from the first batch (n=8 for all groups) and are presented as mean ± SEM.
Figure 8.
Insulin tolerance tests in WT male and female mice orally dosed with an OPFR mixture (1 mg/kg) for ~6 weeks. (A & C) ITT and (B & D) AUC of ITT. Data were analyzed by a two-way ANOVA (B, D) or a repeated-measures, three-way ANOVA (A, C) with post-hoc Newman-Keuls multiple comparisons test. Capped letters denote diet effects within treatment group and lowercase letters denote treatment effect within diet (A/a=P<.05). Data was collected only from the first batch (n=8 for all groups) and are presented as mean ± SEM.
Peptide Hormones
Terminal plasma hormone levels of insulin, leptin, and ghrelin were measured. Alterations in these hormones indicate perturbed energy homeostatic control in peripheral endocrine organs. Insulin levels were elevated in HFD-fed males as compared to LFD-fed males in the OPFR group (Figure 9A), where there was no significant difference in controls. Interestingly, in female mice OPFR exposure increased insulin in LFD-fed females compared to oil-treated counterparts, producing a HFD-induced fall in insulin (Figure 9D). In males, HFD elevated plasma leptin concentrations in both treatment groups (Figure 9B). In female mice, OPFR induced a rise in leptin in HFD-fed group compared to oil-treated (Figure 9E). OPFR treatment suppressed ghrelin in males (Figure 9C) and eliminated the HFD-induced reduction in ghrelin. In females, HFD increased ghrelin levels in both treatments (Figure 9F). A summary of these results is presented in Table 1.
Figure 9.
Terminal plasma peptide hormone concentrations in WT male and female mice orally dosed with an OPFR mixture (1 mg/kg) for ~7 weeks. (A & D) Insulin; (B & E) Leptin; and (C & F) Ghrelin. Data were analyzed by a two-way ANOVA with post-hoc Newman-Keuls multiple comparisons test. Uppercase letters denote diet effects within treatment group and lowercase letters denote treatment effects within diet (A/a=P<.05). Data was collected only from the first batch (n=8 for all groups) and are presented as mean ± SEM.
Table 1.
Summary of data comparing oil- and OPFR-treated groups.
| Endpoint | Males | Females | ||
|---|---|---|---|---|
| LFD | HFD | LFD | HFD | |
| Bodyweight Gain | n.s. | ↑↑ | n.s. | n.s. |
| Feeding Efficiency | n.s. | n.s. | n.s. | n.s. |
| Fat Mass | n.s. | ↑↑ | n.s. | n.s. |
| Lean Mass | n.s. | ↓↓ | n.s. | n.s. |
| V.O2 | ↑ | n.s. | ↓ | n.s. |
| V.CO2 | ↑ | n.s. | ↓ | n.s. |
| RER | n.s. | n.s. | ↓↑ | n.s. |
| Energy Expenditure | ↑ | n.s. | ↓ | ↓ |
| Activity | n.s. | n.s. | ↑↑ | ↓↓ |
| Water Intake | ↑↑ | ↑↑ | ↓↓ | ↓↑ |
| 96 hr Food Intake | n.s. | n.s. | n.s. | ↓↓ |
| Hourly Food Intake | n.s. | ↓↑ | n.s. | ↓↑ |
| Meal Frequency | n.s. | n.s. | n.s. | ↓↓ |
| Meal Duration | n.s. | n.s. | n.s. | n.s. |
| Meal Size | n.s. | n.s. | n.s. | n.s. |
| Fasting Glucose | n.s. | n.s. | n.s. | n.s. |
| Glucose Tolerance | n.s. | n.s. | n.s. | n.s. |
| Insulin Tolerance | n.s. | n.s. | n.s. | n.s. |
| Insulin | n.s. | n.s. | ↑↑ | n.s. |
| Leptin | n.s. | n.s. | n.s. | ↑↑ |
| Ghrelin | ↓↓ | n.s. | n.s. | n.s. |
Up arrows denote an OPFR-induced increase and down arrows denote an OPFR-induced decrease. One arrow indicates a modest effect and two arrows indicate a strong effect. One up and one down arrow indicates a mixed effect dependent on time of day. N.S = not significant.
DISCUSSION
Our understanding of the influence of OPFR exposure and its actions as an EDC primarily focused on developmental exposure (Wang et al. 2019a; 2019b). Previous perinatal studies with FM550 identified TPP as a candidate for metabolic alterations through actions on PPARγ (Pillai et al. 2014), as well as changes in behavior and glucose homeostasis (Patisaul et al. 2013; Baldwin et al. 2017; Du et al. 2016). In the current study using a subchronic adult exposure model, weight gain in male mice was detected when exposed to OPFR and fed HFD compared to HFD-fed oil-treated males. The rise in body weight was due to enhanced fat accumulation, suggesting OPFR augmentation of fat accumulation occurs on HFD, a function mediated through PPARγ (Janani and Ranjitha Kumari 2015). Conversely, OPFR-treated males fed LFD gained less weight compared to oil-treated males, which is in agreement with our previous findings (Krumm et al. 2018). Interestingly, our current observations in intact wild-type (WT) females are not similar to our earlier results wherein adult OPFR exposure increased weight gain in ovariectomized WT females, which is known to enhance body weight gain in WT females (Krumm et al. 2018). This discrepancy may also be due to differences between a semi-purified LFD in the current investigation and a standard chow diet used in the previous experiment. Regardless, there does appear to be a disruptive capacity of OPFR on weight gain, perhaps differentially dependent upon sex, diet, or ovarian status. Aside from weight gain, a decrease in food intake and meals per day was observed in HFD-fed male mice, suggesting that OPFR treatment alters ingestive behaviors and metabolism. Further, circulating insulin levels were elevated by OPFR in female mice, as was leptin, offering a potential explanation for why female mice did not undergo a weight-gain effect noted in male mice. Conversely, male mice showed no marked change in insulin or leptin, but exhibited lowered ghrelin levels. Taken together, these alterations demonstrated an overall anorexigenic effect of OPFR, and support OPFR’s capacity as an EDC to disrupt energy homeostasis in a sex-dependent fashion.
Adult exposures to other EDC previously reported metabolic effects including and not limited to glucose homeostasis, thyroid hormone levels, and fat metabolism (Ding et al. 2014; Marmugi et al. 2014; Moghaddam et al. 2015; Brulport et al. 2017; Bertuloso et al. 2015; Sharan et al. 2014; Jansen et al 2017). Data indicate that OPFRs disrupt metabolism (Fernie et al. 2015; Wang et al. 2019a; Du et al. 2016), supporting our examination of metabolic parameters including V.O2, V.CO2, RER, and energy expenditure. In males, significant perturbations in metabolism were observed during the nighttime. However, in female mice, OPFR treatment consistently decreased V.O2, V.CO2, and energy expenditure on a LFD during the night. In addition, RER was significant elevated on LFD. The shift in RER may indicate an enhanced carbohydrate utilization by OPFR on LFD, as opposed to lipids, during the night. However, this rise occurred concomitantly with a fall in carbohydrate usage during the later afternoon/early evening, prior to nighttime when the mice are more active. More notable than subtle substrate utilization alterations, the decreased V.O2, V.CO2, and energy expenditure indicates that mice are simply using less energy overall i.e., less active, when dosed with OPFRs. This response may be due to a variety of mechanisms, but the simplest cellular targets are mitochondria. Mitochondria are responsible for respiration and energy production at the cellular level, and if OPFRs are impinging on mitochondrial function, it may result in basal perturbations of metabolic mitochondrial activity. This hypothesis is supported by a recent study in which both TPP and TDCPP were found to decrease basal mitochondrial respiration in zebrafish embryos (Lee et al. 2019). These investigators also reported a reduction in maximal mitochondrial respiration attributed to TDCPP exposure (Lee et al. 2019). Further, in the nematode C. elegans, TPP and TDCPP both disrupted mitochondrial membrane permeability in a similar manner as brominated FR predecessors, indicating mitochondrial toxicity (Behl et al. 2016). These studies establish a precedent to support a hypothesis that respiratory effects that were noted may in part be due to a mitochondrial effect of OPFR. However, regardless of the cellular mechanism, our data suggest that even subchronic exposures to OPFRs altered respiration and suppressed metabolism, which over a lifespan may influence energy homeostasis leading to greater weight gain and adiposity.
Mice are generally nocturnal animals, and thus their activity is greatest during the night. In our experiment on mouse locomotor activity and wheel running, female mice were more susceptible to OPFR exposure than males displaying a marked reduction in both X-plane movement (general locomotor activity) and wheel running during the nighttime on both LFD and HFD. These data indicate that OPFR ingestion is interfering with neurological pathways that control activity, producing reduced locomotor motivation in females, but not males. Many of these pathways express ERα, a steroid receptor that is known to increase activity upon activation (Ogawa et al. 1998; 2003; Shughrue et al. 1997; Hatcher et al. 2018), or PPARγ, a nuclear receptor that is also involved in exploratory behaviors (Domi et al. 2016; Moreno et al. 2004). Interestingly, developmental exposure to the commercial OPFR mixture, FM550, in rats induced hyperactivity in females (Baldwin et al. 2017). The conflicting data between our findings and the FM550 study may be accounted for differences in species, exposure window, and chemical content. Regardless, there is a striking effect of OPFR exposure in adults on activity, and in regards to energy homeostasis, a reduction of activity (an increase sedentary behavior) is associated with an obese phenotype and its sequelae – metabolic syndrome and type 2 diabetes in rodent models and humans (de Rezende et al. 2014; Hamilton et al. 2014).
Locomotor motivation is a complex and multifaceted behavioral characteristic, which is influenced by more than just the search for food. Through the lens of energy homeostasis, activity is associated with energy expenditure, a process tightly under hormonal control and particularly through 17β-estradiol (Rettberg et al. 2014; Lopez and Tena-Sempere 2015). Energy expenditure is controlled, in part, by actions of hypothalamic neurons in the arcuate nucleus (Nahon 2006), and activity of these neurons may be modulated by 17β-estradiol in an energy state-sensitive manner (Roepke et al. 2011; Gao et al. 2007; Xu et al. 2011). Because OPFR interact with ERs, their exposure may be impinging on estrogenic mediation of energy homeostasis, and increasing the risk of metabolic disruption. Further, activity is not only dictated by energy status, motivation and mood may also be involved, and lack of motivation to move is symptomatic and causative for a variety of mood disorders (Zhai et al. 2015; Schwartz et al. 2000). Future studies may benefit from exploring OPFR action on brain regions involved in motivation such as the ventral tegmental area, ventral striatum, prefrontal cortex, amygdala, and dorsal media habenula (Hsu et al. 2014; Kim 2013).
In addition to energy expenditure, water intake was also dysregulated. Males exhibited an increase in water intake during the night on both diets, while females displayed an ablation of typical diurnal drinking patterns. A possible explanation for female mice is that dysregulation of water intake is a direct result of OPFR actions decreasing activity and energy expenditure. If animals are moving less, then the motivation to drink also diminishes. However, there are likely to be additional complexities to this simple explanation. Although female mice are moving less, these animals maintained the nocturnal pattern of enhanced activity. OPFR exposure seems to exert a specific effect of reducing nighttime water intake or increase daytime intake in females, thus eliminating differences between night and day. Further, despite no marked activity alterations in males, OPFR treatment elevated nighttime fluid consumption that indicating an impact of OPFR on control of fluid balance, either centrally or peripherally in the kidneys. Indeed, TDCPP produced cytotoxic effects on cultured renal cells (Killilea et al. 2017), and in human population study of approximately 1,500 patients. TDCPP exposure also correlated with markers of kidney damage and chronic renal disease (Kang et al. 2019).
Centrally, there are also many areas of the brain that control fluid balance including the paraventricular hypothalamus, supraoptic nucleus, median preoptic area, organum vasculosum laminae terminalis, and subfornical organ (Curtis 2009). Many of these nuclei express ERs and are involved in the control of fluid balance in response to 17β-estradiol (Shughrue et al. 1997; Santollo and Daniels 2015a; 2015b; Santollo et al. 2013; Curtis 2009). In hormone replacement therapies, E2 produced a direct effect on water intake (Krause et al. 2003; Santollo et al. 2013), its actions mediated in part through dampening of angiotensin II (AngII) signaling (Kisley et al. 1999; Findlay et al. 1979; Danielsen and Buggy 1980; Jonklaas and Buggy 1984). Potentially, OPFRs interfere with this estrogen-sensitive balance leading to changes in fluid intake. However, like any homeostatic function, thirst is regulated through a multitude of pathways, allowing for alternate avenues of OPFR actions. Thirst is closely related to energy homeostasis, and the powerful “hunger” hormone ghrelin is also known to exert effects on fluid intake, reducing water consumption by inhibiting Ang II (Mietlicki et al. 2009; Hashimoto et al. 2010; Plyler and Daniels 2017), which as previously indicated, is also under the influence of E2. Conversely, intracerebroventricular infusionsof Ang II diminishes food and enhances energy expenditure, establishing an Ang II link between food and fluid intake mediated by ghrelin (Porter and Potratz 2004). In our current study, OPFR decreased circulating ghrelin in male mice on LFD, supporting a ghrelin-mediated hypothesis for the dipsogenic effect of OPFR on male mice. Finally, somatostatin, produced both centrally in the ventromedial nucleus of the hypothalamus, and peripherally by delta cells in the digestive system, is involved in thirst generation and may be a target for OPFR dysregulation. Central action of somatostatin increases food and water intake (Karasawa et al. 2014; Stengel et al. 2010), and was shown to be altered by exposure to bisphenol A, another well-known estrogenic EDC (Facciolo et al. 2002; 2005). Taken together, these data offer a precedented route for OPFR EDC action on fluid regulation.
The collective observations of this study demonstrate a clear sex-dependent effect of OPFR exposure within adult mice. Body composition was only altered in males, while feeding behavior and activity were largely only modified within females. In addition, circulating ghrelin was diminished in males, while leptin and insulin were elevated within female mice. These differences are likely attributed to the innate biological differences between male and female mice. Energy homeostasis is well-documented to be a sexually-dimorphic function (Shi et al. 2009; Woods et al. 2003; Wu and O’Sullivan 2011), through which estrogen plays a substantial role (Rettberg et al. 2014; Nestor et al. 2014; Lopez and Tena-Sempere 2015). The decline in circulating estrogen following menopause is associated with adverse health effects such as weight gain, hot flashes, and increased risk of diabetes and cardiovascular disease (Cignarella and Bolego 2010; Clegg et al. 2017), and estrogen replacement therapies show marked protection against these effects (Warren et al. 2015). Estrogen is typically present at roughly 10-fold higher serum concentration within women than men, therefore OPFR interference with estrogenic signaling is likely to exert differing impacts depending upon sex. Further, Krumm et al., (2018) reported decreased expression of ERα in males, but not in ovariectomized females, demonstrating a sex-specific interaction of OPFR with estrogenic signaling. Expression of the other OPFR-target PPARγ was found to be upregulated, but not dependent upon sex. Sex-differences in PPARγ signaling is not well-known, but few studies implicate differential PPARγ expression and regulation of adipose tissue and immune function (Kadowaki et al. 2007; Park and Choi 2017). Fernandez et al. (2017) found that brain-specific knockout (KO) of PPARγ KO resulted in additional weight gain in female mice, whereas male KO mice gained less than their WT littermates. Taken together, these implicate that OPFR interactions with ERα and PPARγ may contribute to the observed sex-dependent effects noted in the current study.
Part of the reason for growing concern over EDCs is what is termed the “cocktail effect,” wherein exposures to multiple different EDCs might induce additive or synergistic effects, depending upon the period of exposure, developmental or throughout the lifespan. This is of particular concern for adult exposures due to the ability of most EDCs to bioaccumulate, resulting in chronic exposure of many, sub-adverse effect EDC exposures combining to culminate in significant disruptions (Lauretta et al. 2019). Thus, consistent with the multiplicity of EDC exposures in human, our experimental protocols used a mixture of organophosphate EDCs. While a more accurate-to-life model than studying the singular effects of one specific OPFR, the fact that different OPFR are known to exert both agonistic and antagonistic effects on ERs and PPARs (Kojima et al. 2013; Liu et al. 2013) makes identifying a mechanistic resolution of our findings more difficult. Future studies need to examine the effects of OPFR exposures in tissue- or cell-specific ERα and PPARγ knockout mouse models. Regardless, what remains is that OPFR appear to be interfering with estrogenic and/or PPARγ control of energy expenditure through receptor-mediated actions.
CONCLUSIONS
In summary, our findings indicated that a mixture of three common OPFR compounds exerted disruptive actions on energy homeostasis that subsequently interact with and potentially exacerbate diet-induced obesity. There were a multitude of sex-dependent effects on metabolism, energy expenditure, weight-gain, activity, water intake and circulating hormone concentrations. Most notable were OPFR alterations to water intake and behavioral activity. Our findings demonstrate the EDC capacity of OPFR to disrupt energy homeostasis, which increases the risk of metabolic disorders such as diet-induced obesity, diabetes, and metabolic syndrome. Despite the apparent risk, OPFR still continue to be the leading FR in the United States. This reality calls for the need of continued research, if there are to be regulatory actions taken to limit human OPFR exposure. With the multitude of OPFR endpoints, it may be a while yet before one knows the full scope of OPFR-mediated toxicity. However, it would be interesting to investigate the mechanistic roots of the dysregulated fluid homeostasis noted herein, as well as additional behavioral studies to tease out whether the sedentary behavior of OPFR-treated females is a mood, or motivation effect. Further, while this study focused primarily on peripheral and behavioral outcomes, energy homeostasis is tightly regulated through central processes in the hypothalamus. Thus, it will also be important to investigate potential OPFR actions on neuronal subpopulations that regulate feeding and reward pathways in the brain.
Acknowledgments
FUNDING SOURCES
This investigation was supported by the US Department of Agriculture–National Institute of Food and Agriculture (NJ06195, TAR) and the National Institutes of Health (R21ES027119 and P30ES005022, TAR). SNW was funded by R21ES027119-S1 and GMV was funded, in part, by T32ES007148.
Footnotes
Disclosure Statement
The authors have no competing interests and have nothing to disclose.
References
- Arora S, and Anubhuti. 2006. Role of neuropeptides in appetite regulation and obesity--a review. Neuropeptides 40 :375–401. [DOI] [PubMed] [Google Scholar]
- Baldwin KR, Phillips AL, Horman B, Arambula SE, Rebuli ME, Stapleton HM, and Patisaul HB 2017. Sex specific placental accumulation and behavioral effects of developmental Firemaster 550 exposure in Wistar rats. Sci. Rep 7:7118–7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl M, Rice JR, Smith MV, Co CA, Bridge MF, Hsieh J-H, Freedman JH, and Boyd WA 2016. Comparative toxicity of organophosphate flame retardants and polybrominated diphenyl ethers to Caenorhabditis elegans. Toxicol. Sci 154: 241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher SM, Cookman CJ, Patisaul HB, and Stapleton HM 2014. In vitro assessment of human nuclear hormone receptor activity and cytotoxicity of the flame retardant mixture FM 550 and its triarylphosphate and brominated components. Toxicol. Lett 228: 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR 2002. Multiple neural systems controlling food intake and body weight. Neurosci. Biobehav. Rev 26 : 393–428. [DOI] [PubMed] [Google Scholar]
- Bertuloso BD, Podratz PL, Merlo E, de Araújo JFP, Lima LCF, de Miguel EC, de Souza LN, Gava Agata L., de Oliveira Miriane, Miranda-Alves Leandro, Carneiro Maria T. W. D, Nogueira Celia R, and Graceli Jones B. 2015. Tributyltin chloride leads to adiposity and impairs metabolic functions in the rat liver and pancreas. Toxicol Lett 235: 45–59. [DOI] [PubMed] [Google Scholar]
- Boudreau DM, Malone DC, Raebel MA, Fishman PA, Nichols GA, Feldstein AC, Boscoe AN, Ben-Joseph RH, Magid DJ, and Okamoto LJ 2009. Health care utilization and costs by metabolic syndrome risk factors. Metab Syndr Relat Disord 7 : 305–314. [DOI] [PubMed] [Google Scholar]
- Brulport A, Le Corre L, and Chagnon MC 2017. Chronic exposure of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induces an obesogenic effect in C57BL/6J mice fed a high fat diet. Toxicology 390:43–52. [DOI] [PubMed] [Google Scholar]
- Butt CM, Congleton J, Hoffman K, Fang M, and Stapleton HM 2014. Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ Sci Technol 48: 10432–10438. [DOI] [PubMed] [Google Scholar]
- Cignarella A, and Bolego C. 2010. Mechanisms of estrogen protection in diabetes and metabolic disease. Horm Mol Biol Clin Investig 4: 575–580. [DOI] [PubMed] [Google Scholar]
- Clegg D, Hevener AL, Moreau KL, Morselli E, Criollo A, Van Pelt RE, and Vieira-Potter VJ 2017. Sex hormones and cardiometabolic health: Role of estrogen and estrogen receptors. Endocrinology 158: 1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis KS 2009. Estrogen and the central control of body fluid balance. Physiol Behav 97: 180–192. [DOI] [PubMed] [Google Scholar]
- Danielsen J, and Buggy J. 1980. Depression of ad lib and angiotensin-induced sodium intake at oestrus. Brain Res Bull 5: 501–504. [DOI] [PubMed] [Google Scholar]
- de Rezende LFM, Lopes MR, Rey-López JP, Matsudo VKR, and do Carmo Luiz O. 2014. Sedentary behavior and health outcomes: An overview of systematic reviews. PloS One 9: e105620–e105620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decherf S. and Demeneix BA 2013. The obesogen hypothesis: A shift of focus from the periphery to the hypothalamus. J. Toxicol. Environ. Health B 14: 423–448. [DOI] [PubMed] [Google Scholar]
- Ding S, Fan Y, Zhao N, Yang H, Ye X, He D, Jin X, Liu J, Tian C, Li H, Xu S, and Ying C. 2014. High-fat diet aggravates glucose homeostasis disorder caused by chronic exposure to bisphenol A. J Endocrinol 221:167–179. [DOI] [PubMed] [Google Scholar]
- Domi E, Uhrig S Soverchia L, Spanagel R, Hansson AC, Barbier E, Heilig M, Ciccocioppo R, and Ubaldi M. 2016. Genetic deletion of neuronal PPARγ enhances the emotional response to acute stress and exacerbates anxiety: An effect reversed by rescue of amygdala PPARγ function. J Neurosci 36 : 12611–12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman DC, Chiu W Hales BF, Hauser R, Johnson KJ, Mantus E, Martel S, Robinson KA, Rooney AA, Rudel R, Sathyanarayana S, Schantz SL, and Waters KM. 2018. Polybrominated diphenyl ehter (PBDE) neurotoxicity: A systemic review and meta-analysis of animal evidence. J. Toxicol. Environ. Health B 21:269–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake I, Sonestedt E, Ericson U, Wallstrom P, and Orho-Melander M. 2018. A Western dietary pattern is prospectively associated with cardio-metabolic traits and incidence of the metabolic syndrome. Br. J. Nutr 119: 1168–1176. [DOI] [PubMed] [Google Scholar]
- Du Z, Zhang Y, Wang G, Peng J, Wang Z, and Gao S. 2016. TPhP exposure disturbs carbohydrate metabolism, lipid metabolism, and the DNA damage repair system in zebrafish liver. Sci Rep 6:21827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facciolo RM, Alo R, Madeo M, Canonaco M, and Dessi-Fulgheri F. 2002. Early cerebral activities of the environmental estrogen bisphenol A appear to act via the somatostatin receptor subtype sst(2). Environ Health Perspect 110 Suppl 3:397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facciolo RM, Madeo M, Alo R, Canonaco M, and Dessi-Fulgheri F. 2005. Neurobiological effects of bisphenol A may be mediated by somatostatin subtype 3 receptors in some regions of the developing rat brain. Toxicol Sci 88: 477–484. [DOI] [PubMed] [Google Scholar]
- Fernandez MO, Sharma S, Kim S, Rickert E, Hsueh K, Hwang V, Olefsky JM, and Webster NJG 2017. Obese neuronal PPARγ knockout mice are leptin sensitive but show impaired glucose tolerance and fertility. Endocrinology 158:121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie KJ, Palace V, Peters LE, Basu N, Letcher RJ, Karouna-Renier NK, Schultz SL, Lazarus RS, and Rattner BA 2015. Investigating endocrine and physiological parameters of captive American kestrels exposed by diet to selected organophosphate flame retardants. Environ Sci Technol 49: 7448–7455. [DOI] [PubMed] [Google Scholar]
- Findlay AL, Fitzsimons JT, and Kucharczyk J. 1979. Dependence of spontaneous and angiotensin-induced drinking in the rat upon the oestrous cycle and ovarian hormones. J Endocrinol 82: 215–225. [DOI] [PubMed] [Google Scholar]
- Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, Gao XB, Mobbs C, Shulman GI, Diano S, and Horvath TL 2007. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med 13: 89–94. [DOI] [PubMed] [Google Scholar]
- Garretson JT, Teubner BJ, Grove KL, Vazdarjanova A, Ryu V, and Bartness TJ 2015. Peroxisome proliferator-activated receptor gamma controls ingestive behavior, agouti-related protein, and neuropeptide Y mRNA in the arcuate hypothalamus. J Neurosci 35 : 4571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert ME, Rovet J, Chen Z, and Koibuchi N. 2012. Developmental thyroid hormone disruption: prevalence, environmental contaminants and neurodevelopmental consequences. Neurotoxicology 33: 842–852. [DOI] [PubMed] [Google Scholar]
- Gray SL, Dalla Nora E, and Vidal-Puig AJ 2005. Mouse models of PPAR-gamma deficiency: Dissecting PPAR-gamma’s role in metabolic homoeostasis. Biochem Soc Trans 33: 1053–1058. [DOI] [PubMed] [Google Scholar]
- Grill HJ, and Hayes MR 2012. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab 16: 296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun F, and Blumberg B. 2009. Endocrine disrupters as obesogens. Mol Cell Endocrinol 304: 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton MT, Hamilton DG, and Zderic TW 2014. Sedentary behavior as a mediator of type 2 diabetes. Med Sport Sci 60:11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H, Otsubo H, Fujihara H, Suzuki H, Ohbuchi T, Yokoyama T, Takei Y, and Ueta Y. 2010. Centrally administered ghrelin potently inhibits water intake induced by angiotensin II and hypovolemia in rats. J Physiol Sci 60: 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher KM, Royston SE, and Mahoney MM 2018. Modulation of circadian rhythms through estrogen receptor signaling. Eur J Neurosci. 51: 217–228 [DOI] [PubMed] [Google Scholar]
- Herbstman JB, Sjodin A, Kurzon M, Lederman SA, Jones RS, Rauh V, Needham LL, Tang D, Niedzwiecki M, Wang RY, and Perera F. 2010. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect 118: 712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Butt CM, Webster TF, Preston EV, Hammel SC, Makey C, Lorenzo AM, Cooper EM, Carignan C, Meeker JD, Hauser R, Soubry A, Murphy SK, Price TM, Hoyo C, Mendelsohn E, Congleton J, Daniels JL, and Stapleton HM 2017. Temporal trends in exposure to organophosphate flame retardants in the United States. Environ Sci Technol Lett 4: 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou R, Liu C, Gao X, Xu Y, Zha J, and Wang Z. 2017. Accumulation and distribution of organophosphate flame retardants (PFRs) and their di-alkyl phosphates (DAPs) metabolites in different freshwater fish from locations around Beijing, China. Environ Pollut 229: 548–556. [DOI] [PubMed] [Google Scholar]
- Hsu Y-WA, Wang SD, Wang S, Morton G, Zariwala HA, de la Iglesia HO, and Turner EE. 2014. Role of the dorsal medial habenula in the regulation of voluntary activity, motor function, hedonic state, and primary reinforcement. J Neurosci 34 : 11366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janani C, and Ranjitha Kumari BD 2015. PPAR gamma gene – A review. Diabetes Metab Syndrome: Clin Res Rev 9 : 46–50. [DOI] [PubMed] [Google Scholar]
- Jansen A, Lyche JL Polder A, Aaseth J, and Skaug MA. 2017. Increased blood levels of persistent organic pollutants (POP) in obese individuals after weight loss - A review. J. Toxicol. Environ. Health B 20: 22–37. [DOI] [PubMed] [Google Scholar]
- Jonklaas J, and Buggy J. 1984. Angiotensin-estrogen interaction in female brain reduces drinking and pressor responses. Am J Physiol 247: R167–R172. [DOI] [PubMed] [Google Scholar]
- Kadowaki K, Fukino K, Negishi E, and Ueno K. 2007. Sex differences in PPARgamma expressions in rat adipose tissues. Biol Pharm Bull 30: 818–820. [DOI] [PubMed] [Google Scholar]
- Kang H, Lee J, Lee JP, and Choi K. 2019. Urinary metabolites of organophosphate esters (OPEs) are associated with chronic kidney disease in the general US population, NHANES 2013–2014. Environ Int 131:105034. [DOI] [PubMed] [Google Scholar]
- Karasawa H, Yakabi S, Wang L, Stengel A, Rivier J, and Taché Y. 2014. Brain somatostatin receptor 2 mediates the dipsogenic effect of central somatostatin and cortistatin in rats: role in drinking behavior. Am J Physiol. Regul, Integr Comp Physiol 307: R793–R801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killilea DW, Chow D, Xiao SQ, Li C, and Stoller ML 2017. Flame retardant tris(1,3-dichloro-2-propyl)phosphate (TDCPP) toxicity is attenuated by N-acetylcysteine in human kidney cells. Toxicol Rep 4:260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-I. 2013. Neuroscientific model of motivational process. Front Psychol 4: 98–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisley LR, Sakai RR, Ma LY, and Fluharty SJ 1999. Ovarian steroid regulation of angiotensin II-induced water intake in the rat. Am J Physiol 276: R90–R96. [DOI] [PubMed] [Google Scholar]
- Kojima H, Takeuchi S, Itoh T, Iida M, Kobayashi S, and Yoshida T. 2013. In vitro endocrine disruption potential of organophosphate flame retardants via human nuclear receptors. Toxicology 314: 76–83. [DOI] [PubMed] [Google Scholar]
- Krause EG, Curtis KS, Davis LM, Stowe JR, and Contreras RJ 2003. Estrogen influences stimulated water intake by ovariectomized female rats. Physiol Behav 79: 267–74. [DOI] [PubMed] [Google Scholar]
- Krumm EA, J Patel V, S Tillery T, Yasrebi A, Shen J, L Guo G, M Marco S, T Buckley B, and A Roepke T. 2018. Organophosphate flame-retardants alter adult mouse homeostasis and gene expression in a sex-dependent manner potentially through interactions with ERα. Toxicol Sci 162: 212–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauretta R, Sansone A, Sansone M, Romanelli F, and Appetecchia M. 2019. Endocrine disrupting chemicals: Effects on endocrine glands. Front Endocrinol 10: 178–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Lee H, and Kim K-T. 2019. Optimization of experimental conditions and measurement of oxygen consumption rate (OCR) in zebrafish embryos exposed to organophosphate flame retardants (OPFRs). Ecotoxicol Environ Safety 182: 109377. [DOI] [PubMed] [Google Scholar]
- Li J, Zhao L, Letcher RJ, Zhang Y, Jian K, Zhang J, and Su G. 2019. A review on organophosphate Ester (OPE) flame retardants and plasticizers in foodstuffs: Levels, distribution, human dietary exposure, and future directions. Environ Int 127: 35–51. [DOI] [PubMed] [Google Scholar]
- Liu C, Wang Q, Liang K, Liu J, Zhou B, Zhang X, Liu H, Giesy JP, and Yu H. 2013. Effects of tris(1,3-dichloro-2-propyl) phosphate and triphenyl phosphate on receptor-associated mRNA expression in zebrafish embryos/larvae. Aquat Toxicol 128–129: 147–57. [DOI] [PubMed] [Google Scholar]
- Lopez M, and Tena-Sempere M. 2015. Estrogens and the control of energy homeostasis: A brain perspective. Trends Endocrinol Metab 26: 411–421. [DOI] [PubMed] [Google Scholar]
- Ma J, Zhu H, and Kannan K. 2019. Organophosphorus flame retardants and plasticizers in breast milk from the United States. Environ Sci Technol Lett 6: 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Cui K, Zeng F, Wen J, Liu H, Zhu F, Ouyang G, Luan T, and Zeng Z. 2013. Microwave-assisted extraction combined with gel permeation chromatography and silica gel cleanup followed by gas chromatography-mass spectrometry for the determination of organophosphorus flame retardants and plasticizers in biological samples. Anal Chim Acta 786 :47–53. [DOI] [PubMed] [Google Scholar]
- Ma Y, Jin J, Li P, Xu M, Sun Y, Wang Y, and Yuan H. 2017. Organophosphate ester flame retardant concentrations and distributions in serum from inhabitants of Shandong, China, and changes between 2011 and 2015. Environ Toxicol Chem 36: 414–421. [DOI] [PubMed] [Google Scholar]
- Mackay H, Patterson ZR, Khazall R, Patel S, Tsirlin D, and Abizaid A. 2013. Organizational effects of perinatal exposure to bisphenol-A and diethylstilbestrol on arcuate nucleus circuitry controlling food intake and energy expenditure in male and female CD-1 mice. Endocrinology 154: 1465–1475. [DOI] [PubMed] [Google Scholar]
- Marmugi A, Lasserre F, Beuzelin D, Ducheix S, Huc L, Polizzi A, Chetivaux M, Pineau T, Martin P, Guillou H, and Mselli-Lakhal L. 2014. Adverse effects of long-term exposure to bisphenol A during adulthood leading to hyperglycaemia and hypercholesterolemia in mice. Toxicology 325: 133–143. [DOI] [PubMed] [Google Scholar]
- Mauvais-Jarvis F, Clegg DJ, and Hevener AL 2013. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev 34: 309–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Cooper EM, Stapleton HM, and Hauser R. 2013. Urinary metabolites of organophosphate flame retardants: Temporal variability and correlations with house dust concentrations. Environ Health Perspect 121: 580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietlicki EG, Nowak EL, and Daniels D. 2009. The effect of ghrelin on water intake during dipsogenic conditions. Physiol Behav 96: 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam HS, Samarghandian S, and Farkhondeh T. 2015. Effect of bisphenol A on blood glucose, lipid profile and oxidative stress indices in adult male mice. Toxicol Mech Meth 25: 507–513. [DOI] [PubMed] [Google Scholar]
- Moreno S, Farioli-Vecchioli S, and Cerù MP 2004. Immunolocalization of peroxisome proliferator-activated receptors and retinoid x receptors in the adult rat CNS. Neuroscience 123: 131–145. [DOI] [PubMed] [Google Scholar]
- Moreno-Fernandez S, Garces-Rimon M, Vera G, Astier J, Landrier JF, and Miguel M. 2018. High fat/high glucose diet induces metabolic syndrome in an experimental rat model. Nutrients 10: 1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahon JL 2006. The melanocortins and melanin-concentrating hormone in the central regulation of feeding behavior and energy homeostasis. Comptes Rendue Biol 329: 623–638; discussion 653–655. [DOI] [PubMed] [Google Scholar]
- Nestor CC, Kelly MJ, and Ronnekleiv OK 2014. Cross-talk between reproduction and energy homeostasis: central impact of estrogens, leptin and kisspeptin signaling. Horm Mol Biol Clin Investig 17: 109–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, and Pfaff DW 1998. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology 139: 5070–5081. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Chan J, Gustafsson J-Å, Korach KS, and Pfaff DW 2003. Estrogen increases locomotor activity in mice through estrogen receptor α: Specificity for the type of activity. Endocrinology 144: 230–239. [DOI] [PubMed] [Google Scholar]
- Pap A, Cuaranta-Monroy I, Peloquin M, and Nagy L. 2016. Is the mouse a good model of human PPARgamma-related metabolic diseases? Int J Mol Sci 17: 1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, and Choi JM 2017. Sex-specific regulation of immune responses by PPARs. Exp Mol Med 49: e364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, Belcher SM, and Stapleton HM 2013. Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster(R) 550 in rats: An exploratory assessment. J Biochem Mol Toxicol 27: 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng B, Yu Z-M, Wu C-C, Liu L-Y, Zeng L, and Zeng EY. 2020. Polybrominated diphenyl ethers and organophosphate esters flame retardants in play mats from China and the exposure risks for children. Environ Int 135:105348. [DOI] [PubMed] [Google Scholar]
- Pillai HK, Fang M, Beglov D, Kozakov D, Vajda S, Stapleton HM, Webster TF, and Schlezinger JJ 2014. Ligand binding and activation of PPARgamma by Firemaster(R) 550: Effects on adipogenesis and osteogenesis in vitro. Environ Health Perspect 122: 1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plyler KS, and Daniels D. 2017. Fourth ventricle injection of ghrelin decreases angiotensin II-induced fluid intake and neuronal activation in the paraventricular nucleus of the hypothalamus. Physiol Behav 178: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JP, and Potratz KR 2004. Effect of intracerebroventricular angiotensin II on body weight and food intake in adult rats. Am J Physiol Regul Integr Comp Physiol 287: R422–R428. [DOI] [PubMed] [Google Scholar]
- Rettberg JR, Yao J, and Brinton RD 2014. Estrogen: A master regulator of bioenergetic systems in the brain and body. Front Neuroendocrinol 35: 8–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Monforte M, Sanchez E, Barrio F, Costa B, and Flores-Mateo G. 2017. Metabolic syndrome and dietary patterns: a systematic review and meta-analysis of observational studies. Eur. J. Nutr 56: 925–947. [DOI] [PubMed] [Google Scholar]
- Roepke TA, Qiu J, Smith AW, Rønnekleiv OK, and Kelly MJ 2011. Fasting and 17β-estradiol differentially modulate the M-current in neuropeptide Y neurons. J Neuroscience 31: 11825–11835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santollo J, and Daniels D. 2015a. Multiple estrogen receptor subtypes influence ingestive behavior in female rodents. Physiol Behav 152: 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santollo J, Marshall A, and Daniels D. 2013. Activation of membrane-associated estrogen receptors decreases food and water intake in ovariectomized rats. Endocrinology 154: 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santollo J, and Daniels D. 2015b. Control of fluid intake by estrogens in the female rat: role of the hypothalamus. Front Syst Neurosci 9: 25–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Chou TC, and Elmquist JK 2002. The need to feed: homeostatic and hedonic control of eating. Neuron 36:199–211. [DOI] [PubMed] [Google Scholar]
- Sarruf DA, Yu F, Nguyen HT, Williams DL, Printz RL, Niswender KD, and Schwartz MW 2009. Expression of peroxisome proliferator-activated receptor-gamma in key neuronal subsets regulating glucose metabolism and energy homeostasis. Endocrinology 150: 707–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D Jr., Seeley RJ, and Baskin DG 2000. Central nervous system control of food intake. Nature 404: 661–671. [DOI] [PubMed] [Google Scholar]
- Sharan S, Nikhil K, and Roy P. 2014. Disruption of thyroid hormone functions by low dose exposure of tributyltin: an in vitro and in vivo approach. Gen Comp Endocrinol 206: 155–165. [DOI] [PubMed] [Google Scholar]
- Shaw SD, Blum A, Weber R, Kannan K, Rich D, Lucas D, Koshland CP, Dobraca D, Hanson S, and Birnbaum LS 2010. Halogenated flame retardants: Do the fire safety benefits justify the risks? Rev Environ Health 25: 261–305. [DOI] [PubMed] [Google Scholar]
- Shi H, Seeley RJ, and Clegg DJ 2009. Sexual differences in the control of energy homeostasis. Front Neuroendocrinol 30: 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, and Merchenthaler I. 1997. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol 388: 507–525. [DOI] [PubMed] [Google Scholar]
- Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Monnikes H, and Tache Y. 2010. Selective central activation of somatostatin receptor 2 increases food intake, grooming behavior and rectal temperature in rats. J Physiol Pharmacol 61: 399–407. [PMC free article] [PubMed] [Google Scholar]
- Strakovsky RS, Wang H, Engeseth NJ, Flaws JA, Helferich WG, Pan YX, and Lezmi S. 2015. Developmental bisphenol A (BPA) exposure leads to sex-specific modification of hepatic gene expression and epigenome at birth that may exacerbate high-fat diet-induced hepatic steatosis. Toxicol Appl Pharmacol 284: 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Le Y, Lu D, Zhao M, Dou X, and Zhang Q. 2019a. Triphenyl phosphate causes a sexually dimorphic metabolism dysfunction associated with disordered adiponectin receptors in pubertal mice. J Hazard Mater 388:121732. [DOI] [PubMed] [Google Scholar]
- Wang D, Yan S, Yan J, Teng M, Meng Z, Li R, Zhou Z, and Zhu W. 2019b. Effects of triphenyl phosphate exposure during fetal development on obesity and metabolic dysfunctions in adult mice: Impaired lipid metabolism and intestinal dysbiosis. Environ Pollut 246: 630–638. [DOI] [PubMed] [Google Scholar]
- Warren MP, Shu AR, and Dominguez JE. 2015. Menopause and Hormone Replacement. South DArtmount (MA): Endotext. [Google Scholar]
- Waye A. and Trudeau VL, 2011. Neuroendocrine disruption: More than hormones are upset. J. Toxicol. Environ. Health B 14: 267–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G, Bing C, Cai XJ, Harrold JA, King PJ, and Liu XH 2001. The hypothalamus and the control of energy homeostasis: Different circuits, different purposes. Physiol Behav 74: 683–701. [DOI] [PubMed] [Google Scholar]
- Woods SC, Gotoh K, and Clegg DJ 2003. Gender differences in the control of energy homeostasis. Exp Biol Med 228: 1175–1180. [DOI] [PubMed] [Google Scholar]
- Wu BN, and O’Sullivan AJ 2011. Sex differences in energy metabolism need to be considered with lifestyle modifications in humans. J Nutr Metab 2011: 391809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, Khan SA, Elias CF, Elmquist JK, and Clegg DJ 2011. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab 14: 453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhao Y, Li M, Du M, Li X, and Li Y. 2019. A review of a class of emerging contaminants: The classification, distribution, intensity of consumption, synthesis routes, environmental effects and expectation of pollution abatement to organophosphate flame retardants (OPFRs). Int J Mol Sci 20: 2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AS, Allen JG, Kim U-J, Seller S, Webster TF, Kannan K, and Ceballos DM 2018. Phthalate and organophosphate plasticizers in nail polish: Evaluation of labels and ingredients. Environ Sci Technol 52: 12841–12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai L, Zhang Y, and Zhang D. 2015. Sedentary behaviour and the risk of depression: A meta-analysis. Br J Sports Med 49:705–709. [DOI] [PubMed] [Google Scholar]
- Zota AR, Park JS, Wang Y, Petreas M, Zoeller RT, and Woodruff TJ 2011. Polybrominated diphenyl ethers, hydroxylated polybrominated diphenyl ethers, and measures of thyroid function in second trimester pregnant women in California. Environ Sci Technol 45: 7896–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]