Abstract
Various psychotropic drugs may affect the hematological and biochemical profiles of plasma and its metabolism. Carbamazepine, the most well-known psychotropic drug, can cause substantial hyponatremia. Methylphenidate, a piperidine derivative structurally related to amphetamines, acts as a central nervous system stimulant. The current study evaluated whether methylphenidate affects hematological and biochemical parameters of patients diagnosed with attention deficit hyperactivity disorder.
Patients undergoing treatment for attention deficit hyperactivity disorder at our Adolescent Psychiatric Clinic were enrolled in the study. Blood samples for complete blood count and common biochemical analyses were collected before patients started methylphenidate and after 3 months of continuous treatment.
Participants included 64 patients comprised the study cohort. There were 48 (75%) males and 16 (25%) females, with a median age of 16 years (range 11–31). The total median potassium level decreased by 0.6 mg/dL (P < .0001), while glucose rose by 15 mg/dL (P < .0001), sodium decreased in 0.7meq/L, (P = .006). The white blood count rose by 1350 cells/μL (P < .033) due to neutrophilia, lymphocytosis and eosinophilia. Hemoglobin rose slightly by 0.1 (P = .041). Changes in calcium, phosphorus, protein, albumin, and liver enzyme levels were not significant.
The results indicate that methylphenidate may cause hypokalemia and elevated glucose, leukocyte, neutrophil, lymphocyte and eosinophil counts.
Keywords: hyperglycemia, hypokalemia, leukocytosis, methylphenidate
1. Introduction
Methylphenidate is one of the drugs shown to elicit behavioral sensitization.[1] It was first synthesized in 1944 by Leandro Panizzon. He named the substance Ritalin, after his wife's nickname, Rita.[2] Methylphenidate is a piperidine derivative, structurally related to amphetamines, and acts as a central nervous system (CNS) stimulant. It has been widely used since 1955 for numerous indications, including attention deficit hyperactivity disorder (ADHD), narcolepsy, cataplexy,[1] and conduct disorder in children, adolescents and adults.[3,4] Although it has been indicated for ADHD since 1957, it gained widespread use only during the last 2 decades.[5] Methylphenidate stimulates CNS activity and produces effects similar to amphetamines. Both drugs increased synaptic and intracellular norepinephrine and dopamine in rodents and baboons.[5,6] As such, it affects the biochemical profile of glucose, electrolytes, minerals, complete blood count, and liver enzymes; similar to those of catecholamines.[5–7] Therapeutic doses of amphetamines and of methylphenidate differentially increase synaptic and extracellular dopamine.[5]
There are abundant data on the impact of catecholamines on laboratory parameters, but surprisingly little comparable information on methylphenidate. Gontokovsky et al[8] reported a 26% decrease in serum glucose values after methylphenidate initiation. Several reports[7–10] found no association between prolonged administration (1–4 years) of methylphenidate on hematopoietic, endocrine (including blood glucose levels), hepatic, or cardiovascular functions in hyperactive boys.
Clinical manifestations of overdose include agitation, hallucinations, psychosis, lethargy, seizures, tachycardia, dysrhythmias, hypertension, and hyperthermia. Hepatotoxicity was first reported in rodents.[11] A possible mechanism for these responses is the inhibition of cytochrome p-450.[12] Various types of organ failure have also been reported. They were manifested by abnormal liver function enzymes, poor urine output, hypotension, tachypnea, tachycardia, abnormal blood gases, rising serum blood urea nitrogen (BUN) and creatinine, and hyperactive deep-tendon reflexes.[13] Spivak et al[7] reported thrombocytopenia and decreased levels of norepinephrine, dopamine, and serotonin in children treated with methylphenidate for 3 months. These findings are indicative of an inhibitory effect on platelet activation.
Few studies have investigated the effects of methylphenidate on the lipid profile. A randomized study that examined the effect of methylphenidate on blood lipid levels[14] found significant decreases in total cholesterol, low-density lipoprotein-cholesterol, and triglyceride levels. Non-significant changes were observed in high-density lipoprotein-cholesterol, apolipoprotein A, and apolipoprotein B levels. Because of its rapidly growing use, especially among children,[10] it is important to examine the effect of this medication on the biochemical and hematological parameters in the blood plasma of young individuals. This study investigated the effects of methylphenidate on youngsters diagnosed with ADHD.
2. Methods
The patients enrolled in this historical cohort (retrospective) study were from the Children and Adolescent Outpatient Psychiatric Clinic of the Tel Aviv Sourasky Medical Center. They had been referred to the clinic for suspected ADHD. Excluded were patients with other psychiatric or neurologic diseases, endocrinopathies, mental retardation, other chronic illness, or taking other medication. The candidates were evaluated, diagnosed with ADHD and prescribed treatment with methylphenidate (Ritalin).
2.1. Ethics statement
Participants received a full explanation about the nature of this 3-month study. Patients were enrolled after consent was provided by themselves or a guardian. This historical cohort study was approved by the local Ethics Committee of the Tel Aviv Sourasky Medical Center. The study was conducted in accordance with the Basic & Clinical Pharmacology & Toxicology policy for experimental and clinical studies.[11]
Before beginning treatment, patients provided a medical history, underwent a physical examination including blood pressure, weight, and height measurements. Blood samples were taken for a complete blood count and biochemical analysis (sodium, potassium, calcium phosphorus, magnesium, total protein, albumin, and liver enzymes) before treatment and after 3 months of continuous treatment. The period of 3 months was considered sufficient for methylphenidate to have had an effect, but not to have caused significant constitutional changes in weight, height and other parameters within that short period. The chemistry tests were analyzed with AU5800, Beckman Coulter analyzers. Sodium and Potassium were analyzed with ISE module. Glucose levels were determined in enzymatic UV test (hexokinase method). Urea levels were determined in kinetic UV test (Urease method)
2.2. Statistical analysis
Sample size was calculated with expected difference of 10% or more between 2 measurements. The required sample was about 30 patients.
Data are expressed as mean and standard deviation. Parameters were checked for normality with the Shapiro–Wilk test and most were not normally distributed. Differences between all parameters were evaluated with Wilcoxon test using Bonferroni correction for multiple testing. Variables which were highly statistically different were also found to be statistically different after applying this correction
Logistic regression was used to identify parameters independently affected by methylphenidate. Five parameters (those with difference at P < .0001) were added to the model in an “enter all selected variables to the model” mode with adjustment for age, gender, and background diagnoses. P < .05 was considered significant. All statistical analyses were done with SPSS-25 software (IBM, Armonk, NY).
3. Results
A total of 83 consecutive outpatients with an established diagnosis of ADHD met the inclusion criteria and were eligible to participate in this study. Among them, 19 were excluded for various reasons, including stopping treatment, noncompliance, or lack of sufficient follow-up data. The remaining 64 patients comprised the study cohort. There were 48 (75%) males and 16 (25%) females, at a median age of 16 years (range 11–31).
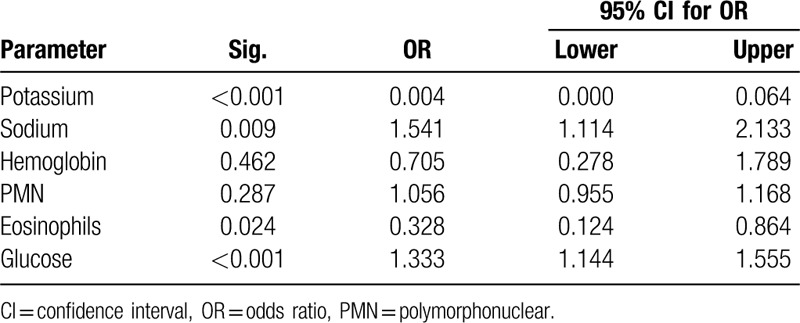
Table 1 and Figure 1 displays the differences between parameters before taking methylphenidate and after 3 months of methylphenidate. Body mass index did not change during the study period. The mean potassium level decreased by 0.6 mg/dL (P < .0001), while glucose rose by 15 mg/dL (P < .0001), sodium increased by 0. 7 meq/L (P = .006) (Table 1). Changes in hemoglobin, calcium, phosphorus, protein, albumin, and liver enzyme levels, white blood count did not reach significance. Neutrophil count, lymphocytosis and eosinophilia were increased 3 months after using of methylphenidate. However, the absolute changes of the significant variables mostly fell within normal ranges. No changes in red blood cells or thrombocyte levels were found. Logistic regression (Table 2) showed that methylphenidate significantly affected blood potassium, glucose, sodium, and eosinophils (p < .001).
Table 1.
Differences in laboratory values between baseline measurements and 3 months after starting methylphenidate.
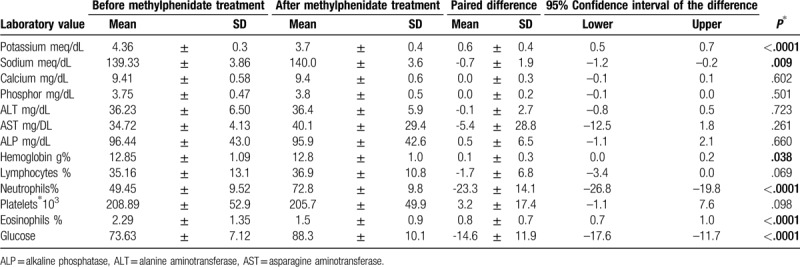
Figure 1.
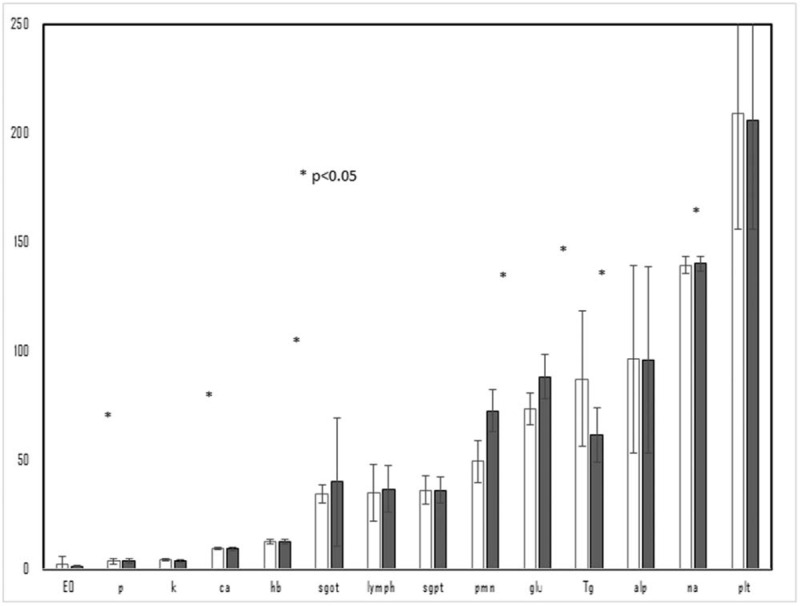
The differences between parameters before taking methylphenidate and after 3 months of methylphenidate.
Table 2.
Logistic regression-dependent variable: methylphenidate by selected parameters.
4. Discussion
Methylphenidate HCl is a CNS stimulant. The mode of therapeutic action in ADHD is not known, although it is thought to block the reuptake of norepinephrine and dopamine into the presynaptic neurons and increase the release of these monoamines into the extra neuronal space. Worldwide, it is the most extensively used medication for treating ADHD in all age groups. A careful search of the literature revealed very little conclusive data about the impact of methylphenidate on the biochemical profile of serum glucose, sodium, potassium, calcium, phosphorus, protein, albumin, and liver enzymes. The following is an interpretation of the findings for each of these factors in the current study.
4.1. Glucose
Methylphenidate had a significant, positive effect on blood glucose levels. It increased by 22%, similar to previous studies.[8,15] Data on amphetamine-like substances and hyperglycemia are inconsistent and the changes are mostly explained by emotional stress. An exception to these studies is Gontokovsky et al[8] who reported a 26% decrease in serum glucose values after methylphenidate initiation. Data on the association between amphetamine-like substances and hyperglycemia are controversial as well, and elevations in serum glucose are also mostly explained by stress.[16,17] The hyperglycemia inhibitory effect of amphetamines was also observed when given shortly before signs of stress, but not several days later.[7]
4.2. Potassium
The mean potassium levels of the study cohort decreased by 18% (P < .0001), indicative of hypokalemia.
Sympathomimetics and their derivatives may lower serum potassium levels, but this is usually clinically significant when there is an overdose.[18] Hypokalemia of pathological or drug-induced origin can shift the resting potential toward less negative values and thus, considerably increase the risk of torsades-de-points. It is therefore recommended to avoid combining neuroleptics with hypokalemia drugs, such as amphetamines.[18–22]
4.3. Sodium
Our results reveal minimal changes in sodium levels. There are no substantial data on the effect of methylphenidate on sodium in the literature. One study explained hyponatremia as the result of a mechanism of inappropriate anti-diuretic hormone secretion.[19] However, others found a non-significant increase in serum sodium.[20,21]
4.4. Calcium and phosphorous
There were no significant fluctuations in serum calcium and phosphorous associated with methylphenidate. The only publication we found regarding this association was a description of severe poisoning by the drug ecstasy, which caused hypocalcemia.[22]
4.5. Liver enzymes
Although the results of clinical trials failed to reveal any association between methylphenidate and serum aminotransferase elevations or instances of hepatic injury, after-market reports of enzyme elevations were received from the sponsor.[11–13,22–26] The elevations were transient, mild-to-moderate in severity, and not associated with jaundice or its symptoms.[11,13,23,26] In addition, there were several case reports of marked serum enzyme elevations and clinically apparent acute liver injury attributed to methylphenidate given intravenously.[22] The pattern of liver enzyme elevations was hepatocellular and the clinical phenotype was typical of acute hepatic necrosis, with rapid onset and rapid recovery.[23] Severe amphetamine liver injury was reported as more common among patients with hepatitis C,[11–13,22–25,27] while immunoallergic features, such as eosinophilic hepatitis were rare.[26] Liver injury due to oral methylphenidate is usually self-limited and resolves spontaneously,[11–13] while acute hepatic necrosis after intravenous injection of methylphenidate caused severe liver injury and even a fatal outcome.[22–25] Eosinophilic hepatitis has also been reported in association with amphetamine derivatives.[26]
4.6. Transaminase
Our findings did not show any significant changes in transaminases; however, there was evidence of elevated alkaline phosphatase. One explanation for this is that most of the patients were young adults and children in whom the bone alkaline phosphatase originated in growing bones. Renal function test results, for example, BUN and creatinine did not change. There have been only a few reports about different types of organ failure during overdose in the setting of intravenous methylphenidate treatment. These were manifested by abnormal liver enzymes, jaundice, poor urine output, hypotension, tachypnea, tachycardia, abnormal blood gases, increasing serum BUN and creatinine, and hyperactive deep-tendon reflexes.[13,22–25]
4.7. Complete blood count
The current study showed 21% elevated white blood count (P = .014) and 19% neutrophilia (P = .038). However, the absolute changes mostly fell within normal ranges for leukocytes. Significant changes in white blood counts have been reported in the literature; mostly cases of leukocytosis stress-induced poisoning by amphetamines and neutrophilia.[22–25,27,28] Our results revealed that erythrocyte and thrombocyte count did not rise significantly after 3 months of treatment.
A limitation of this study was the relatively small sample size of 64 patients. Controlled studies of patients according to age subgroups will provide additional important information about the effect of methylphenidate on various laboratory parameters. It is worth mentioning, however, that decrease in potassium level, increase in glucose level and increase in WBC count bear a potential clinical importance. For instance, in individuals with glucose level at the high end of the normal range an elevation of glucose level by 15 mg/dL or more would establish the diagnosis of diabetes mellitus. Along the same line, toward the low end of the normal range, a decrease of 18% or greater in potassium level increases the likelihood of cardiac arrhythmia.
5. Conclusions
Methylphenidate affects the biochemical and hematological parameters in the blood of patients diagnosed with ADHD. The results showed that it causes hyperglycemia, hypokalemia, slight hyponatremia, and elevation of total leukocyte count including, neutrophils, lymphocytes, eosinophils.
5.1. Uncited references
Acknowledgments
We thank Faye Schreiber, the institutional medical and scientific editor, who provided editorial assistance.
Author contributions
Conceptualization: Gideon Charach, Itamar Grosskopf.
Data curation: Gideon Charach.
Formal analysis: Gideon Charach, Itamar Grosskopf.
Funding acquisition: Gideon Charach.
Investigation: Gideon Charach, Itamar Grosskopf.
Project administration: Gideon Charach, Itamar Grosskopf, Alexander Rabinovich.
Software: Lior Charach
Supervision: Eli Karniel.
Validation: Gideon Charach, Itamar Grosskopf, Alexander Rabinovich.
Writing – original draft: Gideon Charach.
Writing – review & editing: Gideon Charach, Itamar Grosskopf.
Footnotes
Abbreviations: ADHD = attention deficit hyperactivity disorder, CNS = central nervous system.
How to cite this article: Charach G, Karniel E, Grosskopf I, Rabinovich A, Charach L. Methylphenidate has mild hyperglycemic and hypokalemia effects and increases leukocyte and neutrophil counts. Medicine. 2020;99:27(e20931).
The authors declare that they have no competing interests whatsoever.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The study was approved by the Ethics Committee of the Tel Aviv Sourasky Medical Center. All participants provided written informed consent prior to data collection.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Leonard B, McCartan D, White J, et al. Methylphenidate: a review of its neuropharmacological, neuropsychological and adverse clinical effects. Hum Psychopharmacol Clin Exp 2004;19:151–80. [DOI] [PubMed] [Google Scholar]
- [2].Mayers RL. Methylphenidate (Ritalin). The 100 Most Important Chemical Compounds: A Reference Guide. 2007;USA: Greenhood Press, 178–180. [Google Scholar]
- [3].Gerardin P, Cohen D, Mazet P, et al. Drug treatment of conduct disorder in young people. Eur Neuropsychopharmacol 2002;12:361–70. [DOI] [PubMed] [Google Scholar]
- [4].Gonzalez de Dios J, Cardo E, Servera M. Methylphenidate in the treatment of attention deficit/hyperactivity disorder: are we achieving an adequate clinical practice? Rev Neurol 2006;43:705–14. [PubMed] [Google Scholar]
- [5].Schiffer WK, Volkow ND, Fowler JS, et al. Therapeutic doses of amphetamine or methylphenidate differentially increase synaptic and extracellular dopamine. Synapse 2006;59:243–51. [DOI] [PubMed] [Google Scholar]
- [6].Knepper S, Grunewald G, Rutledge C. Inhibition of norepinephrine transport into synaptic vesicles by amphetamine analogs. J Pharmacol Exp Ther 1988;247:487–94. [PubMed] [Google Scholar]
- [7].Spivak B, Veref Y, Yoran-Hegesh R, et al. The influence of three months of methylphenidate treatment on platelet: poor biogenic amine levels in boys with attention deficit hyperactivity disorder. Hum Psychopharmacol 2001;16:333–7. [DOI] [PubMed] [Google Scholar]
- [8].Gontokovsky SR, Nevel R, McDonald NB, et al. Decreased serum glucose levels after initiation of methylphenidate in a patient status post-cerebellar tumour resection: a potential interaction with glipizide. Clin Drug Investig 2007;217:719–25. [DOI] [PubMed] [Google Scholar]
- [9].Samuels JA, Eranco K, Wan F, et al. Effect of stimulants on 24 ambulatory blood pressure in children with ADHD. Pediatr Nephrol 2006;21:92–5. [DOI] [PubMed] [Google Scholar]
- [10].Satterfield JH, Scell AM, Barb SD. Potential risk of prolonged administration of stimulant medication for hyperactive children. Dev Behav Pediatr 1980;1:102–7. [PubMed] [Google Scholar]
- [11].Tveden-Nyborg P, Bergmann TK, Lykkesfeldt J. Basic & clinical pharmacology & toxicology policy for experimental and clinical studies. Basic Clin Pharmacol Toxicol 2018;123:233–5. [DOI] [PubMed] [Google Scholar]
- [12].Roberts S, Harbison R, Roth L, et al. Methylphenidate-induced hepatotoxicity in mice and its potentiation by beta-adrenergic agonist drugs. Life Sci 1994;55:269–81. [DOI] [PubMed] [Google Scholar]
- [13].Le Nedelec M, Rosengren R. Methylphenidate inhibits cytochrome P450 in Swiss Web mouse. Hum Exp Toxicol 2002;21:273–80. [DOI] [PubMed] [Google Scholar]
- [14].Stechyc O, Loludice T, Demeter S, et al. Multiple organ failure resulting from intravenous abuse of methylphenidate hydrochloride. Ann Emerg Med 1985;14:597–9. [DOI] [PubMed] [Google Scholar]
- [15].Charach G, Kaysar N, Rabinovich A. Jill Dr, Norvilitis M, et al. Methylphenidate and dyslipidemia. Current Directions in ADHD and Its Treatment.. Croatia: InTech; 2012. 185–92. [Google Scholar]
- [16].Schmidt MJ. Dopamine agonist-induced hyperglycemia in rats: effects of lergotrile mesylate. Eur J Pharmacol 1979;59:95–101. [DOI] [PubMed] [Google Scholar]
- [17].Gagliano H, Ortega-Sanchez JA, Nadal R, et al. Psychostimulants and forced swim stress interaction: how activation of the hypothalamic-pituitary-adrenal axis and stress-induced hyperglycemia are affected. Psychopharmacology (Berl) 2017;234:2859–69. [DOI] [PubMed] [Google Scholar]
- [18].Markiewicz K, Cholewa M, Lutz W. Influence of coffee and amphetamine on the concentration of free fatty acids, triglycerides and glucose during exertion and in the restitution phase. Z Gesamte Inn Med 1977;32:74–7. [PubMed] [Google Scholar]
- [19].Frimas V, Roberge C, Perroux D, et al. Cardiological monitoring of antipsychotic-treated patients: evaluation and evolution of a hospital protocol. Encephale 2008;34:467–76. [DOI] [PubMed] [Google Scholar]
- [20].Satchell SC, Connaughton M. Inappropriate antidiuretic hormone secretion and extreme rises in serum creatinine kinase following MDMA ingestion. Br J Hosp Med 1994;51:495. [PubMed] [Google Scholar]
- [21].Ligtenberg JJ, Olgers TJ, van de Meeberg EK, et al. Re: MDMA-associated cerebral edema resembling psychogenic polydipsia? J Emerg Med 2015;48:81–2. [DOI] [PubMed] [Google Scholar]
- [22].Ghatol A, Kazory A. Ecstasy-associated acute severe hyponatremia and cerebral edema: a role for osmotic diuresis? J Emerg Med 2012;42:137–40. [DOI] [PubMed] [Google Scholar]
- [23].Refstad S. Paramethoxyamphetamine (PMA) poisoning; a ’party drug’ with lethal effects. Acta Anaesthesiol Scand 2003;47:1298–9. [DOI] [PubMed] [Google Scholar]
- [24].Mehta H, Murray B, LoIudice TA. Hepatic dysfunction due to intravenous abuse of methylphenidate hydrochloride. J Clin Gastroenterol 1984;6:149–51. [DOI] [PubMed] [Google Scholar]
- [25].Vakde T, Diaz M, Uday K, et al. Rapidly reversible multiorgan failure after ingestion of ”Molly" (pure 3, 4-methylenedioxymethamphetamine): a case report. J Med Case Rep 2014;8:204–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Stecyk O, Loludice TA, Demeter S, et al. Multiple organ failure resulting from intravenous abuse of methylphenidate hydrochloride. Ann Emerg Med 1985;14:597–9. [DOI] [PubMed] [Google Scholar]
- [27].Hood B, Nowicki MJ. Eosinophilic hepatitis in an adolescent during lisdexamfetamine dimesylate treatment for ADHD. Pediatrics 2010;125:1510–3. [DOI] [PubMed] [Google Scholar]
- [28].Wiergowski M, Anand JS, Krzyżanowski M, et al. Acute methoxetamine and amphetamine poisoning with fatal outcome: a case report. Int J Occup Med Environ Health 2014;27:683–90. [DOI] [PubMed] [Google Scholar]


