Abstract
Disabled-2 (Dab2) is a clathrin and cargo-binding endocytic adaptor protein that plays a role in cellular trafficking of low-density lipoprotein receptor (LDLR). However, little is known about its involvement in coronary artery disease (CAD). Here, we aimed to investigate the association between Dab2 single-nucleotide polymorphisms (SNPs) and CAD in Chinese Han and Uyghur populations.
We performed a case-control study in CAD group that consisted of 621 Han and 346 Uygurs, and the age and gender matched control group consisted of 611 Han and 405 Uygurs. The clinicopathological characteristics of these subjects were analyzed. Genotyping of 4 SNPs (rs1050903, rs2855512, rs11959928, and rs2255280) of the Dab2 gene was performed in all subjects with an improved multiplex ligase detection reaction method.
The distribution of the genotype, dominant model (AA vs. AC + CC), as well as allele frequencies of both rs2855512 and rs2255280, was significantly different between CAD patients and control subjects in Han population but not in Uyghur population. AA genotype may be a risk factor for CAD. For Han population, statistical significant correlation between dominant model for both SNPs (AA) and CAD was found after multivariate adjustment. After multivariate adjustment in the Han population, we speculate that rs285512 A allele and rs2255280 A allele may be potentially associated with the onset of coronary heart disease. Individuals with the AA genotype had an OR of 1.44 (95% CI: 1.10–1.88, P = .01, rs2855512) and 1.41 (95% CI: 1.08–1.85, P = .01, rs2255280) for CAD compared with individuals with the AC or CC genotype, respectively.
Our data indicates that the AA genotype of rs2855512 and rs2255280 in the Dab2 gene may be a genetic marker of CAD risk in Chinese Han population.
Keywords: coronary artery disease, disabled-2 (Dab2), single-nucleotide polymorphism
1. Introduction
Coronary artery disease (CAD) is the main cause of death worldwide and brings considerable emotional and economic burden.[1] It remains one of the world's major health problems, accounting for 12.7% of global mortality.[2] Several risk factors, such as lipid levels, hypertension, family history, smoking, diabetes, obesity, and genetic factors, are involved in the development of CAD.[2–5] For example, Miao et al[6] reported that the BRCA2 (breast susceptibility gene 2) rs9534275 correlated with increased risk of CAD. For over half a century, hyperlipidaemia has been considered an important risk factor for CAD.[7] High levels of total cholesterol and low-density lipoprotein cholesterol (LDL-C) increase the risk of CAD.[8,9] Blood lipids are heritable, modifiable risk factors for CAD.[10] Therefore, by studying the human lipid genes, we can identify new therapeutic targets for cholesterol management and prevention of heart disease.[11]
The human Disabled-2 (Dab2) gene, which is located on chromosome 5p13, consists of 15 exons and encodes a protein consisting of 770 amino acids. Dab2 was originally characterized as a protein with 2 alternatively spliced forms named p96 (also known as p82) and p67 (also known as p59), which lacks a central exon.[12] Dab2 is a putative tumor suppressor that was first recognized because of its down regulation in ovarian tumor.[13] Tissue-dependent expression analysis showed that Dab2 is mainly expressed in the brain,[14] kidneys,[15] intestine,[16] and ovaries.[17] Single-nucleotide polymorphism (SNP) of Dab2 has been widely studied. For example, it is shown that Dab2 rs11959928 is associated with renal function and/or renal disease,[18] whereas Dab2 rs268091 is a significant predictor of prostate cancer-specific mortality.[19] The rs2255280 variant of Dab2 is associated with a lower risk of pancreatic cancer.[20] However, the relationship of Dab2 SNP with CAD is unclear.
Sochacki et al[21] found that Dab2 located on both the edge and center of clathrin-coated structures. Dab2 is a clathrin adaptor that binds to the membrane, clathrin, and other components.[19] As an adaptor protein, Dab2 can bind to and transport cell surface receptors, including low-density lipoprotein receptor (LDLR)[22] and type 1 and 2 transforming growth factor-β receptors.[23] In studies using cultured cells, Dab2 was shown to play a role in LDLR endocytosis.[22,24] In liver sinusoid endothelial cells, Dab2 participated in LDLR-mediated LDL uptake, was responsible for the majority of adaptor functions in LDLR endocytosis and LDLR-mediated cholesterol clearance from the circulation, and regulated cholesterol synthesis.[25] Previous studies also showed that different adaptors may be complementary in the endocytosis of certain proteins, such as autosomal-recessive hypercholesterolemia and Dab2 in LDLR endocytosis;[24,26] and Dab2 and Numb in integrin endocytosis.[27] Consistently, Wei et al[28] showed that Dab2 modulated cholesterol homeostasis by selectively participating in the process of LDLR-mediated cholesterol uptake. As an adaptor protein, Dab2 also plays roles in growth factor signaling,[29] cytoskeleton reorganization,[15] and cell adhesion control.[30] These studies demonstrate that Dab2 plays an important role in the regulation of cholesterol absorption.
Therefore, in the present study, we determined to investigate the association between SNP of Dab2 and CAD.
2. Materials and methods
2.1. Ethical approval of the study protocol
This study was approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University (Xinjiang, China). We conducted the study according to the standards of the Declaration of Helsinki. All of the patients provided written informed consents and explicitly provided permission for DNA analyses, as well as the collection of relevant clinical data.
2.2. Subjects
CAD patients and control subjects were recruited from The First Affiliated Hospital of Xinjiang Medical University and from January 2012 to December 2016. Han Chinese patients and Uighur Chinese patients were studied independently. CAD group included 611 Han Chinese patients and 346 Uyghur Chinese patients and the control group included 621 Han Chinese individuals and 405 Uyghur Chinese individuals. CAD was defined as the presence of at least 1 significant coronary artery stenosis with more than 50% luminal diameter on coronary angiography. Control subjects also underwent coronary angiography and were confirmed to be free of coronary artery stenosis. Moreover, the control subjects did not show clinical or electrocardiogram evidence of myocardial infarction or CAD.[31] Clinical data were collected from all study subjects. Subjects with malignant tumors, connective tissue disease, chronic kidney disease, valvular heart disease, and chronic inflammatory diseases were excluded.
2.3. Laboratory examination and definition of cardiovascular risk factors
Fasting blood samples were collected from all participants and serum was isolated. Serum concentrations of blood urea nitrogen (BUN), creatinine (Cr), fasting plasma glucose (FPG), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and LDL-C were measured using standard methods as previously described.[32] Major CAD risk factors were defined based on current national guidelines.[33,34] Dyslipidemia was defined as TG ≥1.70 mmol/L, TC ≥ 5.20 mmol/L, LDL-C ≥ 3.40 mmol/L, HDL-C < 1.00 mmol/L, or a prior Dyslipidemia diagnosis and/or receiving a lipid-lowering drug.[33] Hypertension was defined as a systolic blood pressure (SBP) ≥ 140 mm Hg, diastolic blood pressure (DBP) ≥ 90 mm Hg, or a prior hypertension diagnosis and/or receiving an antihypertensive drug.[34] Diabetes was defined as FPG ≥ 6.99 mmol/L, or a prior diabetes diagnosis and/or using a diabetes drug.[35] Smoking was defined as currently or previously smoking. Drinking was defined as drinking at least once a week and last for more than 1 year.
2.4. DNA extraction
The blood samples were collected and centrifuged at 4000 × g for 5 minutes to separate the peripheral blood leukocytes. DNA was extracted from these leukocytes using a whole blood genome extraction kit (Beijing Bioteke Corporation, Beijing, China), as described previously.[32] The DNA samples were stored at − 80°C until use.
2.5. SNP selection and genotyping
In the current study, the data from the 1000 Genomes Project (http://www.1000genomes.org) was analyzed by Haploview 4.2 software (Broade Institute, MA). After analyzing, we obtained 4 tagging SNPs for Chinese Hans and Uygurs using a minor allele frequency (MAF)≥0.05 and linkage disequilibrium patterns, with r2≥0.8 as a cutoff.
SNP genotyping was performed using an improved multiplex ligase detection reaction method (iMLDR, Genesky Bio-Tech Cod., Ltd, Shanghai, China). The primers were shown in Table 1. The PCR reaction mixture contained 1x GC-I buffer (Takara, Dalian, China), 3.0 mM Mg2+, 0.3 mM dNTP, 1 U HotStarTaq polymerase (Qiagen Inc, USA), 1 μM multiplex PCR primer, and 50 ng DNA sample. PCR amplification was performed under the following conditions: 95°C for 2 minutes and 11 cycles at 94°C for 20 seconds, 65°C for 40 seconds, 72°C for 1.5 minutes, and 24 cycles at 94°C for 20 seconds, 59°C for 30 seconds, and 72°C for 1.5 minutes, and a final extension at 72°C for 2 minutes. PCR products were purified with 5U SAP enzyme and 2U Exonuclease I enzyme, 37°C warm bath for 1 hour, and then 75 inactivation for 15 minutes. Ligation primer sequences were shown in Table 2. The ligation system includes: 1 uL connection buffer (10×), 0.25 μL high-temperature ligase, 0.4 uL 5 ’ ligation primer mixture (1 μM), 0.4 μL 3’ ligation primer mixture (2 μM), 2 μL purified multiple PCR products and 6 μL ddH2O. PCR ligation procedure was performed under the following conditions: 38 cycles at 94°C for 1.0 minutes and 56°C for 4 minutes. A total of 0.5 μL of the diluted ligation product was mixed with 0.5 μL Liz500 SIZE STANDARD (Thermo Fisher Scientific) and 9 μL Hi-Di (ABI), denatured at 95°C for 5 minutes and then sequenced on ABI3730XL sequencer (Applied Biosystems, Foster City, CA). Genotyping was performed in a blinded manner without knowledge of the patients’ clinical data, and a total of 10% of the genotyped samples were duplicated to monitor genotyping quality.
Table 1.
PCR primers used in this study.
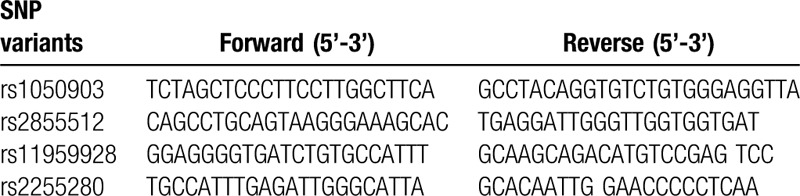
Table 2.
Ligation primers used in this study.

2.6. Statistical analysis
All statistical analyses were performed using SPSS 17.0 software for Windows (SPSS Institute, Chicago, IL). The normality of distribution of continuous variables was analyzed with D’Agostino-Pearson test. All continuous variables were expressed as mean ± standard deviation (SD) and the differences in continuous variables were compared by using an independent-sample t test. Chi-square analysis was used to test the deviations of genotype distribution from the Hardy-Weinberg equilibrium and to determine the differences of allele or genotype frequencies. The variables with significant differences between control and patients were included in the Logistic regression models. Logistic regression analyses with effect ratios (odds ratio [OR] and 95% CI [confidence interval]) were used to assess the contribution of the major risk factors. The frequency distribution of the haplotypes was calculated by performing a permutation test using the bootstrap method. P < .05 was considered to be statistically significant.
3. Results
3.1. Characteristics of CAD patients and control subjects
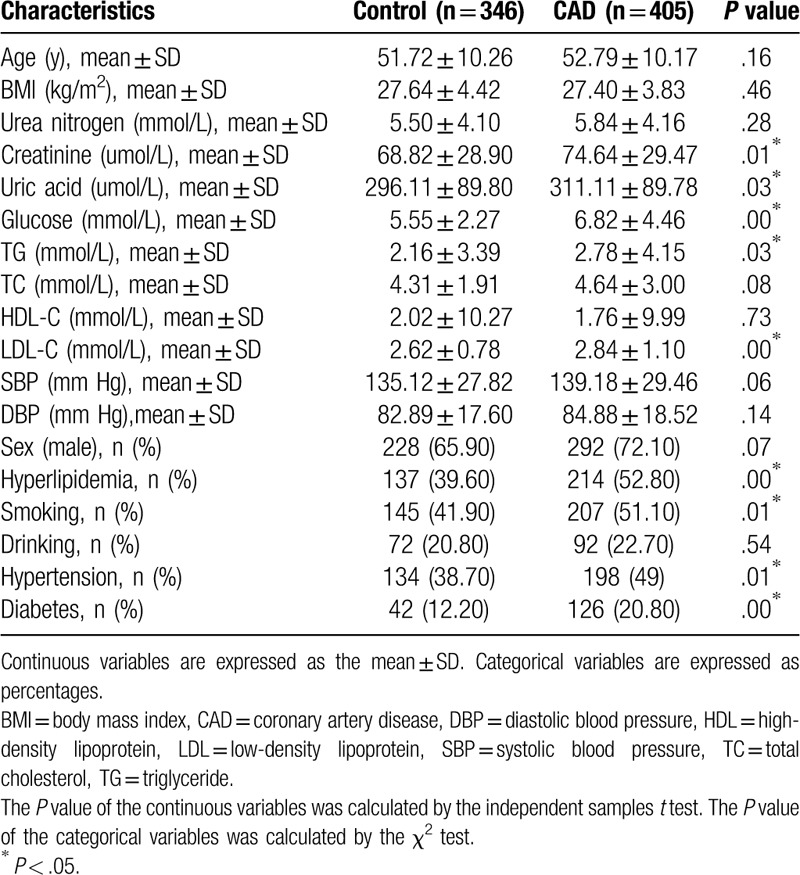
The general data and clinical characteristics of CAD patients and the control group subjects of Han and Uygur population are described in Tables 3 and 4, respectively. For both populations, there was no significant difference in age between CAD patients and control subjects, indicating that the subjects are age-matched. For Han population, the body mass index (BMI) (P < .01), FPG (P < .01), TG (P < .01), TC (P < .01), LDL-C (P < .01), SBP (P < .01), hyperlipidemia (P < .01), the prevalence rate of hypertension (P < .01), and the prevalence rate of diabetes mellitus (P < .01) were significantly higher in patients with CAD than that in control participants. For Uygur populations, the serum Cr (P = .01), uric acid (P = .03), FPG (P < .01), TG (P = .03), LDL-C (P = .00), hyperlipidemia (P < .01), smoking (P = .01), the prevalence rate of hypertension (P = .01), and the prevalence rate of diabetes mellitus (P < .01) were significantly higher in patients with CAD than that in control participants. These data show that some risk factors for CAD are common in both Han and Uygur population.
Table 3.
Characteristics of Han Chinese subjects.
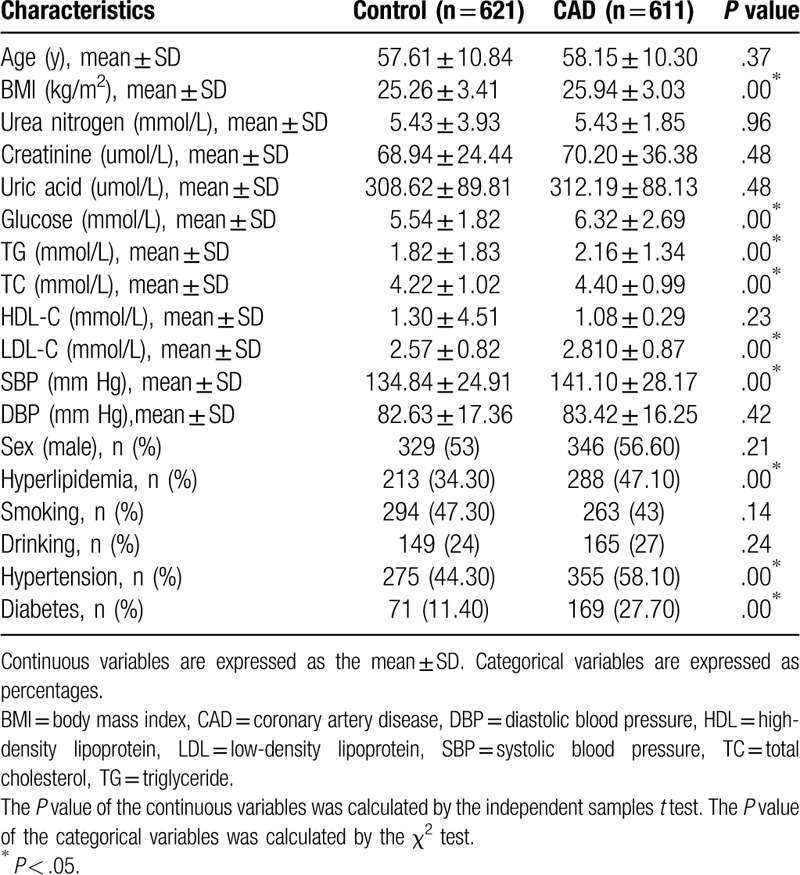
Table 4.
Characteristics of Uygur Chinese subjects.
3.2. Hardy-Weinberg equilibrium test
The genotype distributions of the 4 SNPs in Han and Uygur controls were analyzed using Hardy-Weinberg equilibrium test. We found that all the SNPs are in line with the Hardy-Weinberg equilibrium (P = .02 for rs1050903, P = .35 for rs2855512, P = .350 for rs11959928, and P = .06 for rs2255280 in Han population; P = .77 for rs1050903, P = .07 for rs2855512, P = .70 for rs11959928, and P = .08 for rs2255280 in Uygur population), which indicated accurate population representation of the samples.
3.3. The distribution of the genotypes and alleles for the Dab2 SNPs between CAD patients and control subjects
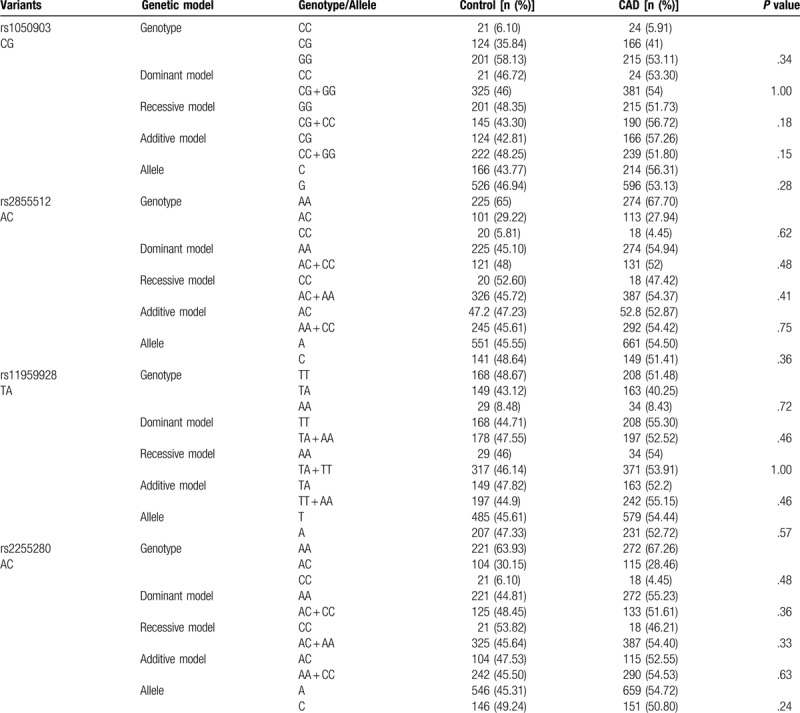
We first compared the distribution of Dab2 SNPs in Han population (Table 5). For rs2855512, the distribution of the genotype, dominant model (AA vs. AC + CC), as well as the allele frequency was significantly different between patients with CAD and control subjects (P = .02, P = .01 and P = .01, respectively). Meanwhile, the distribution of the genotype, dominant model (AA vs. AC + CC), and recessive model (CC vs. AC + AA) as well as the allele frequency was significantly different between patients with CAD and control subjects (P = .03, P = .02, P = .05, and P = .01, respectively) for rs2255280. However, there was no difference in the genotype, allele, and distribution frequencies between CAD patients and health controls for rs1050903 and rs11959928. In contrast, all the 4 SNPs in the Dab2 gene showed similar genotype, gene model, and the allele frequencies between CAD patients and control participants among the Uygur populations (P > .05; Table 6). These results indicated that the distribution of rs2855512 and rs2255280 genotypes in CAD patients and control group was different.
Table 5.
Genotype and allele distribution among control subjects and patients with CAD in the Han population.
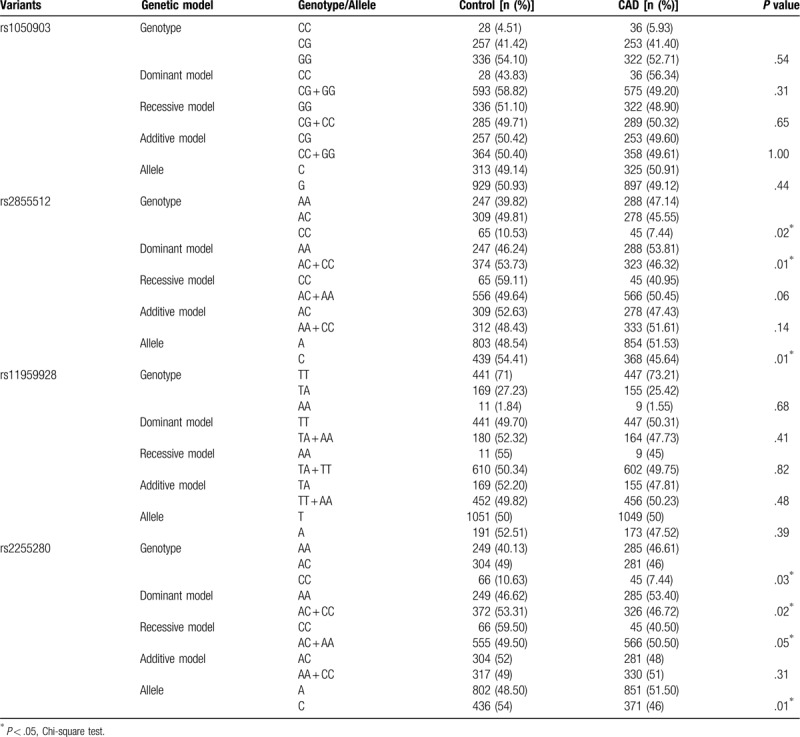
Table 6.
Genotype and allele distribution among control subjects and patients with CAD in the Uygur population.
3.4. Association of Dab2 polymorphisms with CAD in Han population
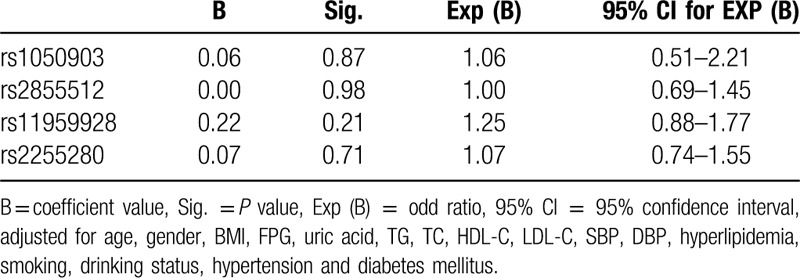
To further investigate the relationship between the significantly different Dab2 SNPs (rs2855512 and rs2255280) and CAD in the Han population, multivariable logistic regression analysis was conducted. The following variables were adjusted: gender, age, BMI, uric acid, FPG, TG, TC HDL-C, LDL-C, SBP, DBP, Hyperlipidemia, smoking, drinking, hypertension, and diabetes (Table 7). Statistical significant correlation between dominant model of both SNPs and CAD was found after multivariate adjustment (P = .01, OR = 1.44, 95% CI: 1.10–1.88 for rs2855512 and P = .01, OR = 1.42, 95% CI: 1.08–1.85 for rs2255280, respectively; Table 7). However, there was no significant difference in the distribution frequency of A allele in rs2855512 and A allele in rs2255280. For Uygur population, there was no significant difference in 4 SNPs gene model (Table 8). Therefore, we speculate that rs285512 A allele and rs2255280 A allele may be potentially associated with the onset of CAD of Xinjiang Han population.
Table 7.
Multiple logistic regression analysis for CAD patients and control subjects in Han Chinese (rs2855512 and rs2255280).
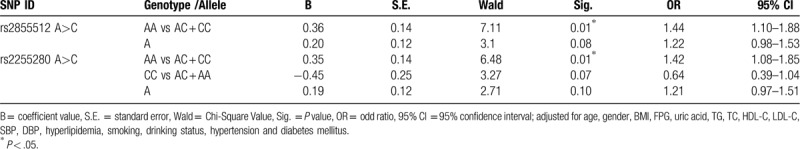
Table 8.
Multiple logistic regression analysis for CAD patients and control subjects in Uygur Chinese (rs1050903, rs2855512, rs2255280).
4. Discussion and conclusion
To the best of our knowledge, this is the first study on the relationship of Dab2 gene variants rs1050903, rs2855512, rs11959928, and rs2255280 with susceptibility to CAD. Here, we identified a significant association of Dab2 gene variants with CAD in Chinese Han population, showing that rs2855512 A > C and rs2255280 A>C may be risk factors of CAD susceptibility.
Abnormal blood lipid often leads to an increased risk of early atherosclerosis, which is also a major risk factor for CAD.[36] Elevated LDL-C and serum cholesterol levels are risk factors of CAD, whereas lowering LDL-C levels could significantly reduce the risk of CAD morbidity and mortality.[37,38] Other types of dyslipidaemia, such as elevated TG and reduced HDL-C, are also associated with an elevated risk of CAD.[39,40]
The results of more than 200 prospective cohort studies, randomized trials, and large-scale research projects involving more than 2 million participants were meta-analyzed.[41] The results showed that there was a significant dose-dependent relationship between the absolute exposure of vascular system to LDL cholesterol and atherosclerotic cardiovascular disease, and the risk increased with exposure time.[41] Thus, effective control of dyslipidaemia is of great importance to the prevention and control of CAD in China.
In this case-control study, 4 SNPs in the Dab2 gene were examined in 1983 subjects to determine whether they were related to the risk of CAD in the Chinese Han and Uygur populations. The overall distribution, genotypes, and alleles of the 4 SNPs were not significantly different between CAD patients and control subjects in Uygur populations. In the Han population, the distribution of the genotype and dominant model (AA vs. AC + CC) of rs2855512 as well as its allele frequency was significantly different between patients with CAD and control subjects. Meanwhile, the distribution of the genotype, dominant model (AA vs. AC + CC), and recessive model (CC vs. AC + AA) of rs2255280 as well as its allele frequency was significantly different between patients with CAD and control subjects. The most valuable finding is that the rs2855512 and rs2255280 polymorphisms of Dab2 were significantly associated with increased risk of CAD. After multivariate adjustment for gender, age, BMI, UA, FPG, TG, TC, HDL-C, LDL-C, SBP, DBP, hyperlipidaemia, smoking, drinking, hypertension, and diabetes in the total Han Chinese population, we found that the rs2855512 A allele and rs2255280 A allele are risk alleles. Individuals with the AA genotype had an odds ratio (OR) of 1.44 (95% confidence interval [CI]: 1.10–1.88, P = .01, rs2855512) and 1.42 (95% CI: 1.08–1.85, P = .01, rs2255280) for CAD, respectively. Our data indicate that the AA genotype of rs2855512 and rs2255280 in the Dab2 gene may be genetic markers of CAD risk. However, the underlying mechanisms remain unclear. We hypothesize that variants of Dab2 rs2243421 and rs2255280 may enhance Dab2 expression and reduce the incidence of CAD.
Dab2 is a functional protein with multiple domains that mediates endocytosis and is a signal transduction phosphoprotein involved in intracellular protein transport.[42] The activity and ability of Dab2 to mediate LDLR endocytosis have been demonstrated in cultured cells.[24] Members of the LDL receptor family contain NPXY motifs that are specifically recognized by Dab2;[43–45] thus, a role for Dab2 in LDL uptake and cholesterol metabolism is expected. As an endocytic adaptor protein,[22,46,47] Dab2 possesses a PTB/PID domain at its N-terminus that can bind the NPXY (Asn-Pro-X-Tyr, X represents any amino acid) motif found in a subset of cell surface receptors.[48] Through its PTB domain, Dab2 mediates the attachment of cargo-containing transmembrane proteins with an NPXY motif, such as the LDL receptor, EGF receptor, megalin, and integrins, to clathrin coats (through DFF motifs).[15,46,47,49] Therefore, Dab2 is able to recruit the LDL receptor into clathrin-coated pits and facilitate endocytosis.[24,50] Based on the findings from these previous studies and from our present study, we hypothesize that variants of Dab2 rs2243421 and rs2255280 may enhance Dab2 expression and reduce the incidence of CAD.
To better interpret the results, the limitations of our analysis should also be acknowledged. First, this study is limited in the small sample size. Further large-scale investigation is needed. Second, the large time interval for subject enrolment may cause certain bias due to differences in the detection of relevant indicators and the collection of clinical data.
In conclusion, we found that Dab2 gene polymorphisms rs2855512 and rs2255280 are associated with CAD in the Han population in China. The AA genotype of rs2855512 and rs2255280 in the Dab2 gene may be a risk genetic marker of CAD in the Han population in China. Therefore, further large-scale investigations should be carried out in different ethnic groups to verify these findings in other populations. Additionally, functional analyses will be needed to confirm the relevance of rs2855512 and rs2255280 with CAD.
Author contributions
Conceptualization: Zhenyan Fu, Yitong Ma.
Data curation: Yinghong Wang, Yongtao Wang.
Funding acquisition: Yitong Ma.
Methodology: Yinghong Wang, Yongtao Wang, Dilare Adi, XiaoDong He, Fen Liu, Asiya Abudesimu.
Project administration: Zhenyan Fu, Yitong Ma.
Resources: Yinghong Wang.
Software: Yinghong Wang, Yongtao Wang.
Writing – original draft: Yinghong Wang, Yongtao Wang.
Writing – review & editing: Zhenyan Fu, Yitong Ma.
Footnotes
Abbreviations: CAD = coronary artery disease, Dab2 = Disabled-2, LDLR = low-density lipoprotein receptor, SNPs =single-nucleotide polymorphisms.
How to cite this article: Wang Y, Wang Y, Adi D, He X, Liu F, Abudesimu A, Fu Z, Ma Y. Dab2 gene variant is associated with increased coronary artery disease risk in Chinese Han population. Medicine. 2020;99:27(e20924).
Yinghong Wang and Yongtao Wang contributed equally to this work.
This work was supported financially by the Xinjiang Uygur Autonomous Region Natural Science Foundation Project (2017D01C295), National Natural Science Foundation of China (91957208,81970380) and the Xinjiang Uygur Autonomous Region science and technology branch project (2017E0269), the Xinjiang graduate research innovation project (XJGRI2016068), and the Postgraduate Innovation Project of Xinjiang Medical University (CXCY001).
All authors reviewed and approved the final draft.
The authors have no conflicts of interest to disclose.
References
- [1].Pagidipati NJ, Gaziano TA. Estimating deaths from cardiovascular disease: a review of global methodologies of mortality measurement. Circulation 2013;127:749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Finegold JA, Asaria P, Francis DP. Mortality from ischaemic heart disease by country, region, and age: statistics from World Health Organisation and United Nations. Int J Cardiol 2013;168:934–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Curry SJ, Krist AH, Owens DK, et al. Risk assessment for cardiovascular disease with nontraditional risk factors: US Preventive Services Task Force Recommendation Statement. JAMA 2018;320:272–80. [DOI] [PubMed] [Google Scholar]
- [4].Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Failure 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- [5].Knowles JW, Ashley EA. Cardiovascular disease: the rise of the genetic risk score. PLoS Med 2018;15:e1002546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Miao L, Yin RX, Yang S, et al. Association between single nucleotide polymorphism rs9534275 and the risk of coronary artery disease and ischemic stroke. Lipids Health Dis 2017;16:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:3168–209. [DOI] [PubMed] [Google Scholar]
- [8].Clarke R, Emberson J, Parish S, et al. Cholesterol fractions and apolipoproteins as risk factors for heart disease mortality in older men. Arch Intern Med 2007;167:1373–8. [DOI] [PubMed] [Google Scholar]
- [9].Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet 2012;380:572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kannel WB, Dawber TR, Kagan A, et al. Factors of risk in the development of coronary heart disease—six-year follow-up experience: the Framingham study. Ann Intern Med. 1961; 49:33–50. [DOI] [PubMed] [Google Scholar]
- [11].Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 2010;466:707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Xu X, Yang W, Jackowski S, et al. Cloning of a novel phosphoprotein regulated by colony-stimulating factor 1 shares a domain with the Drosophila disabled gene product. J Biol Chem 1995;270:14184–91. [DOI] [PubMed] [Google Scholar]
- [13].Mok S, Wong K, Chan R, et al. Molecular cloning of differentially expressed genes in human epithelial ovarian cancer. Gynecol Oncol 1994;52:247–52. [DOI] [PubMed] [Google Scholar]
- [14].Huang CH, Cheng JC, Chen JC, et al. Evaluation of the role of Disabled-2 in nerve growth factor-mediated neurite outgrowth and cellular signalling. Cell Signal 2007;19:1339–47. [DOI] [PubMed] [Google Scholar]
- [15].Morris S, Arden S, Roberts R, et al. Myosin VI binds to and localises with Dab2, potentially linking receptor-mediated endocytosis and the actin cytoskeleton. Traffic 2002;3:331–41. [DOI] [PubMed] [Google Scholar]
- [16].Vazquez-Carretero MD, Garcia-Miranda P, Calonge ML, et al. Regulation of Dab2 expression in intestinal and renal epithelia by development. J Cell Biochem 2011;112:354–61. [DOI] [PubMed] [Google Scholar]
- [17].Fazili Z, Sun W, Mittelstaedt S, et al. Disabled-2 inactivation is an early step in ovarian tumorigenicity. Oncogene 1999;18:3104–13. [DOI] [PubMed] [Google Scholar]
- [18].Boger CA, Gorski M, Li M, et al. Association of eGFR-related loci identified by GWAS with incident CKD and ESRD. PLoS Genet 2011;7:e1002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 2011;12:517–33. [DOI] [PubMed] [Google Scholar]
- [20].Wu C, Miao X, Huang L, et al. Genome-wide association study identifies five loci associated with susceptibility to pancreatic cancer in Chinese populations. Nat Genet 2011;44:62–6. [DOI] [PubMed] [Google Scholar]
- [21].Sochacki KA, Dickey AM, Strub MP, et al. Endocytic proteins are partitioned at the edge of the clathrin lattice in mammalian cells. Nat Cell Biol 2017;19:352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Morris S, Cooper J. Disabled-2 colocalizes with the LDLR in clathrin-coated pits and interacts with AP-2. Traffic 2001;2:111–23. [DOI] [PubMed] [Google Scholar]
- [23].Hocevar B, Smine A, Xu X, et al. The adaptor molecule Disabled-2 links the transforming growth factor beta receptors to the Smad pathway. EMBO J 2001;20:2789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Maurer ME, Cooper JA. The adaptor protein Dab2 sorts LDL receptors into coated pits independently of AP-2 and ARH. J Cell Sci 2006;119:4235–46. [DOI] [PubMed] [Google Scholar]
- [25].Tao W, Moore R, Meng Y, et al. Endocytic adaptors Arh and Dab2 control homeostasis of circulatory cholesterol. J Lipid Res 2016;57:809–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].He G, Gupta S, Yi M, et al. ARH is a modular adaptor protein that interacts with the LDL receptor, Clathrin, and AP-2. J Biol Chem 2002;277:44044–9. [DOI] [PubMed] [Google Scholar]
- [27].Teckchandani A, Toida N, Goodchild J, et al. Quantitative proteomics identifies a Dab2/integrin module regulating cell migration. J Cell Biol 2009;186:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wei J, Fu ZY, Li PS, et al. The clathrin adaptor proteins ARH, Dab2, and numb play distinct roles in Niemann-Pick C1-Like 1 versus low density lipoprotein receptor-mediated cholesterol uptake. J Biol Chem 2014;289:33689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhou J, Hsieh JT. The inhibitory role of DOC-2/DAB2 in growth factor receptor-mediated signal cascade. DOC-2/DAB2-mediated inhibition of ERK phosphorylation via binding to Grb2. J Biol Chem 2001;276:27793–8. [DOI] [PubMed] [Google Scholar]
- [30].Rosenbauer F, Kallies A, Scheller M, et al. Disabled-2 is transcriptionally regulated by ICSBP and augments macrophage spreading and adhesion. EMBO J 2002;21:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li X, Ma Y, Xie X, et al. Association of Egr3 genetic polymorphisms and coronary artery disease in the Uygur and Han of China. Lipids Health Dis 2014;13:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Xie X, Ma Y-T, Yang Y-N, et al. Polymorphisms in the SAA1/2 gene are associated with carotid intima media thickness in healthy Han Chinese subjects: the cardiovascular risk survey. PLoS One 2010;5:e13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].JciCgftmodi. 2016 Chinese guideline for the management of dyslipidemia in adults. Zhonghua Xin Xue Guan Bing Za Zhi 2016; 44:833–53. [DOI] [PubMed] [Google Scholar]
- [34].Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. National Institutes of Health. Obes Res 1998;6: suppl 2: 51S–209S. [PubMed] [Google Scholar]
- [35].Bild DE. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- [36].Stamler J, Wentworth D, Neaton JD. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA 1986;256:2823–8. [PubMed] [Google Scholar]
- [37].Baigent C, Keech A, Kearney P, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267–78. [DOI] [PubMed] [Google Scholar]
- [38].Levy R. Cholesterol and coronary artery disease. What do clinicians do now? Am J Med 1986;80:18–22. [DOI] [PubMed] [Google Scholar]
- [39].Ren J, Grundy SM, Liu J, et al. Long-term coronary heart disease risk associated with very-low-density lipoprotein cholesterol in Chinese: the results of a 15-Year Chinese Multi-Provincial Cohort Study (CMCS). Atherosclerosis 2010;211:327–32. [DOI] [PubMed] [Google Scholar]
- [40].Wang M, Zhao D, Wang W, et al. [Serum triglyceride is an independent risk factor for acute coronary heart disease events in 35-64 years old Chinese-Chinese Multi-provincial Cohort Study.]. Zhonghua Xin Xue Guan Bing Za Zhi 2008;36:940–3. [PubMed] [Google Scholar]
- [41].Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2017;38:2459–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chetrit D, Ziv N, Ehrlich M. Dab2 regulates clathrin assembly and cell spreading. Biochem J 2009;418:701–15. [DOI] [PubMed] [Google Scholar]
- [43].Willnow T, Nykjaer A, Herz J. Lipoprotein receptors: new roles for ancient proteins. Nat Cell Biol 1999;1:E157–62. [DOI] [PubMed] [Google Scholar]
- [44].Herz J, Gotthardt M, Willnow T. Cellular signalling by lipoprotein receptors. Curr Opin Lipidol 2000;11:161–6. [DOI] [PubMed] [Google Scholar]
- [45].Stolt PC, Bock HH. Modulation of lipoprotein receptor functions by intracellular adaptor proteins. Cell Signal 2006;18:1560–71. [DOI] [PubMed] [Google Scholar]
- [46].Hasson T. Myosin VI: two distinct roles in endocytosis. J Cell Sci 2003;116:3453–61. [DOI] [PubMed] [Google Scholar]
- [47].Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem 2003;72:395–447. [DOI] [PubMed] [Google Scholar]
- [48].Bork P, Margolis B. A phosphotyrosine interaction domain. Cell 1995;80:693–4. [DOI] [PubMed] [Google Scholar]
- [49].Mishra S, Keyel P, Hawryluk M, et al. Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J 2002;21:4915–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mulkearns EE, Cooper JA. FCH domain only-2 organizes clathrin-coated structures and interacts with Disabled-2 for low-density lipoprotein receptor endocytosis. Mol Biol Cell 2012;23:1330–42. [DOI] [PMC free article] [PubMed] [Google Scholar]


