Abstract
Primary nephrotic syndrome (PNS) is one of the most common primary glomerular diseases in children. Patients complicated nephrotic syndrome with pancreatic lesions are rarely reported, and the clinical manifestations in children are atypical. This study has observed the incidence, clinical types, and prognosis of acute pancreatitis (AP) in children with primary nephrotic syndrome, and analyzed its related factors, early diagnosis, and treatment.
Seven children with PNS and AP in Shanghai Children's Hospital from January 2015 to December 2017 were reviewed. The clinical data including age, height, weight, body mass index (BMI), diet, biliary tract disease, PNS durations, drugs, proteinuria, creatinine, glucose, glycated hemoglobin, amylase and lipase, albumin, cholesterol, triglyceride, ultrasound, computerized tomography (CT), renal pathology and estimated glomerular filtration rate (eGFR) were retrospectively analyzed. All patients were followed for >2 years.
Ten in 589 patients with PNS were detected pancreatic lesions by abdominal ultrasound. Seven were diagnosed as AP, which the incidence was 1.2%. Only 1 of 7 patients had elevated serum amylase. Lesions of pancreas were found by ultrasound and/or enhanced CT. Four of 7 patients had been treated with tacrolimus. All patients with AP were improved after octreotide acetate injection and supportive treatment. Only 1 patient suffered recurrent AP during the relapse of PNS 10 months later.
AP in children with PNS is not common, and the clinical manifestations are not typical. Abdominal ultrasound and enhanced CT are of high value in diagnosis. The adverse effects of tacrolimus should be concerned. Early diagnosis and timely treatment can be helpful for a prognosis.
Keywords: children, nephrotic syndrome, pancreatitis, tacrolimus
1. Introduction
Primary nephrotic syndrome (PNS) is a very common kidney diseases in children, which is characterized by a large number of proteinuria, hypoproteinemia, hyperlipidemia, and edema. The annual incidence rate is 1.15 to 16.9/100,000 and the prevalence rate is 12 to 16/100,000.[1] The most common complications in children with PNS include infection, electrolyte imbalance, low blood volume, hypercoagulability, and acute renal injury. Pancreatitis is inflammation of the pancreas. It happens when digestive enzymes digest the pancreas itself. AP is a common clinical acute abdomen disease, which could manifest acute upper abdominal pain, abdominal distension, nausea, vomiting, and other gastrointestinal symptoms. Though AP in children is less than adults, the incidence increased gradually in recent years.[2] The incidence of AP in children in the United States is 6.1 to 8.8/100,000.[3] Patients complicated nephrotic syndrome with pancreatic lesions are rarely reported, and clinical manifestations in children are very atypical. So the aim of this study is to observe clinical manifestations, laboratory tests, and related imaging of children complicated in our hospital, and to explore its clinical features.
2. Patients and methods
2.1. Patients
Children with PNS were diagnosed as acute pancreatitis in Shanghai Children's Hospital from January 2015 to December 2017. All patients were diagnosed with PNS according to the diagnostic criteria as following[1]: edema with protein excretion >40 mg/m2 per hour or urineprotein:creatinine ratio ≥2000 mg/g (≥200 mg/mmol) or >3+ proteinuria on dipstick with serum albumin <2.5 g/dL (25 g/L), excluded congenital hereditary renal disease, secondary renal disease, hypertension, renal vein thromboembolism, hemodialysis, chronic virus infection (such as epstein-barr virus [EBV], hepatitis B virus [HBV], hepatitis C virus [HCV]), etc. The diagnostic criteria of AP are as below[4]: abdominal pain (acute onset of persistent and severe epigastric pain, often radiating to the back); serum lipase (or amylase) activity at least 3 times the upper limit of normal; characteristic findings of acute pancreatitis on contrast-enhanced CT or, less often, MRI or transabdominal ultrasonography. Acute pancreatitis can be diagnosed if at least 2 of the above 3 criteria are fulfilled. The diagnosis of all patients was determined by both nephrologists and gastroenterologists.
2.2. Methods
All patients with PNS received conventional abdominal ultrasound (liver, bile, pancreas, spleen, kidney, ureter, and bladder), and who with pancreatic abnormalities got abdominal enhanced CT scans. This study is approved by the Institutional Review Board of Shanghai Children's Hospital. We recorded the age, height, weight, body mass index (BMI), improper diet (high-fat diet, irregular eating) or not, 24 hours urinary protein (24hUP, g), serum amylase (Amy, U/L) and lipase (Lip, U/L), urinary amylase (UAm, U/L), serum fasting blood glucose (Glu), glycated hemoglobin (HbA1c), creatinine (Scr, umol/L), direct bilirubin (DB, umol/L), alanine transferase (ALT, U/L), Albumin (Alb, g/L), total cholesterol (TC, mmol/L), triglyceride (TG, mmol/L), abdominal ultrasound, abdominal contrast-enhanced CT scan, chest x-ray, renal pathology, eGFR calculated according to Schwatz formula (ml/min/1.73 m2), and other related clinical data of the patients. All the data were retrospectively analyzed. The ultrasound and CT reports were confirmed by 2 different radiologists. All patients were followed for >2 years.
3. Results
3.1. General
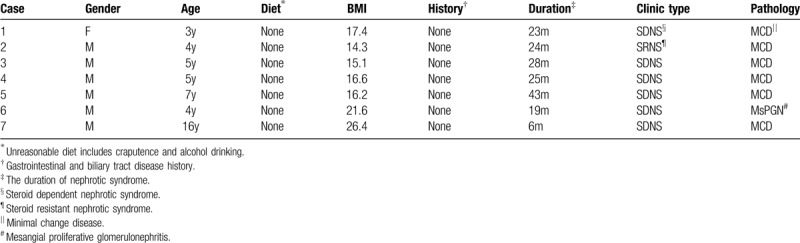
There are 589 cases of PNS in children, including 388 male patients, 201 female patients. The median age is 7 years (range 3–16). Ten patients were detected pancreatic lesions by ultrasound, in which 7 were diagnosed as AP, including 6 men and 1 woman (Table 1). The clinical type of all patients with AP was interstitial edematous pancreatitis (mild). No obviously improper diet and gastrointestinal, biliary tract disease history was found. The incidence was 1.2%. All 7 patients were followed for >2 years.
Table 1.
The basic situation of the patients with acute pancreatitis includes gender, onset age, improper diet, history of other diseases, disease duration, clinical type, and kidney biopsy pathology.
3.2. Clinical and pathologic types
Seven cases with acute pancreatitis were refractory nephrotic syndrome, including 1 case with steroid resistance, 6 cases with steroid dependent. Renal pathological types of 6 cases were minimal change disease (MCD), while 1 case was mesangial proliferative glomerulonephritis (MsPGN) (Table 1).
3.3. Used medications
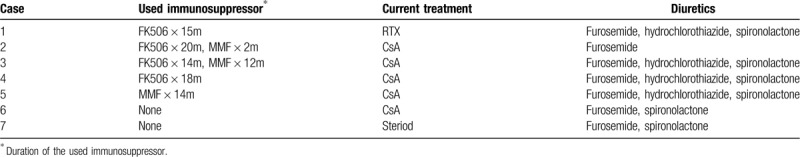
All of 7 patients had been treated with glucocorticoid. In the period of AP treatment the doses of the steroid were equivalent to 2 mg/kg of prednisone. Four patients received tacrolimus and 3 patients received mycophenolate mofetil (MMF) before AP onset. Cyclosporine A (CsA) was administered to 5 patients after AP onset. Diuretics were for severe edema (Table 2).
Table 2.
Used medication of the patients includes immunosuppressive agents and diuretics that had been ever taken by the patients and current medicine for nephrotic syndrome.
3.4. Clinical manifestations
The 7 children had varying degrees of abdominal pain, bloating, nausea, vomiting, who could not clearly describe the quality, character of abdominal pain and whether it radiated to the back, while physical examination had obvious epigastric tenderness. One case performed early shock due to the pain. In addition to abdominal pain and other signs/symptoms, all patients with AP were in the recurrence of nephrotic syndrome, and presented significant edema (peritoneal effusion, pleural effusion), massive proteinuria, and varying degrees of electrolyte imbalance (low sodium, low potassium) and hypercoagulable state.
3.5. Laboratory tests
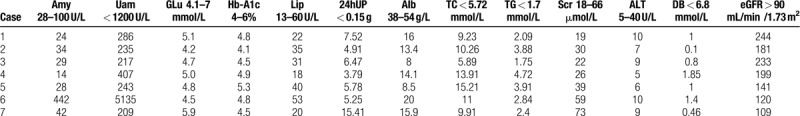
Serum amylase was found increased in only 1 patient. The remaining 6 cases had normal serum amylase, lipase, while most of patients had hypertriglyceridemia and hypercholesterolemia. Glu, HbA1c, DB, ALT, SCr, eGFR level were normal in the all patients with AP, as characteristic indicators of nephrotic syndrome included massive proteinuria and hypoalbuminemia (Table 3).
Table 3.
Laboratory tests of the patients with acute pancreatitis includes serum amylase (Amy), lipase (Lip), fasting blood glucose (Glu), glycated hemoglobin (HbA1c), direct bilirubin (DB), albumin (ALB), Creatine (Cr), alanine aminotransferase (ALT), 24-hour urine protein (24UP) and estimated glomerular filtration rate (eGFR) which calculated by Schwartz formula.
3.6. Imaging examinations
All patients with PNS underwent abdominal ultrasound, of which 10 cases were found pancreatic lesions in pancreatic morphology or structure, which mostly showed pancreas was enlarged with enhanced internal echo, effusion around the pancreas, and gallbladder. All 7 patients received routine plain chest and x-rays which showed pleural effusion only due to PNS. The patients with AP were found enlargement of pancreas, peripancreatic effusion, peritoneal effusion in CT scan, of which 4 cases had obvious pancreatic edema.
3.7. Treatment and prognosis
The 7 children with AP were given fasting, gastrointestinal decompression, and infusion for 3 to 7 days. Proton pump inhibitor, octreotide acetate were used for 1 week. The symptoms of the patients were significantly alleviated within 72 hours, then they gradually resumed diet. All patients had good prognoses at 2-year follow-up. But 1 suffered recurrent AP during the relapse of nephrotic syndrome 10 months later, and also improved by the same treatment.
4. Discussion
Previously, AP mostly occurs in young adults which is relatively rare in children, but recently the incidence of children increased year by year with the emphasis on disease and progress of detective methods. AP could threaten the children's life,[5] with viscera perforation, intestinal ischemia, bowel obstruction, or myocardial infarction.[6] AP is not a common complication in children with primary nephrotic syndrome, and the incidence in our center is 1.2%. Classic screening laboratory test is serum amylase and lipase, but the simple detection of amylase sensitivity (75%–90%) and specificity (20%–60%) is not high.[7] Serum lipase has a sensitivity of 86.5% to 100% and specificity of 84.7% to 99.0% for diagnosing acute pancreatitis. Thus, its sensitivity is higher compared with serum amylase. In severe pancreatitis, serum lipase levels 7 times higher than normal have been reported within 24 hours after onset of pancreatitis.[8] However, there have been a number of reported cases of AP patients with normal amylase and/or lipase, but the specific mechanism is unknown. The reasons could be: acute hemorrhagic necrotizing pancreatitis leads to massive necrosis of pancreatic enzyme-producing tissues, resulting in normal amylase; the time of detecting amylase is too early or too late; amylase inhibiting factor were released in hyperlipidemia and inhibits the activity of amylase; previous basic pancreatic diseases have deprived the pancreas of secretory function.[9,10] In our group, only 1 patient had abnormal amylase and lipase, so children with AP might manifest atypically, especially in the patients with nephrotic syndrome. We speculated that the following factors may be involved: all PNS children in this group were in the state of hyperlipidemia and hypercholesterolemia. PNS caused edema in organs and tissues without serious damage to pancreatic tissues; corticosteriod and/or other immunosuppressive therapy may impair the secretion function of the pancreas. Therefore, the misdiagnosis rate of PNS combined with AP may be high. Ultrasound is currently the best choice to screen for suspected pancreatitis and to evaluate for biliary tract abnormalities in pediatric patients,[11] which can display the morphology and size of the pancreas and the echo of the substantial changes. Contrast-enhanced CT is the imaging procedure of choice if clinical and biochemical findings are inconclusive.[12] It is helpful to determine diagnosis, stage, severity, and complications.[8,13] However, imaging examinations including ultrasound and CT are sometimes subjective, so it is necessary to diagnose by >2 radiologists in conjunction with the clinicians.
The main causes of AP are cholelithiasis and alcohol abuse in adults, which is different from children. The common causes of childhood AP are drugs, infections, trauma, and anatomic anomalies, but it is reported that there are 31.3% of children with unknown etiology[5] (idiopathic). The AP patients with nephrotic syndrome in our group had been taking long-term related drugs (glucocorticoids and immunosuppressive agents). So the causes of AP were likely to be related with the primary disease and/or drugs. Although in the state of nephrotic syndrome with hyperlipidemia/hypercholesterolemia, systemic edema and peritoneal effusion may induce pancreatic edema and other lesion, AP might be associated with nephrotic syndrome. In our group, 589 patients with PNS had varying degrees of edema, ascites, in which only 7 cases occurred acute pancreatitis-related symptoms. Drug-induced pancreatitis (DIP) has been estimated to account for 0.1% to 2% of all AP cases.[14] According to a report from Netherland, 41.6% of AP patients used pancreatitis-associated drugs at admission,[15] including commonly used diuretics (such as furosemide, hydrochlorothiazide, etc), L-aspartase, glucocorticoids, Azathioprine, sodium valproate, and so on. Although some reports in adult suggested a significant increase in the risk of AP in patients who are taking glucocorticoids,[16] all patients with PNS in our group are prescribed glucocorticoids therapy which is the preferred treatment for nephrotic syndrome. Diuretics are commonly used for severe edema, which may lead to hypokalemia, pancreatic cell dysfunction, poor blood circulation of the pancreas, pancreatic duct fluid viscosity, allergies, and other factors of pancreatitis. So we can not rule out the factor of diuretics, but relief of edema is helpful for the treatment of acute pancreatitis. According to the current study,[17] it is safe to use glucocorticoids and furosemide for the treatment of nephrotic syndrome, whether acute pancreatitis occurs or not. Many children with refractory nephrotic syndrome (steroid-dependent and steroid-resistant) use different immunosuppressive agents. In our group, 4 cases had been used tacrolimus before the onset of AP. The drug description mentions its possible adverse effects include pancreatitis (it is often difficult to determine whether the adverse effects are associated with immunosuppressive drugs because other drugs used at the same time). Tacrolimus has been associated with higher rates of hyperglycemia[18] and may be an risk factor of pancreas lesion, but there have been no report that it could cause pancreatitis. Although there is no evidence that tacrolimus is related to AP, while children still need glucocorticoids and diuretic therapy, so we stopped giving tacrolimus to the patients as the onset of AP.
AP treatment depends on the type of disease. The vast majority of AP type in children is edema, which pancreatic lesions are generally mild. So supportive and symptomatic treatment is more important, including fasting, rehydration, inhibiting the pancreatic enzyme secretion (somatostatin and antacids, etc). If the fasting time is estimated >72 hours, the patients should undertake nutritional support. Now it is thought that enteral nutrition could reduce the risk of sepsis,[19] which is better than parenteral nutrition. Therefore, if conditions permits, the jejunum tube can be placed in to supply children with the necessary nutrients, unless concurrent obstruction, perforation, bleeding, and other serious complications. Prophylactic use of antibiotics for mild AP is still controversial. Somatostatin not only inhibits pancreatic exocrine, protects pancreatic cells and stimulates growth, but also induces apoptosis of pancreatic cells to reduce the severity of pancreatitis. So short-term use of somatostatin in AP can effectively control AP symptoms, and it is safe in children.[7] In our group, 7 patients with mild edema AP were treated by fasting for 3 to 7 days, intravenous nutrition, octreotide (8 peptide derivative of the natural somatostatin). Clinical symptoms and signs of the patients were rapidly ameliorated, without obvious adverse reactions. Accordingly, we believe that pancreatic secretion inhibitor should be used timely for AP, to achieve control of the disease and prevent the progress.
In summary, acute pancreatitis is not rare in children with nephrotic syndrome. Some atypical acute pancreatitis is easy to miss in the past, especially in patients with nephrotic syndrome. These patients sometimes have normal amylase. Ultrasound and enhanced CT are important for the diagnosis. If the characteristics of this disease could be recognized, early diagnosis and treatment will achieve a good prognosis. Special attention should be paid to the use of drugs that may cause pancreatic injury and timely adjustment of treatment is needed. It is still necessary to collect a larger sample to do further study.
In our clinical practice, patients with nephrotic syndrome with abdominal pain should be routinely screened for pancreatitis by ultrasound, especially those with long-term use of hormones, diuretics, and tachrolimus. Unexplained abdominal pain that meets the diagnostic criteria should be diagnosed as pancreatitis, even if serum amylase is normal.
4.1. Limitations of the study
This study is an observational study, and the mechanism and causes of the disease are not further discussed. The diagnosis of pancreatitis in children is still controversial, due to the lack of amylase abnormality which is the main biomarker of pancreatitis, although it meets the criteria. However, for these patients, we have discussed with the gastroenterologist before diagnosing. And the last, the sample size of this study is too small to explain the characteristics of this disease, and a larger clinical study is needed to reach a clearer conclusion.
Author contributions
Sheng Hao conceptualized and designed the study, collected and analyzed the clinical data, developed all tables, provided data interpretation, and drafted, revised and gave final approval of the manuscript. Ying Wu, Yu-lin Kang, Xiaoling Niu and Guanghua Zhu managed the cases and collected the data. Wenyan Huang conceptualized and designed the study and reviewed and revised the initial manuscript.
Footnotes
Abbreviations: 24hUP = 24 hours urinary protein, Alb = albumin, ALT = alanine transferase, Amy = serum amylase, AP = acute pancreatitis, BMI = body mass index, CsA = cyclosporine A, CT = computerized tomography, CTX = cyclophosphamide, DB = direct bilirubin, eGFR = estimated glomerular filtration rate, Glu = fasting blood glucose, HbA1c = glycated hemoglobin, Lip = serum lipase, MCD = minimal change disease, MMF = mycophenolate mofetil, MsPGN = mesangial proliferative glomerulonephritis, PNS = primary nephrotic syndrome, Scr = serum creatinine, TC = total cholesterol, TG = triglyceride, UAm = urinary amylase.
How to cite this article: Hao S, Wu Y, Kang Y, Niu X, Zhu G, Huang W. A single-center analysis of primary nephrotic syndrome with acute pancreatitis in children. Medicine. 2020;99:27(e21056).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Noone DG, Iijima K, Parekh R. Idiopathic nephrotic syndrome in children. Lancet 2018;392:61–74. [DOI] [PubMed] [Google Scholar]
- [2].Morinville VD, Barmada MM, Lowe ME. Increasing incidence of acute pancreatitis at an American pediatric tertiary care center: is greater awareness among physicians responsible? Pancreas 2010;39:5–8. [DOI] [PubMed] [Google Scholar]
- [3].Hornung LN, Szabo FK, Kalkwarf HJ, et al. Stabilized incidence of pediatric acute pancreatitis. Pancreas 2018;47:e60–2. [DOI] [PubMed] [Google Scholar]
- [4].Paul Georg L, Minoti A, Banks PA. Acute pancreatitis. Lancet 2015;386:85–96. [DOI] [PubMed] [Google Scholar]
- [5].Lautz TB, Chin AC, Radhakrishnan J. Acute pancreatitis in children: spectrum of disease and predictors of severity. J Pediatr Surg 2011;46:1144–9. [DOI] [PubMed] [Google Scholar]
- [6].Wu BU, Banks PA. Clinical management of patients with acute pancreatitis. Gastroenterology 2013;144:1272–81. [DOI] [PubMed] [Google Scholar]
- [7].Pietzak MM. Guandalini S. Acute and chronic pancreatitis. Textbook of Pediatric Gastroenterology and Nutrition. United Kingdom: Taylor & Francis; 2004. 303–18. [Google Scholar]
- [8].Suzuki M, Sai JK, Shimizu T. Acute pancreatitis in children and adolescents. World J Gastrointest Pathophysiol 2014;5:416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mathur AK, Whitaker A, Kolli H, et al. Acute pancreatitis with normal serum lipase and amylase: a rare presentation. JOP 2016;17:98–101. [Google Scholar]
- [10].Kitagawa S, Sawai K. Hypertriglyceridemia-induced acute pancreatitis with normal pancreatic enzymes. Am J Med 2018;131:299–300. [DOI] [PubMed] [Google Scholar]
- [11].Restrepo R, Hagerott HE, Kulkarni S, et al. Acute pancreatitis in pediatric patients: demographics, etiology, and diagnostic imaging. AJR Am J Roentgenol 2016;206:632–44. [DOI] [PubMed] [Google Scholar]
- [12].Baddeley RNB, Skipworth JRA, Pereira SP. Acute pancreatitis. Medicine (Baltimore) 2011;39:108–15. [Google Scholar]
- [13].Türkvatan A, Erden A, Türkoğlu MA, et al. Imaging of acute pancreatitis and its complications. Part 1: Acute pancreatitis. Diagn Interv Imaging 2015;96:151–60. [DOI] [PubMed] [Google Scholar]
- [14].Nitsche C, Maertin S, Scheiber J, et al. Drug-induced pancreatitis. Curr Gastroenterol Rep 2012;14:131–8. [DOI] [PubMed] [Google Scholar]
- [15].Spanier BM, Tuynman HA, Van Der Hulst RW, et al. Acute pancreatitis and concomitant use of pancreatitis-associated drugs. Am J Gastroenterol 2011;106:2183–8. [DOI] [PubMed] [Google Scholar]
- [16].Sadr-Azodi O, Mattsson F, Bexlius TS, et al. Association of oral glucocorticoid use with an increased risk of acute pancreatitis: a population-based nested case-control study. JAMA Intern Med 2013;173:444–9. [DOI] [PubMed] [Google Scholar]
- [17].Lopez MJ. The changing incidence of acute pancreatitis in children: a single-institution perspective. J Pediatr 2002;140:622–4. [DOI] [PubMed] [Google Scholar]
- [18].Viau-Colindres J, Astudillo M, Redondo MJ, et al. Medication-induced hyperglycemia: pediatric perspective. BMJ Open Diab Res Care 2020;8:e000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Roberts KM, Nahikian-Nelms M, Ukleja A, et al. Nutritional aspects of acute pancreatitis. Gastroenterol Clin 2018;47:77–94. [DOI] [PubMed] [Google Scholar]


