Abstract
Background and Objective:
Undiagnosed pleural effusions (UPEs) are a common problem of respiratory medicine, leading to an increased diagnostic burden globally. However, the most efficient and cost-effective approaches to UPEs remain controversial. This study aimed to assess the diagnostic value of ultrasound-guided needle biopsy (UGNB) in UPEs.
Methods:
We conducted a search of PubMed, Embase, the Cochrane Library and reference lists of retrieved studies with no publication data limitation. Articles that investigated the diagnostic accuracy of UGNB in UPEs were included. The quality of eligible studies was assessed using Quality Assessment of Diagnostic Accuracy Studies-2. The diagnostic value of UGNB was evaluated by calculating the pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, diagnostic odds rate, and the area under the curve for the summary receiver operating characteristic curve using a random effects model.
Results:
Seven studies comprising 165 patients with UPEs met the inclusion criteria. UGNB had a pooled sensitivity of 83% (95% confidence intervals [CI], 75% - 89%), a specificity of 100% (95% CI, 90% - 100%), a positive likelihood ratio of 8.89 (95% CI, 3.29 - 24.02), a negative likelihood ratio of 0.23 (95% CI, 0.16 - 0.33), a diagnostic odds rate of 51.47 (95% CI, 14.70 - 180.16), and an area under the curve of 0.94. Six pneumothorax cases (3.6%), 5 local wound infections (3.0%), and 1 empyema case (less than 1%) were observed. There was no significant heterogeneity or publication bias in this study.
Conclusions:
Based on current evidence, UGNB is a safe and convenient procedure with a high accuracy for diagnosing UPEs. However, physicians should still be cautious in interpreting negative UGNB results.
Keywords: meta-analysis, ultrasound-guided needle biopsy, undiagnosed pleural effusion
1. Introduction
Pleural effusions (PEs) are a common problem in respiratory medicine, with more than 50 identified causes.[1] An estimated 1.5 million diagnoses are made annually in the United States alone.[2] Congestive heart failure, cirrhosis, pneumonia, malignancy, and tuberculosis are some of the representative and important aetiologies.[3] Clinical evaluation, imaging, thoracocentesis and fluid analysis are crucial approaches that explore the aetiology of PEs.[4] However, despite standard diagnostic procedures, approximately 20% of PEs remain undiagnosed[5,6] and histological confirmation is often necessary for undiagnosed PEs (UPEs).[1] Pleural biopsy can be performed using several methods including blind-closed needle biopsy, image-guided needle biopsy, Local Anaesthetic (Medical) Thoracoscopy (LAT) and Video-Assisted Thoracoscopic surgery (VATs).[7]
The traditional Abrams needle biopsy (ANB) has the benefit of being a low-cost procedure with ease of accessibility. However, the diagnostic sensitivity for malignant PEs is lower than 60%.[1,4,8] In addition, complications from this procedure, such as site pain (up to 15%), pneumothorax (up to 15%) and vasovagal reaction (about 5%) are common.[1]
In the past few years, image-guided needle biopsy has attracted increased attention, with recent reports showing that image guidance could significantly increase the accuracy of closed needle biopsy in pleural diseases while reducing the incidence of complications.[9,10] A prospective study enrolling 100 consecutive patients with undiagnosed pleural exudates reported that ultrasound-guided needle biopsy (UGNB) achieved high diagnostic yield (88%) with less complications (3%) and was therefore recommended as a first-line investigation in exudative UPEs.[11]
Ultrasound (US) and computed tomography (CT) have been the most applied methods for image-guided needle biopsy and previous studies have indicated that the 2 different procedures have similar diagnostic yields.[12] However, UGNB offers shorter procedural times, fewer complications and lower costs, without the need for ionizing radiation.[13]
Thoracoscopic biopsy is considered the gold standard for investigating pleural diseases and has a high diagnostic sensitivity of over 90% and a specificity of 100%.[1,6,14] Despite this, it is limited by its relatively invasiveness, complexity, long examination times and high cost.[7,9,15]
UGNB provides a clear and real-time visualization of the pleural lesions, effusions and needle movement without exposure to ionizing radiation.[7] Furthermore, it has the advantages of being tolerable, less invasive, with a good diagnostic yield, short examination time, good mobility, low cost and it is widely available in resource-constrained countries. These advantages provide evidence that UGNB is a potential and convenient methodology for use in the diagnosis of UPEs.[1,8,16–23]
Several studies have been published on the diagnostic efficacy of needle biopsy with direct US guidance. However, no studies have systematically assessed and summarized the current existing evidence. Therefore, in this study we performed a meta-analysis aimed at determining the diagnostic value of UGNB in UPEs.
2. Methods
2.1. Search strategy
A systematic literature search of PubMed, Embase and the Cochrane Library were performed for publications that accessed the accuracy of UGNB for the diagnosis of UPEs, up to the January 8, 2020. The search was conducted using the following keywords and Medical Subject Headings terms: Ultrasonography, Ultrasound, Needle Biopsy, and Pleural Effusion. The search strategies applied for each database are presented in Table 1. Searches were restricted to publications in English. In order to expand our search, the reference list of each retrieved article was also screened for other appropriate studies.
Table 1.
Search strategy.

2.2. Inclusion criteria
Studies that investigated the diagnostic accuracy of UGNB in patients with UPEs were included. The inclusion criteria comprised prospective or retrospective studies, enrolled patients with UPEs, biopsy procedures under the guidance of real-time US, sufficient information provided to construct a 2 × 2 table, and articles published in English.
2.3. Exclusion criteria
We excluded the following studies: no relevance to our subject, case reports, conference abstracts, reviews, letters and animal experiments, biopsy procedures performed using a “free-hand” technique without direct US guidance and number of enrolled patients <10.
2.4. Study selection and data extraction
Two reviewers (ZD Lin, DH Wu) independently screened titles and abstracts to identify potential studies. The full text of eligible studies was retrieved and disagreements on study selection were resolved by discussion. We extracted the following information from the included studies: author's name, year of publication, country of origin, method of study, patient characteristics (number and age), technical device used (needle type and needle size), complications, and diagnostic performance of UGNB (true positive, false positive, true negative and false negative). Data extraction was performed by 2 reviewers (ZD Lin, DH Wu).
2.5. Quality assessment
Two investigators (ZD Lin, DH Wu) independently extracted the data and assessed the quality and risk of bias for each study, according to the Quality Assessment of Diagnostic Accuracy Studies 2.[24] Any disagreement among the reviewers was resolved by consensus.
2.6. Statistical analyses
On the basis of the 2 × 2 tables, DerSimonian-Laird random effects models were used to produce summary estimates for sensitivity (SEN), specificity (SPE), positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds rate (DOR).[25] Forest plots were performed for pooled analyses for SEN, SPE, PLR, NLR, and DORs, including 95% confidence intervals (CIs). In addition, the summary receiver operating characteristic (SROC) curve was constructed using the random effects model. The area under the curve (AUC), including the standard error, was computed to assess the diagnostic performance of US-guided pleural biopsy. A test with a good discriminating ability will have a value of AUC close to 1.
The Cochran Q test and the inconsistency index (I2 index) were calculated by forest plots to evaluate heterogeneity. A P-value of less than .1 and an I2 value above 50% were defined as statistically significant and as showing meaningful heterogeneity. Deek funnel plot was constructed to assess publication bias and Egger test was performed. All statistical analyses were performed using Meta-DiSc (version 1.4; Ramon Cajal Hospital) and STATA (version 14; STATA Corporation, College Station, TX).[26]
3. Results
3.1. Study selection and characteristics
A flow diagram summarizing the literature search process and the study selection is presented in Figure 1. A total of 1187 potentially relevant studies were identified and screened by title and abstract, of which 57 full text articles were reviewed. Seven studies including 165 patients met the inclusion criteria and were included in the analyses.[16,17,27–31]
Figure 1.
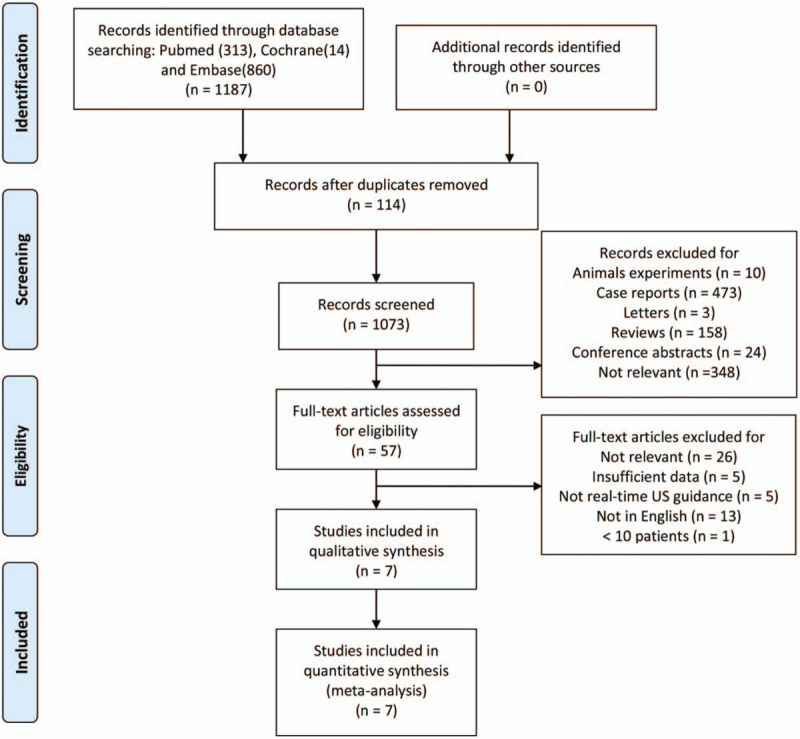
Flow diagram of the study selection. US = ultrasound.
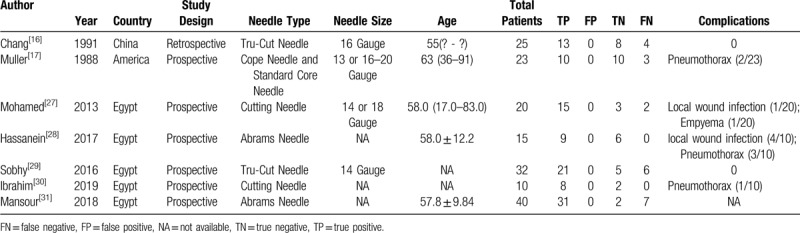
Table 2 shows the characteristics of each included study. The included studies originated in 3 countries: Egypt[27–31] China[16] and the USA.[17] Of these, 6 studies were prospective[17,27–31] and 1 study was retrospective.[16]
Table 2.
Characteristics of included studies.
3.2. Quality assessment
Assessments for the risk of bias and applicability concerns are shown in Figure 2. The results of the quality assessment were satisfactory, with 2 studies fulfilling all the items. Nevertheless, 2 studies were concluded to have a high risk of bias.
Figure 2.
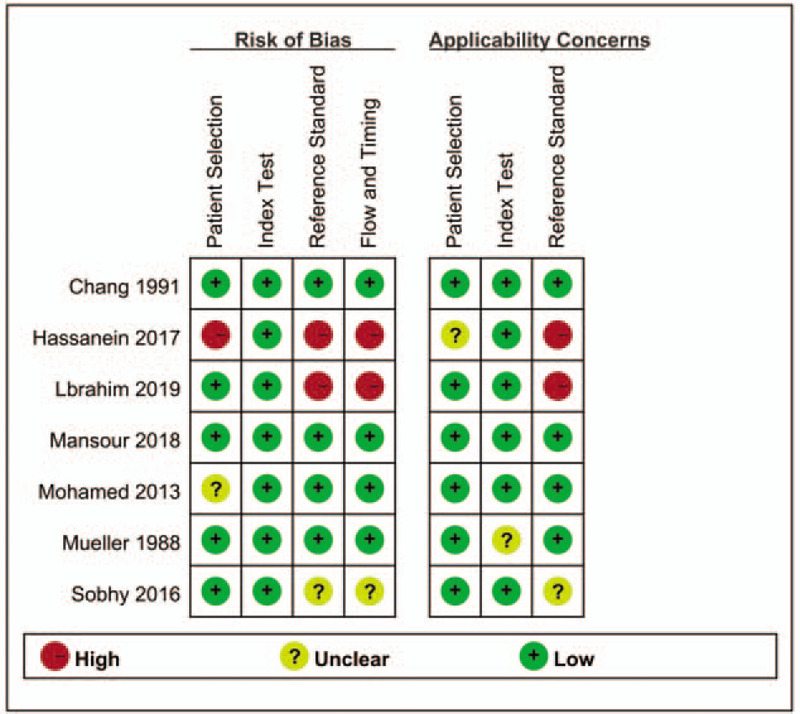
Quality assessment of the methodological quality of each individual study, using the QUADAS-2. QUADAS-2 = quality assessment of diagnostic accuracy studies 2.
3.3. Pooled analysis
Figures 3–5 shows the forest plots for SEN, SPE, PLR, NLR and DOR, and Figure 6 shows the SROC curves. The pooled SEN of US-guided pleural biopsy for the diagnosis of PEs was 83% (95% CI: 75% - 89%; I2 = 25.2%; Cochran Q statistic = 8.02, P = 0.237). Specificities in all studies were 100%, therefore the pooled specificity was 100% (95% CI: 100% - 100%; I2 = 0%; Cochran Q statistic = 0, P = 0). The pooled PLR was 8.89 (95% CI: 3.29 - 24.02; I2 = 0%; Cochran Q statistic = 0.77, P = .993) and the NLR was 0.23 (95% CI: 0.16 - 0.33; I2 = 0%; Cochran Q statistic = 2.86, P = .827). The DOR was 51.47 (95% CI: 14.70 - 180.16; I2 = 0%; Cochran Q statistic = 1.02, P = .985) and the AUC was 0.94. There were negligible noted complications, including 6 pneumothorax cases (3.6%), five local wound infections (3.0%), and 1 empyema case (less than 1%).
Figure 3.
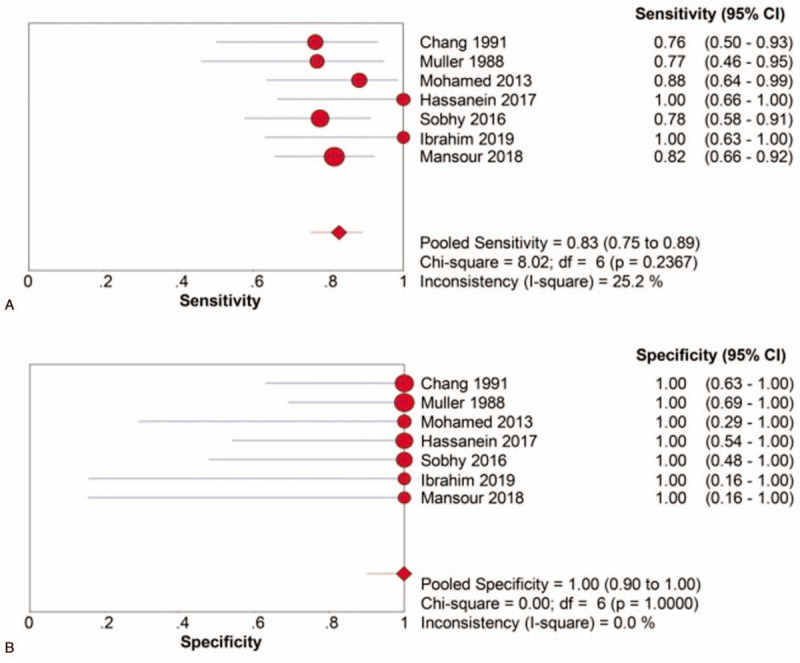
Pooled SEN (a) and SPE (b) of UGNB for the diagnosis of UPEs. SEN = sensitivity, SPE = specificity, UGNB = ultrasound-guided needle biopsy, UPEs = undiagnosed pleural effusions.
Figure 5.
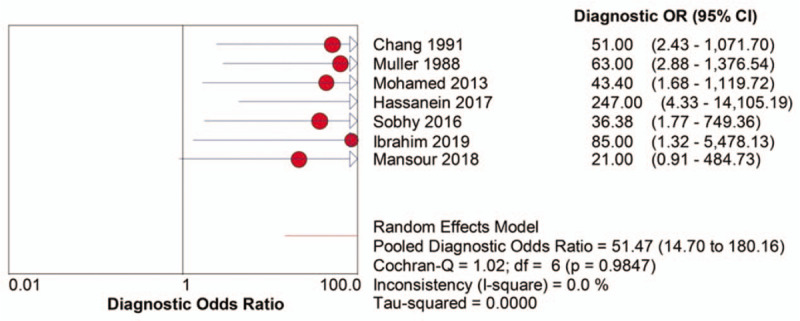
DOR of UGNB for the diagnosis of UPEs. DOR = diagnostic odds rate, UGNB = ultrasound-guided needle biopsy, UPEs = undiagnosed pleural effusions.
Figure 6.
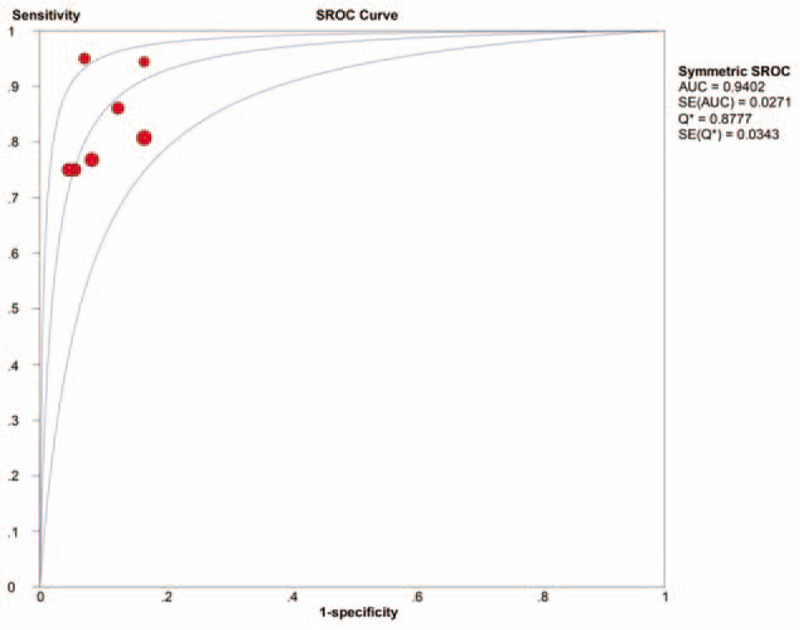
SROC curve of UGNB for the diagnosis of UPEs. AUC = area under the curve, SROC = summary receiver operating characteristic, UGNB = ultrasound-guided needle biopsy, UPEs = undiagnosed pleural effusions.
Figure 4.
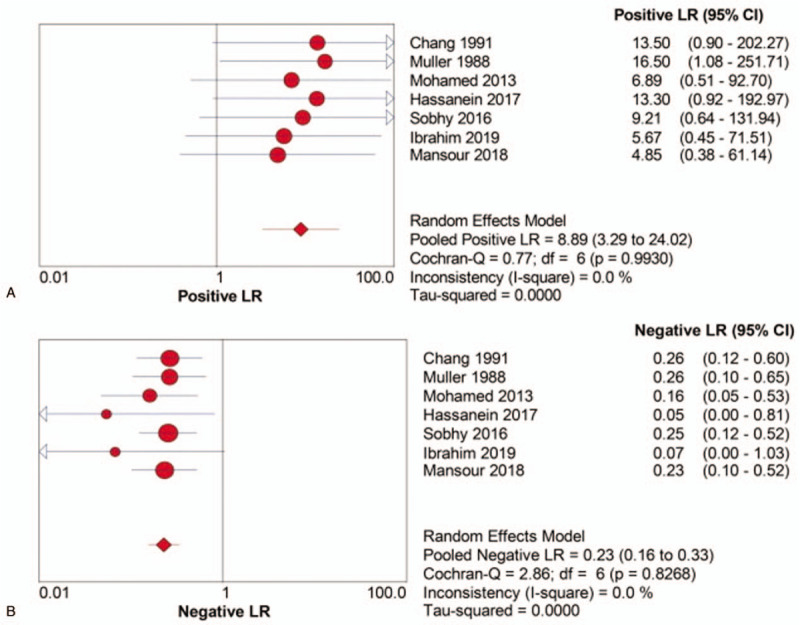
Pooled PLR (a) and NLR (b) of UGNB for the diagnosis of UPEs. NLR = negative likelihood ratio, PLR = positive likelihood ratio, UGNB = ultrasound-guided needle biopsy, UPEs = undiagnosed pleural effusions.
3.4. Publication bias
A Deek's funnel plot is shown in Figure 7 for the assessment of publication bias. No publication bias was observed either on visual examination of the funnel plot or after performance of the Egger test (P = .91).
Figure 7.
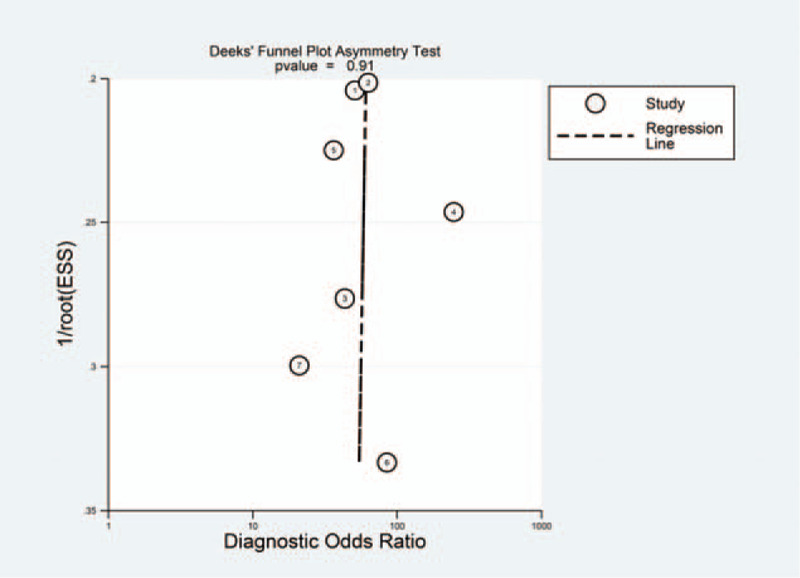
Assessment of publication bias using the Deek's funnel plot.
4. Discussion
Our findings demonstrated that UGNB had a satisfactory pooled sensitivity of 83% (95% CI, 75% - 89%) and a high pooled specificity of 100% (95% CI, 90% - 100%) in UPEs. Both PLR and NLR were used to assess the diagnostic accuracy due to their easiness of interpretation and their meaningful use in clinical practice.[32] The observed pooled PLR was 8.89 (95% CI, 3.29 - 24.02) and the NLR was 0.23 (95% CI, 0.16 - 0.33). A PLR value of 8.89 signifies that patients with PEs of definite aetiology will have an 8.89-fold higher probability of testing positive in the UGNB test compared with those with nonspecific pleuritis. In contrast, an NLR value of 0.23 indicates that despite a negative UGNB test result, the probability that a patient has PE of definite aetiology is approximately 23%. Therefore, the pooled PLR was sufficiently high to present proof for ruling in diagnoses, whereas the pooled NLR was not sufficiently low to rule out diagnoses, especially considering the high prevalence of this disease.[33] Accordingly, physicians should be cautious when interpreting negative UGNB test results.
DOR is a single indicator of test performance combining the strengths of sensitivity and specificity. DOR values range from 0 to infinity and a high DOR value demonstrates a superior test's discriminatory performance.[34] In the present study, a pooled DOR of 51.47 (95% CI, 14.70 - 180.16) was observed, which illustrates that UGNB has a high diagnostic accuracy. A SROC curve was also generated to summarize the global test efficacy from the different diagnostic studies and the AUC value assessed the discriminating capability of the test.[35,36] An AUC value approaching 1 indicates a test with high discrimination. We found that the AUC value was 0.94 in our study, showing that UGNB has a high level of diagnostic performance. The UGNB procedures were generally well tolerated among the included 165 patients. Six pneumothorax cases (3.6%), 5 local wound infections (3.0%), and 1 empyema case (less than 1%) were observed, and there was no mortality, indicating that the procedure is both safe and feasible.
In recent years, US guidance has been increasingly applied for pleural biopsy. UGNB is able to ensure that biopsy samples are obtained from abnormal pleural tissue.[37] US guidance can significantly increase the diagnostic accuracy of ANB and minimize the incidence of complications.[9,10] The present study provided evidence that UGNB is a better technique to blind-closed pleural biopsy for the diagnosis of UPEs.[1,10] Although ANB has the benefits of low cost and ease of accessibility, the sensitivity for malignant pleural effusions is less than 60%.[1,4,8] In a large review including 2893 examinations, the diagnostic rate of ANB was only 57% for malignant disease.[38] However, ANB had a higher sensitivity, ranging from 80% to 87%, for the diagnosis of tuberculosis.[39,40] This is because tuberculosis widely affects the pleura, whereas tumours tend to invade the pleura close to the midline and diaphragm and that area is dangerous to access with the Abrams needle.[1] Moreover, there were complications related to ANB including site pain (up to 15%), pneumothorax (up to 15%) and vasovagal reaction (about 5%). Therefore, ANB was only diagnostically effective in poor resourced areas with high prevalence of tuberculosis.[1]
In the past decades, image-guided pleural biopsies were usually performed under US or CT guidance and previous studies have demonstrated that the 2 different procedures have similar diagnostic yields.[12] The choice between a CT-guided needle biopsy (CGNB) or UGNB depends on the operator's personal expertise and preference.[41] However, UGNB has several advantages over CGNB, including real-time visualization, no exposure to ionizing radiation, good mobility, easy availability and low cost.[9,41] In a retrospective review of 273 biopsies sampling a consecutive series of pleural and peripheral lung lesions, the sample accuracy of UGNB was comparable to CGNB (97.1% vs 96.5%, P = .999). Furthermore, UGNB offers a less mean procedural time (321 seconds vs 556 seconds, P < .001), fewer post-procedural pneumothorax complications (5.8% vs 14.7%, P = .025) and lower cost ($125 for both pleural and peripheral lung lesions vs $185 for pleural lesions and $220 for peripheral lung lesions) when compared to CGNB.[13]
Thoracoscopy is considered the gold standard for the diagnosis of UPEs.[1] Both LAT and VATS have a high diagnostic sensitivity in excess of 90% and a remarkable specificity of 100%, particularly for the diagnosis of tuberculous pleuritis.[1,6,7] This is due to fact that thoracoscopic techniques allow for the direct and clear visualization of pleural lesions and targeted sampling areas.[7] Furthermore, the most important advantage of thoracoscopy over image-guided biopsy is that both diagnostic and therapeutic procedures can be performed simultaneously,[1,42,43] and drainage of the effusion may alleviate symptoms for most patients.[7] Talc poudrage is also commonly used to prevent further PE recurrence.[44] Although LAT is relatively invasive, it has a low incidence of complications and mortality.[41] In a review combining data of 47 studies with 4756 patients who underwent LAT, major complications were reported to occur in 1.8% of cases, minor complications in 7.8% and mortality in 0.34%.[43] VATS is performed by thoracic surgeons and requires general anaesthetic, therefore frail patients are not suitable for this procedure.[1] The incidence of complications was higher in patients who underwent VATS than LAT, with major complications having been reported in up to 15% of cases.[45] However, in a UK centre, major complications were reported in just 1.2% of cases and minor complications in 15.1% in a more recent retrospective study.[46] Thoracoscopy is still described as the gold standard for UPEs, however, in certain contexts, UGNB has clear advantages over thoracoscopy. While LAT and VATS require sufficient amount of PE to separate the visceral and parietal pleura safely, UGNB can be conducted safely in patients with small amounts or no PEs.[6] For LAT, patients must be able to tolerate lying flat or on their side during the procedure for at least 30 minutes. Therefore, uncontrollable cough, poor cardiac and pulmonary functions or other causes may contraindicate the use of LAT.[7] Compared with LAT, UGNB not only can be operated in the sitting position, but also take less procedure time.[41] The equipment for LAT and VATS are not universally applicable due to high cost, particularly in resource-poor areas. Furthermore, thoracoscopy requires physicians to have some degree of capacity and experience to perform the procedure.[10] In contrast, UGNB can be performed in most medical institutions, as it is a simpler, more accessible, faster and less expensive procedure.[9]
The present study had some limitations to consider. First, it may have a publication bias since it selected for articles published in English only. However, this factor was not significant in our meta-analysis. Second, the presence of some degree of methodological weakness in all eligible studies may have led to an overestimated diagnostic accuracy. Different reference standards including various clinical follow-up schedules were used in the included studies and inadequate follow-up may increase the risk of potential false negative results. Due to the invasiveness nature of thoracoscopy, it is difficult to allow for all patients to receive standard tests. Therefore, thoracoscopy was performed only in those with negative UGNB test results, whereas all positive results were recognized as a true positive result. More importantly, 7 studies originated from 3 countries and 165 patients were included in our study and that may have restricted the reliability and generalization of the data. However, validated systematic review methods were used to make our conclusions robust. Last, the type and size of biopsy needles were not limited in the inclusion criteria, though no significant heterogeneity existed among the included studies. This is probably because US guidance is more important than needle selection.
In summary, this study indicated that UGNB is a safe and feasible technique for use in the diagnosis of UPEs, though physicians should still be cautious in interpreting negative UGNB results. Additionally, it is an easily accessible, less invasive, well-tolerated, quick, and radiation-free method to obtain pleural tissue. The diagnosis of UPEs is individualized according to different factors. The choice among different techniques for pleural biopsies cannot be based on simple comparisons of diagnostic accuracy and complications but should take into account several other factors including the patients’ preference, the clinical status of the patient, pleural characteristics, amount of effusion, disease prevalence, regional economy and the level of expertise of the medical staff.
Acknowledgments
We thank all authors who provided published information for this meta-analysis.
Author contributions
Conceptualization: Zhidi Lin.
Data curation: Zhidi Lin, Donghong Wu, Jinlin Wang, Mingkai Huang.
Formal analysis: Zhidi Lin, Donghong Wu, Jinlin Wang, Chuqiao Wang, Mingkai Huang.
Investigation: Zhidi Lin, Jinlin Wang.
Methodology: Zhidi Lin, Donghong Wu, Chuqiao Wang, Mingkai Huang.
Project administration: Zhidi Lin, Donghong Wu, Jinlin Wang.
Resources: Jinlin Wang, Chuqiao Wang.
Software: Zhidi Lin, Donghong Wu, Chuqiao Wang, Mingkai Huang.
Supervision: Zhidi Lin, Jinlin Wang.
Validation: Zhidi Lin, Donghong Wu, Jinlin Wang, Chuqiao Wang, Mingkai Huang.
Visualization: Zhidi Lin, Donghong Wu, Chuqiao Wang, Mingkai Huang.
Writing – original draft: Zhidi Lin, Donghong Wu, Jinlin Wang, Chuqiao Wang, Mingkai Huang.
Writing – review & editing: Zhidi Lin, Donghong Wu, Jinlin Wang, Chuqiao Wang, Mingkai Huang.
Footnotes
Abbreviations: ANB = Abrams needle biopsy, AUC = the area under the curve, CGNB = CT-guided needle biopsy, CIs = confidence intervals, CT = computed tomography, DOR = diagnostic odds rate, LAT = local anaesthetic thoracoscopy, NLR = negative likelihood ratio, PEs = pleural effusions, PLR = positive likelihood ratio, SEN = sensitivity, SPE = specificity, SROC = the summary receiver operating characteristic, UGNB = ultrasound-guided needle biopsy, UPEs = undiagnosed pleural effusions, US = ultrasound, VATS = video-assisted thoracoscopic surgery.
How to cite this article: Lin Z, Wu D, Wang J, Wang C, Huang M. Diagnostic value of ultrasound-guided needle biopsy in undiagnosed pleural effusions: a systematic review and meta-analysis. Medicine. 2020;99:27(e21076).
ZL and DW contributed equally to this work.
Ethics approval and consent to participate: All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Hooper C, Lee YC, Maskell N. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65: Suppl 2: ii4–17. [DOI] [PubMed] [Google Scholar]
- [2].Light RW. Clinical practice. Pleural effusion. N Engl J Med 2002;346:1971–7. [DOI] [PubMed] [Google Scholar]
- [3].Light RW. Pleural effusions. Med Clin North Am 2011;95:1055–70. [DOI] [PubMed] [Google Scholar]
- [4].Porcel JM, Light RW. Pleural effusions. Dis Mon 2013;59:29–57. [DOI] [PubMed] [Google Scholar]
- [5].Porcel JM, Azzopardi M, Koegelenberg CF, et al. The diagnosis of pleural effusions. Expert Rev Respir Med 2015;9:801–15. [DOI] [PubMed] [Google Scholar]
- [6].Rahman NM, Gleeson FV. Image-guided pleural biopsy. Curr Opin Pulm Med 2008;14:331–6. [DOI] [PubMed] [Google Scholar]
- [7].Bibby AC, Maskell NA. Pleural biopsies in undiagnosed pleural effusions; Abrams vs image-guided vs thoracoscopic biopsies. Curr Opin Pulm Med 2016;22:392–8. [DOI] [PubMed] [Google Scholar]
- [8].Whitaker D, Shilkin KB. Diagnosis of pleural malignant mesothelioma in life--a practical approach. J Pathol 1984;143:147–75. [DOI] [PubMed] [Google Scholar]
- [9].Koegelenberg CF, Diacon AH. Image-guided pleural biopsy. Curr Opin Pulm Med 2013;19:368–73. [DOI] [PubMed] [Google Scholar]
- [10].Koegelenberg CF, Diacon AH. Pleural controversy: close needle pleural biopsy or thoracoscopy-which first? Respirology 2011;16:738–46. [DOI] [PubMed] [Google Scholar]
- [11].Koegelenberg CF, Irusen EM, von Groote-Bidlingmaier F, et al. The utility of ultrasound-guided thoracentesis and pleural biopsy in undiagnosed pleural exudates. Thorax 2015;70:995–7. [DOI] [PubMed] [Google Scholar]
- [12].Qureshi NR, Gleeson FV. Imaging of pleural disease. Clin Chest Med 2006;27:193–213. [DOI] [PubMed] [Google Scholar]
- [13].Sconfienza LM, Mauri G, Grossi F, et al. Pleural and peripheral lung lesions: comparison of US- and CT-guided biopsy. Radiology 2013;266:930–5. [DOI] [PubMed] [Google Scholar]
- [14].Blanc FX, Atassi K, Bignon J, et al. Diagnostic value of medical thoracoscopy in pleural disease: a 6-year retrospective study. Chest 2002;121:1677–83. [DOI] [PubMed] [Google Scholar]
- [15].Bibby AC, Dorn P, Psallidas I, et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur Respir J 2018;52:1800349. [DOI] [PubMed] [Google Scholar]
- [16].Chang DB, Yang PC, Luh KT, et al. Ultrasound-guided pleural biopsy with Tru-Cut needle. Chest 1991;100:1328–33. [DOI] [PubMed] [Google Scholar]
- [17].Mueller PR, Saini S, Simeone JF, et al. Image-guided pleural biopsies: indications, technique, and results in 23 patients. Radiology 1988;169:1–4. [DOI] [PubMed] [Google Scholar]
- [18].Wrightson JM, Fysh E, Maskell NA, et al. Risk reduction in pleural procedures: sonography, simulation and supervision. Curr Opin Pulm Med 2010;16:340–50. [DOI] [PubMed] [Google Scholar]
- [19].Yang PC. Ultrasound-guided transthoracic biopsy of peripheral lung, pleural, and chest-wall lesions. J Thorac Imaging 1997;12:272–84. [DOI] [PubMed] [Google Scholar]
- [20].Koegelenberg CF, von Groote-Bidlingmaier F, Bolliger CT. Transthoracic ultrasonography for the respiratory physician. Respiration 2012;84:337–50. [DOI] [PubMed] [Google Scholar]
- [21].Diacon AH, Schuurmans MM, Theron J, et al. Safety and yield of ultrasound-assisted transthoracic biopsy performed by pulmonologists. Respiration 2004;71:519–22. [DOI] [PubMed] [Google Scholar]
- [22].Koegelenberg CF, Bolliger CT, Theron J, et al. Direct comparison of the diagnostic yield of ultrasound-assisted Abrams and Tru-Cut needle biopsies for pleural tuberculosis. Thorax 2010;65:857–62. [DOI] [PubMed] [Google Scholar]
- [23].Adams RF, Gleeson FV. Percutaneous image-guided cutting-needle biopsy of the pleura in the presence of a suspected malignant effusion. Radiology 2001;219:510–4. [DOI] [PubMed] [Google Scholar]
- [24].Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–36. [DOI] [PubMed] [Google Scholar]
- [25].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [26].Zamora J, Abraira V, Muriel A, et al. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mohamed EE, Talaat IM, Abd Alla AEDA, et al. Diagnosis of exudative pleural effusion using ultrasound guided versus medical thoracoscopic pleural biopsy. Egypt J Chest Dis Tuberc 2013;62:607–15. [Google Scholar]
- [28].Hassanein EG, El Ganady AA, El Hoshy MS, et al. Comparative study between the use of image guided pleural biopsy using abram's needle and medical thoracoscope in diagnosis of exudative pleural effusion. Egyptian Journal of Chest Diseases and Tuberculosis 2017;66:435–40. [Google Scholar]
- [29].Sobhy K, Kamel K, Ahmed S, et al. Ultrasound guided closed pleural biopsy versus medical thoracoscopic pleural biopsy in diagnosis of pleural diseases. Egyptian Journal of Chest Diseases and Tuberculosis 2017;66:97–106. [Google Scholar]
- [30].Ibrahim E, Daabis R, Abdallah A, et al. Medical thoracoscopy versus image-guided pleural biopsy for diagnosing pleural diseases. Egyptian Journal of Chest Diseases and Tuberculosis 2018;67:79–86. [Google Scholar]
- [31].Mansour OF, Abd-Aziz AA, El-Habashy MM, et al. Comparison between ultrasound-guided Abrams needle and medical thoracoscopic pleural biopsies in exudative pleural effusion. Egyptian Journal of Chest Diseases and Tuberculosis 2018;67:93–8. [Google Scholar]
- [32].Deville WL, Buntinx F, Bouter LM, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol 2002;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ 2004;329:168–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Glas AS, Lijmer JG, Prins MH, et al. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol 2003;56:1129–35. [DOI] [PubMed] [Google Scholar]
- [35].Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982;143:29–36. [DOI] [PubMed] [Google Scholar]
- [36].Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med 1993;12:1293–316. [DOI] [PubMed] [Google Scholar]
- [37].Wang J, Zhou X, Xie X, et al. Combined ultrasound-guided cutting-needle biopsy and standard pleural biopsy for diagnosis of malignant pleural effusions. BMC Pulm Med 2016;16:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tomlinson J, Sahn S. Invasive Procedures in the Diagnosis of Pleural Disease. Seminars in Respiratory and Critical Care Medicine - SEMIN RESPIR CRIT CARE MED 1987;9:30–6. [Google Scholar]
- [39].Vorster MJ, Allwood BW, Diacon AH, et al. Tuberculous pleural effusions: advances and controversies. J Thorac Dis 2015;7:981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Diacon AH, Van de Wal BW, Wyser C, et al. Diagnostic tools in tuberculous pleurisy: a direct comparative study. Eur Respir J 2003;22:589–91. [DOI] [PubMed] [Google Scholar]
- [41].Dixon G, de Fonseka D, Maskell N. Pleural controversies: image guided biopsy vs. thoracoscopy for undiagnosed pleural effusions? J Thorac Dis 2015;7:1041–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Harris RJ, Kavuru MS, Rice TW, et al. The diagnostic and therapeutic utility of thoracoscopy. A review. Chest 1995;108:828–41. [DOI] [PubMed] [Google Scholar]
- [43].Rahman NM, Ali NJ, Brown G, et al. Local anaesthetic thoracoscopy: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65: Suppl 2: ii54–60. [DOI] [PubMed] [Google Scholar]
- [44].Dresler CM, Olak J, Herndon JE, 2nd, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest 2005;127:909–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Harris RJ, Kavuru MS, Mehta AC, et al. The impact of thoracoscopy on the management of pleural disease. Chest 1995;107:845–52. [DOI] [PubMed] [Google Scholar]
- [46].Medford AR, Awan YM, Marchbank A, et al. Diagnostic and therapeutic performance of video-assisted thoracoscopic surgery (VATS) in investigation and management of pleural exudates. Ann R Coll Surg Engl 2008;90:597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]


