Abstract
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide; its morbidity and mortality have both recently increased. Lately, the role played by the neutrophil-lymphocyte ratio (NLR) in the development of HCC has attracted attention. However, the exact relationship is not fully understood.
A total of 538 participants diagnosed with HCC were recruited between 2010 and 2018. Their relevant routine blood parameters were measured, including NLR. Pearson Chi-Squared test, Spearman Rho test, and logistic regression analysis were performed to explore any correlations between NLR and HCC. A receiver operating characteristic (ROC) curve analysis was performed to determine the usefulness of NLR for predicting HCC. Univariate and multivariate Cox regression analysis for relevant routine blood parameters and any relationships with overall survival (OS) were performed. The Kaplan–Meier method was used to explore any further relationships with OS.
NLR was significantly correlated with HCC tumor size by Pearson Chi-Squared test (P = .008). Furthermore, Spearman correlation coefficient showed that HCC tumor size was significantly correlated with NLR (P = .115, P = .008). NLR could sensitively and specifically predict HCC tumor size (area under the curve [AUC], 0.605; 95% confidence interval [CI], 0.429–0.743; P = .000). Higher NLR in patients with HCC was correlated with better OS (hazard ratio [HR] = 0.584; P = .000).
A close correlation existed between increased NLR and HCC; NLR could sensitively and specifically predict HCC. High NLR might be an independent protective factor in the prognosis of patients with HCC.
Keywords: hepatocellular carcinoma, inflammation, neutrophil–lymphocyte ratio, overall survival, tumor size
1. Introduction
Hepatocellular carcinoma (HCC) is one of the more common malignant tumors worldwide. In 2018, the global cancer statistics report indicated that there were 841,000 new cases of HCC and 781,000 deaths due to this disease around the world, with nearly half accounted for in China.[1] Due to the occult onset of HCC and the absence of obvious symptoms during the early stages, HCC is usually diagnosed in the middle or late stages, with poor prognosis, which is a serious threat to peoples health. Currently, the most important treatments for HCC are surgical resection and liver transplantation, but due to the high recurrence and metastasis rates of postoperative tumors, long-term survival results remain unsatisfactory. Recent studies have shown that patients with HCC suffer from long-term chronic inflammatory stimuli; this led to the concept of cancer-associated inflammation being proposed.[2–4]
The neutrophil–lymphocyte ratio (NLR) has been recognized as an effective indicator of systemic inflammatory responses. There is some correlation between NLR and blood inflammatory-factor levels. Evidence from studies into tumor immunity and the underlying molecular mechanisms suggests that the inflammatory response is an important factor that can affect the prognosis of patients with tumors.[5] Increased neutrophil counts and/or decreased lymphocyte counts lead to an increased NLR. At present, the mechanism of how neutrophils are involved in malignant tumor progression is not clear. Some scholars believe that neutrophils are often distributed in the tissues surrounding tumors, where they secrete large quantities of vascular endothelial growth factor, thus providing an appropriate microenvironment for the promotion of local tumor invasion and metastasis.[6] Studies have shown that an elevated NLR is closely associated with poor prognoses in patients with HCC.[7] However, Li and colleagues[8] proposed the concept of “manufacturing inflammation”, that is, the development of low-grade inflammation into anti-tumor response inflammation. An increased number of neutrophils lead to an increased NLR; as a consequence, the inflammatory response is enhanced, which then activates the immune system. These activated immune cells could further kill tumor cells and play an anti-cancer role.
In the present study, we collected clinical prognostic information and routine blood index data of patients with hepatocellular carcinoma. We then used Pearson Chi-Squared test, Spearman correlation coefficient, logistic regression analysis, receiver operating characteristic (ROC) curve analysis, Cox proportional regression analysis, and the Kaplan–Meier method to explore correlations between NLR and HCC, and to evaluate the usefulness of NLR for judging the prognosis of patients with HCC.
2. Methods
2.1. Ethics and informed consent
This research conformed to the Declaration of Helsinki, and it was authorized by the Human Ethics and Research Ethics Committees of the Fourth Hospital of Hebei Medical University, China. The written informed consent was obtained from all participants.
2.2. Recruitment of participants
Pathological sections were obtained from participants who attended the Fourth Hospital of Hebei Medical University between 2010 and 2018; from pathological reports, it was determined that there was a total of 538 participants who were diagnosed with HCC. The inclusion criteria were patients aged 20 to 80 years, with histologically confirmed HCC, who had not received treatment for tumors, and who had no history of surgery. Exclusion criteria were aged <20 years or >80 years, a combination of other malignant tumors, an operation more than 1 month since their last examination, and severe heart disease. Basic histopathological and clinical parameters were collected. All participants were followed-up to obtain the overall survival (OS) information for all participants. Participates were divided into groups according to their sex, age, tumor size (<5 cm vs ≥5 cm), and overall survival.
2.3. Detection of relevant routine blood parameters
Blood samples were obtained from all participants; 0.4 ml blood from each patient was stored in an EDTA anticoagulant tube. A full blood count analyzer (Beckman, USA) was used to measure relevant parameters, including white blood cell count, monocyte count, neutrophil count, lymphocyte count, blood platelet count, platelet–lymphocyte ratio, and NLR. We calculated the average value of each cell count from the 538 participants diagnosed with HCC. If the cell count of a participant was less than or equal to the average value, we defined it as “low”; when the cell count of a participant was more than the average value, we defined it as “high”.
2.4. Statistical analysis
The data were expressed as percentages of the totals and means ± SD. Pearson Chi-Squared test was used to explore associations between the underlying routine blood parameters and HCC tumor size. The Spearman Rho test was used to compare HCC tumor size with the relevant routine blood parameters for correlation analysis. Univariate logistic regression analysis was used to calculate the odds ratios (ORs) of each variable for HCC tumor size. A receiver operating characteristic (ROC) curve analysis was performed to determine the usefulness of the relevant routine blood parameters in predicting tumor size.
The follow-up period ended when the study period ended (December 2018), or if a participant had died. Overall survival (OS) of a participant defined as the time from the earliest date of diagnosis to the date of death due to HCC. Univariate and multivariate Cox regression analysis for relevant routine blood parameters with OS were performed. The Kaplan–Meier method was used to conduct further analyses.
All statistical analyses were performed using SPSS software, version 21.0 (IBM Corp., USA). A P value <.05 was considered to be statistically significant.
3. Results
3.1. Associations between routine blood parameters and tumor size based on the Chi-Squared test
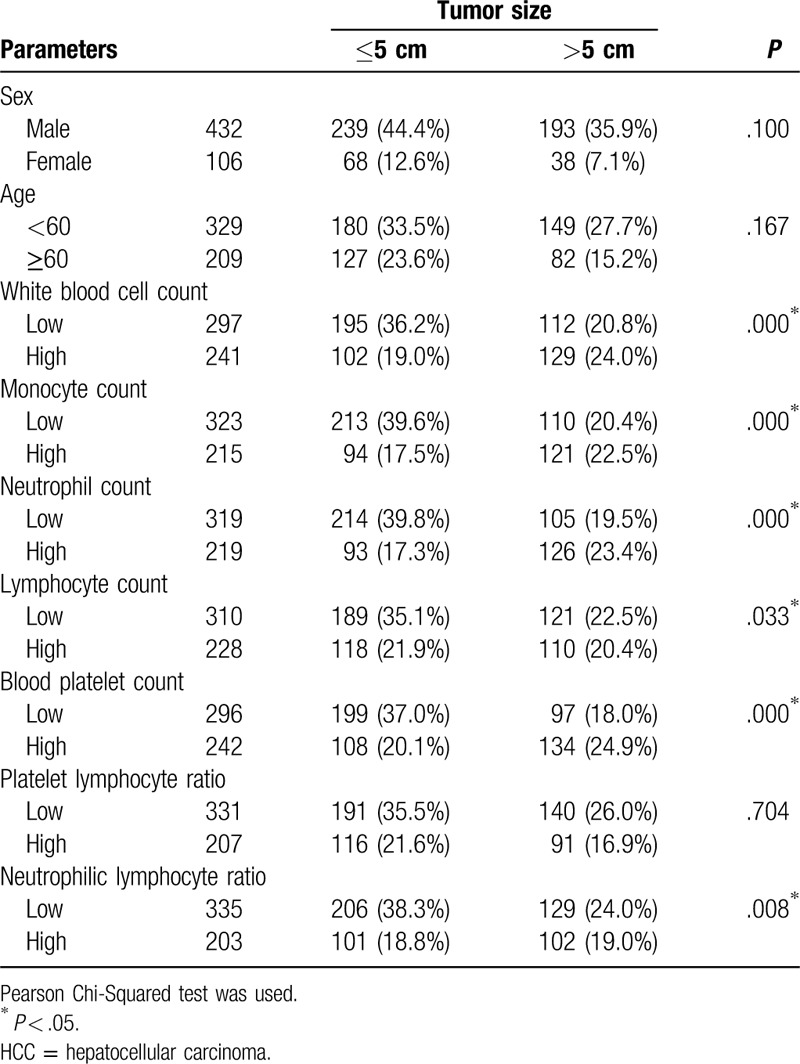
The results of Pearson Chi-Squared test summarized any associations between relevant routine blood parameters and HCC tumor size. Among the participants, it was found that the white blood cell count (P = .000), monocyte count (P = .000), neutrophil count (P = .000), lymphocyte count (P = .033), blood platelet count (P = .000), and NLR (P = .008) were all significantly correlated with HCC tumor size. However, no significant associations were found between tumor size and sex (P = .100), age (P = .167), or platelet–lymphocyte ratio (P = .704) (Table 1).
Table 1.
Relevant parameters of blood routine and the tumor size of HCC.
3.2. Further associations by Spearman correlation test
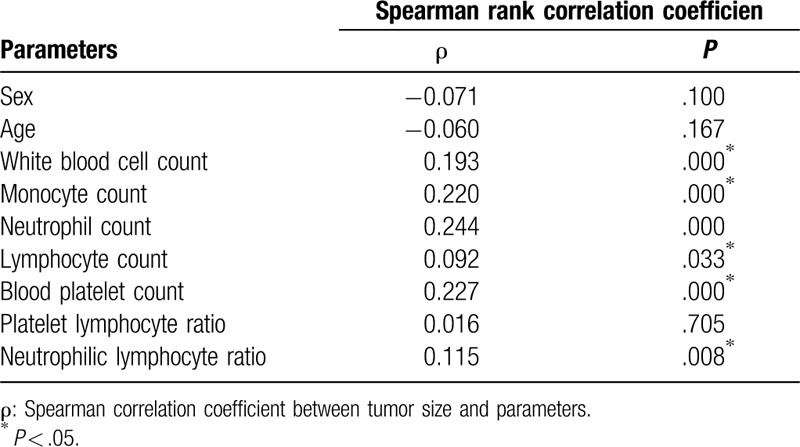
To confirm whether blood factor characteristics played an important role relating to tumor size, a further correlation analysis was performed. Spearman correlation coefficient showed that HCC tumor size was significantly correlated with the white blood cell count (P = .193, P = .000), monocyte count (P = .220, P = .000), neutrophil count (P = .244, P = .000), lymphocyte count (P = .092, P = .033), blood platelet count (P = .227, P = .000), and NLR (P = .115, P = .008). However, there were no significant correlations between any of the other factors and tumor size (Table 2).
Table 2.
Associations between tumor size of HCC and relevant parameters of blood routine.
3.3. Univariate logistic regression for proportional hazard analysis of blood parameters and their relationship with tumor size
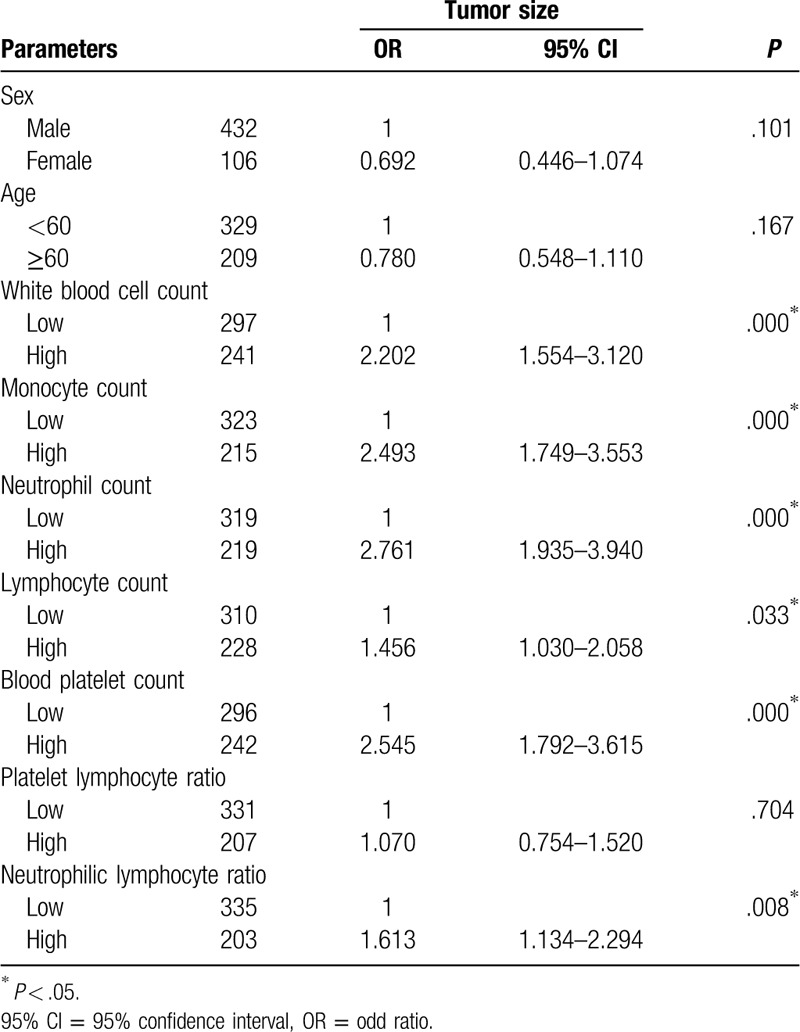
The univariate ORs and 95% confidence intervals (95%CI) for tumor size in participants were determined. The OR for tumor size was 2.202 (95%CI, 1.554–3.120, P = .000) in the group with a high white blood cell count compared with that in the group with a low white blood cell count. For tumor size, participants with a high monocyte count had a higher OR of 2.493 (95%CI, 1.749–3.553, P = .000) than participants with a low monocyte count. Participants who had a high neutrophil count had a clearly larger tumor size than participants who had a low neutrophil count (OR 2.761, 95%CI, 1.935–3.940, P = .000). The OR for tumor size was 1.456 (95%CI, 1.030–2.058, P = .033) in the group with high a lymphocyte count compared with that in the group with a low lymphocyte count. For tumor size, participants with a high blood platelet count had a higher OR, of 2.545 (95%CI, 1.792–3.615, P = .000), than participants with a low blood platelet count. Participants who had a high NLR exhibited a clearly larger tumor size than participants who had a low NLR (OR, 1.613, 95%CI, 1.134–2.294, P = .008) (Table 3).
Table 3.
Correlative parameters effect on tumor size based on univariate logistic regression analysis.
3.4. ROC curves can sensitively and specifically predict HCC tumor size
We constructed ROC curves to identify an accurate threshold for the use of blood parameters in predicting tumor size. White blood cell count was most closely associated with a higher risk of tumor size (area under the curve [AUC], 0.671; 95%CI, 0.506–0.743; P = .000) (Fig. 1A); followed by monocyte count (AUC, 0.653; 95%CI, 0.619–0.629; P = .000) (Fig. 1B); neutrophil count (AUC, 0.685; 95%CI, 0.606–0.661; P = .000) (Fig. 1C); lymphocyte count (AUC, 0.555; 95%CI, 0.417–0.701; P = .029) (Fig. 1D); blood platelet count (AUC, 0.686; 95%CI, 0.385–0.883; P = .000) (Fig. 1E); and NLR (AUC, 0.605; 95%CI, 0.429–0.743; P = .000) (Fig. 1F).
Figure 1.
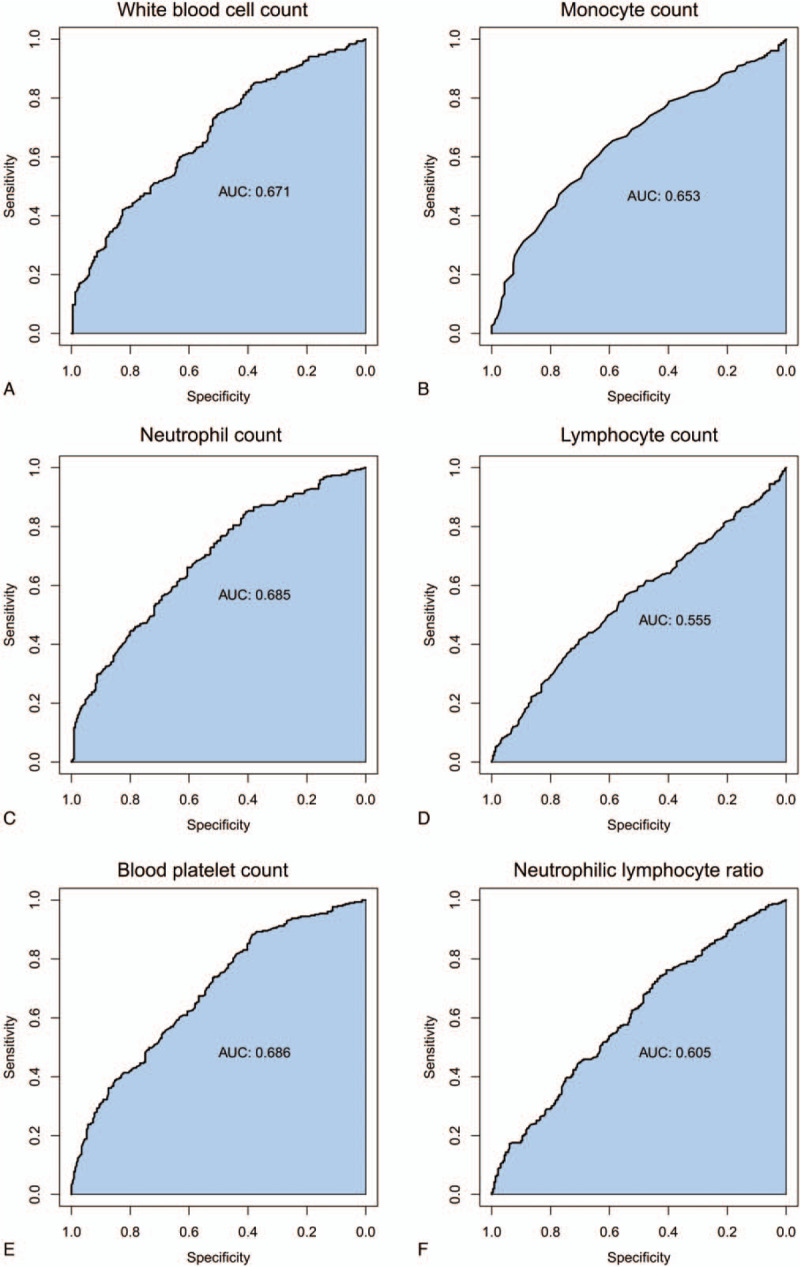
Receiver operating characteristic (ROC) curve of the predictive value between relevant routine blood parameters and HCC tumor size. (A) The ROC curve of white blood cell count for tumor size. (B) The ROC curve of monocyte count. (C) The ROC curve of neutrophil count. (D) The ROC curve of lymphocyte count. (E) The ROC curve of blood platelet count. (F) The ROC curve of neutrophil-lymphocyte ratio (NLR).
3.5. Higher NLR in patients with HCC was correlated with better OS
Univariate Cox analyses were performed and Kaplan–Meier curves for OS were determined. The white blood cell count, monocyte count, neutrophil count, lymphocyte count, blood platelet count, platelet–lymphocyte ratio, and NLR were not associated with the OS of HCC patients based on univariate Cox analyses and Kaplan–Meier curves (Table 4, Fig. 2A-E). A higher NLR was predictive of a better OS (HR = 0.584; P = .000) (Table 4 and Fig. 2F).
Table 4.
Correlative parameters effect on OS based on univariate Cox proportional regression analysis.
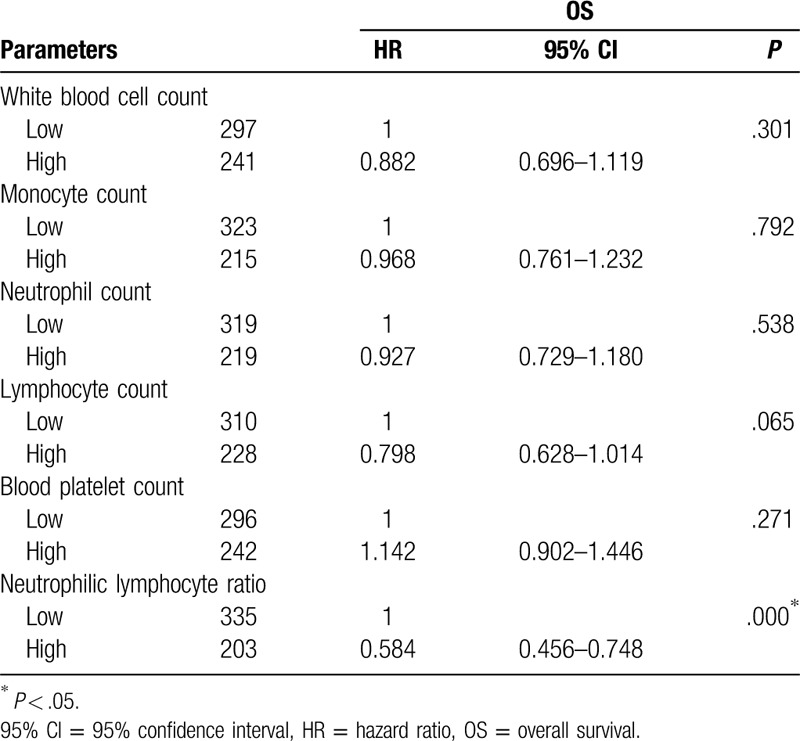
Figure 2.
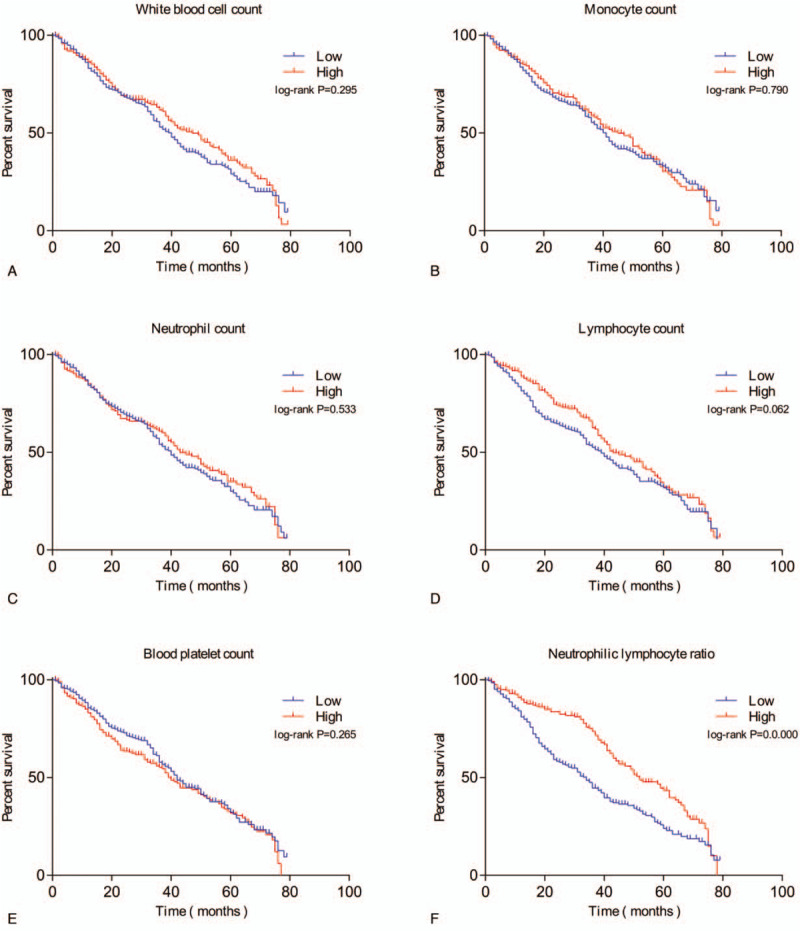
Overall survival (OS) curves of individuals with HCC. (A) Association of OS with white blood cell count. (B) Association of OS with monocyte count. (C) Association of OS with neutrophil count. (D) Association of OS with lymphocyte count. (E) Association of OS with blood platelet count. (F) Association of OS with neutrophil-lymphocyte ratio (NLR). P values were determined by comparing survival distributions using the log-rank test.
3.6. NLR is an independent prognostic factor for HCC
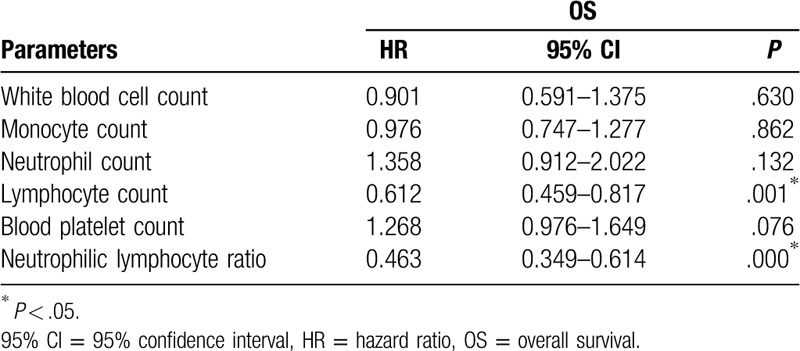
Multivariate Cox regression analyses were performed and the results are shown in Table 5. NLR (P = .000) was a significant independent prognostic factor for OS. Individuals with HCC who had a higher NLR had a significantly better prognosis (P = .000) (Table 5).
Table 5.
Correlative parameters effect on OS based on multivariate Cox proportional regression analysis.
4. Discussion
The results of this research showed that, based on a correlation analysis and a logistic regression analysis, HCC tumor size was significantly correlated with white blood cell count, monocyte count, neutrophil count, lymphocyte count, blood platelet count, platelet–lymphocyte ratio, and NLR. Furthermore, these parameters could sensitively and specifically predict tumor size. However, based on an overall survival analysis, only NLR was correlated with HCC prognosis, with a higher NLR in patients with HCC being correlated with improved OS.
HCC is one of the most common malignant tumors worldwide, and its morbidity and mortality have both been increasing year on year. Recently, the role played by chronic inflammation in the development of HCC has been attracting an increasing level of attention.[9,10]
Contrary to the results of the present study, previous studies have shown that elevated NLR could indicate poor prognosis in patients with HCC.[11] The reasons for this were mainly as follows. First, activated inflammatory cells, such as neutrophils and macrophages, could promote DNA damage in proliferating cells, through the production of reactive oxygen and nitrogen species. Continuous stimulation leading to chronic inflammation could result in the body developing immune tolerance, meaning that mutated cells may not be recognized and cleared in a timely fashion.[12,13] Tissue inflammatory responses exist prior to the malignant transformation of solid tumors,[14] and such inflammatory responses could provide an appropriate microenvironment for the occurrence, development, and metastasis of tumors.[15] Second, neutrophils generated by inflammatory reactions are important mediators in the process of tumor development, promoting the occurrence and development of tumors by secreting angiogenic factors, tumor necrosis factor (TNF), interleukin 4 (IL4), and other cytokines.[16] In addition, neutrophils could also inhibit the activity of T lymphocytes by producing nitric oxide, reactive oxygen species, and arginase, thereby weakening the T lymphocyte-based immune response. Furthermore, lymphocytes can destroy tumors by activating the bodys immune system, while a decrease in the number of lymphocytes could lead to the occurrence and development of tumors.[17]
However, the current study suggested that a higher NLR in patients with HCC was correlated with better OS. We consider there to be a few reasons for this. First, from the perspective of neutrophil function, neutrophils have chemotactic, phagocytosis, and bactericidal effects. Their cytoplasm contains a large number of fine particles that are neither basophilic nor acidophilic.[18,19] Most of these particles are lysosomes, which contain abundant enzymes, such as myeloperoxidase, lysozyme, alkaline phosphatase, and acid hydrolase, all of which play a role in cell phagocytosis and digestion.[20] Neutrophils engulf abnormal structures in the body, including dead tissue, foreign bodies, and mutated cells. When HCC cells undergo excessive proliferation, accompanied by an inflammatory response, neutrophils migrate from the blood vessels to the tissues surrounding the blood vessels to eliminate inflammatory factors, such as mutant cancer cells.[10]
Second, the elevation of neutrophil levels is closely related to the promotion of γδT cells. γδT cells do not require specific antigen stimulation to recognize antigens and are not restricted by the major histocompatibility complex. They function to connect natural immunity with adaptive immunity, providing anti-tumor immunity, and are powerful tools used for tumor cell immunotherapy. Activated γδT cells exert cytotoxic effects on tumor cells through a variety of pathways and show good anti-tumor effects, both in vivo and in vitro.[21] γδT cells also play an indirect anti-tumor role by producing inflammatory cytokines, such as IFN and TNF; by promoting dendritic cell maturation, which enhances cytotoxicity and immune regulation; and by inducing NK cell-mediated anti-tumor cytotoxicity.[22] γδT cell immunotherapy represents a promising application as a novel type of tumor immunotherapy.
Third, neutrophils are an important component of the tumor microenvironment. The extracellular matrix is composed of collagen, proteoglycan, and other soluble factors, which may assist in anti-tumor immune responses.[23] It is now increasingly believed that cancer cells do not work alone, but interact closely with the extracellular matrix, stromal cells, and immune cells, to co-form the tumor microenvironment, thereby promoting chronic inflammation, immunosuppression, and an microenvironment that promotes angiogenesis. The importance of the immune system in protecting the body from internal threats had been described as the cancer–immunity cycle.[24,25] The cycle includes the stimulation of the inflammatory response during the process of tumor formation, the release of antigens, an increase in the number of neutrophils, and the capture of antigen-presenting cells, as well as the activation of cytotoxic T lymphocytes. T lymphocytes then migrate to and permeate a tumor to kill cancer cells.
However, we accept and acknowledge that this study has some shortcomings. First, no animal experiments were conducted to verify the correlation between NLR and HCC. Second, a cross-sectional study design was used, which cannot effectively prove a causal relationship between NRL and HCC, and further studies are needed to clarify the role of NLR as a protective factor in HCC.
5. Conclusions
In summary, this study confirmed the close correlation between increased NLR and HCC. NLR could sensitively and specifically predict HCC. A high NLR was an independent protective factor in the prognosis of patients with HCC. As a simple biomarker in the blood, NLR could provide a better guide for clinicians when evaluating the prognosis of patients with HCC and making clinical treatment decisions.
Acknowledgments
We are thankful to all participants in this study as well as the staff from the long-term care facilities for their cooperation. We also wish to thank Qing-qing Wang for providing statistical assistance and suggestions during the submission process. The authors wrote the manuscript themselves; the English language was subsequently edited by a professional scientific editor.
Author contributions
Sheng-chao Li performed the investigation and was a major contributor toward the writing and submission of the manuscript. Yu-ming Jia made a substantial contribution to conceptualizing the research. She also designed a draft of the research process. Zhuo Xu was involved in developing the intervention and the study protocol. He also provided support for the avoidance of confounding factors in the study design. Yan-li Deng critically revised the manuscript for important intellectual content. Ya-ni Wang modified the manuscript format, discussed reviewer opinions, and clarified the professional terms. She also analyzed the data. All authors read and approved the final manuscript.
Author contributions
Conceptualization: Sheng-chao Li.
Data curation: Zhuo Xu.
Formal analysis: Yan-li Deng.
Investigation: Ya-ni Wang.
Methodology: Yu-ming Jia.
Project administration: Yu-ming Jia.
Resources: Zhuo Xu.
Software: Ya-li Deng.
Validation: Ya-ni Wang.
Visualization: Sheng-chao Li.
Writing – original draft: Sheng-chao Li.
Writing – review & editing: Yu-ming Jia.
Footnotes
Abbreviations: AUC = area under the curve, CI = confidence interval, HCC = Hepatocellular carcinoma, HR = hazard ratio, IL4 = interleukin 4, NLR = neutrophil-lymphocyte ratio, ORs = odds ratios, OS = overall survival, ROC = receiver operating characteristic, TNF = tumor necrosis factor.
How to cite this article: Li Sc, Xu Z, Deng Yl, Wang Yn, Jia Ym. Higher neutrophil-lymphocyte ratio is associated with better prognosis of hepatocellular carcinoma. Medicine. 2020;99:27(e20919).
The authors declare no conflict of interest.
The study funders played no role in the design of the study; in the collection, analysis, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Hasche D, AUID- Oho, Stephan S, et al. The interplay of UV and cutaneous papillomavirus infection in skin cancer development. PLoS Pathog 2017;13:e1006723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Liao CP, Booker RC, Brosseau JP, et al. Contributions of inflammation and tumor microenvironment to neurofibroma tumorigenesis. J Clin Invest 2018;128:2848–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Venneman K, Huybrechts I, AUID- Oho, et al. The epidemiology of Helicobacter pylori infection in Europe and the impact of lifestyle on its natural evolution toward stomach cancer after infection: a systematic review. Helicobacter 2018;23:e12483. [DOI] [PubMed] [Google Scholar]
- [5].Li XH, Chang H, Xu BQ, et al. An inflammatory biomarker-based nomogram to predict prognosis of patients with nasopharyngeal carcinoma: an analysis of a prospective study. Cancer Med 2017;6:310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mizuno R, Kawada K, A.O., et al. The role of tumor-associated neutrophils in colorectal cancer. Int J Mol Sci 2019;20.0000-0003-4336-6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yang HJ, Guo Z, Yang YT, et al. Blood neutrophil-lymphocyte ratio predicts survival after hepatectomy for hepatocellular carcinoma: a propensity score-based analysis. World J Gastroenterol 2016;22:5088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li X, Shao C, Shi Y, et al. Lessons learned from the blockade of immune checkpoints in cancer immunotherapy. J Hematol Oncol 2018;11:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Markowitz GJ, Yang P, Fu J, et al. Inflammation-dependent il18 signaling restricts hepatocellular carcinoma growth by enhancing the accumulation and activity of tumor-infiltrating lymphocytes. Cancer Res 2016;76:2394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].van der Windt DJ, Sud V, Zhang H, et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology 2018;68:1347–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang Y, Peng C, Cheng Z, et al. The prognostic significance of preoperative neutrophil-lymphocyte ratio in patients with hepatocellular carcinoma receiving hepatectomy: a systematic review and meta-analysis. Int J Surg 2018;55:73–80. [DOI] [PubMed] [Google Scholar]
- [12].Rogovskii VS. The linkage between inflammation and immune tolerance: interfering with inflammation in cancer. Curr Cancer Drug Targets 2017;17:325–32. [DOI] [PubMed] [Google Scholar]
- [13].Skopelja-Gardner S, Jones JD, WFC R. NETtling” the host: breaking of tolerance in chronic inflammation and chronic infection. J Autoimmun 2018;88:1–0. [DOI] [PubMed] [Google Scholar]
- [14].Zhou L, Rui JA, Wang SB, et al. Prognostic factors of solitary large hepatocellular carcinoma: the importance of differentiation grade. Eur J Surg Oncol 2011;37:521–5. [DOI] [PubMed] [Google Scholar]
- [15].Oh BS, Jang JW, Kwon JH, et al. Prognostic value of C-reactive protein and neutrophil-to-lymphocyte ratio in patients with hepatocellular carcinoma. BMC Cancer 2013;13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Keizman D, Ish-Shalom M, Huang P, et al. The association of pre-treatment neutrophil to lymphocyte ratio with response rate, progression free survival and overall survival of patients treated with sunitinib for metastatic renal cell carcinoma. Eur J Cancer 2012;48:202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Martinez-Lostao L, Anel A, Pardo J. How do cytotoxic lymphocytes kill cancer cells. Clin Cancer Res 2015;21:5047–56. [DOI] [PubMed] [Google Scholar]
- [18].Carvalho LO, Aquino EN, Neves AC, et al. The neutrophil nucleus and its role in neutrophilic function. J Cell Biochem 2015;116:1831–6. [DOI] [PubMed] [Google Scholar]
- [19].Kubes P. The enigmatic neutrophil: what we do not know. Cell Tissue Res 2018;371:399–406. [DOI] [PubMed] [Google Scholar]
- [20].Bening U, Castino R, Harth N, et al. Lysosomal segregation of a mannose-rich glycoprotein imparted by the prosequence of myeloperoxidase. J Cell Biochem 1998;71:158–68. [DOI] [PubMed] [Google Scholar]
- [21].Jin C, Lagoudas GK, Zhao C, et al. Commensal microbiota promote lung cancer development via gammadelta t cells. Cell 2019;176:998–1013. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Van Acker HH, Anguille S, Van Tendeloo VF, et al. Empowering gamma delta T cells with antitumor immunity by dendritic cell-based immunotherapy. Oncoimmunology 2015;4:e1021538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012;21:309–22. [DOI] [PubMed] [Google Scholar]
- [24].Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39:1–0. [DOI] [PubMed] [Google Scholar]
- [25].Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017;541:321–30. [DOI] [PubMed] [Google Scholar]


