Abstract
Background:
The serum albumin, albumin-to-globulin ratio (AGR), and prognostic nutritional index (PNI) have been recommended to represent the nutritional and inflammatory status. Thus, they may be potential prognostic biomarkers for cancer. However, contradictory results were reported in different studies on glioma. The goal of this study was to perform a meta-analysis to re-evaluate their prognostic potential for glioma.
Methods:
Databases of PubMed, EMBASE, and Cochrane Library were systematically searched to enroll all the studies investigating the prognostic significance of albumin, AGR, and PNI for glioma. Pooled hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using STATA 13.0 software to indicate the intensity of association.
Results:
Eleven studies with 2928 cases were included. Overall meta-analysis showed that the prognostic values of albumin, AGR, and PNI were limited for glioma (P > .05). However, subgroup analysis demonstrated a high preoperative serum albumin was significantly related with excellent OS of patients with GBM (HR = 0.95, 95% CI: 0.91–0.99, P = .018), while high PNI (HR = 0.56, 95% CI: 0.43–0.73, P < .001) and AGR (HR = 0.57, 95% CI: 0.34–0.96, P = .034) may be a protective factor of favorable OS for patients with high-grade gliomas. Furthermore, integration of all studies with multivariate analysis and clear cut-off also proved reduced preoperative serum albumin, AGR, and PNI were predictors of poor prognosis for patients with gliomas.
Conclusion:
Preoperative serum albumin, AGR, and PNI may represent promising biomarkers to predict the prognosis in patients with glioma, especially for high-grade.
Keywords: albumin, albumin-to-globulin ratio, glioma, prognosis, prognostic nutritional index
1. Introduction
Glioma is one of the most common primary central nervous system tumors, with age-adjusted annual incidence of 7.3 cases/100,000 persons.[1] Clinically, gliomas are subdivided into 4 grades, with the malignant degree gradually increasing from grade I to grade IV (glioblastoma multiforme, GBM). Despite advance has been made in the treatment of glioma (including radical surgery, radiotherapy, and chemotherapy), some patients still face a poor prognosis, especially for the high-grade population [the 5-year overall survival (OS) rate: grade I, 82%; grade II, 54%; grade III, 22%; and grade IV, 3%].[1] Therefore, it is very important to early identify patients who may have an unfavorable outcome and then schedule individualized treatment regimens for them in order to further improve OS, in which exploring an effective prognostic biomarker is the first demand.
There is increasing evidence to suggest preoperative malnutrition and inflammatory status are associated with the OS of patients with cancer.[2–4] Albumin synthesized by the liver is a major component (accounting for 50%–65%) of the total protein in human serum and thus serum albumin has been recommended as an indicator to represent the nutritional status of patients.[5] Patients with hypoalbuminemia were considered to be malnourished.[5] Furthermore, supplementation of long-term high-dose albumin was found to reduce the levels of inflammatory cytokines [interleukin (IL)-6, granulocyte colony-stimulating factor, interleukin 1 receptor antagonist, and vascular endothelial growth factor] in patients with decompensated cirrhosis[6]; in vitro albumin dialysis experiments also demonstrated albumin added to the dialysate solution induced a significant decrease of IL-6,[7] indicating albumin may also be an anti-inflammatory protein. Moreover, there was direct evidence to show that tumor-bearing animals became hypoalbuminemic, which was reported to be resulted from depressed albumin synthesis and increased albumin degradation.[8] According to these findings, we hypothesize serum albumin may be a potential prognostic biomarker for glioma, with low level predicting poor survival. This hypothesis had been confirmed in the study of Han et al [9] and Wang et al.[10] However, there were also some contradictory conclusions, showing no significant associations between serum albumin level and OS in patients with glioma.[11–15] These inconsistent results may be attributed to 2 aspects:
-
1.
small sample size of each individual study; and
-
2.
possible limited predictive ability of serum albumin.
To resolve these 2 problems, on one hand, a meta-analysis that integrates all the evidence should be performed to enhance the statistical power; on the other hand, biomarkers that combined with serum albumin should be investigated. For the latter, recent studies put forward to use prognostic nutritional index [PNI, calculated as albumin (g/L) + 5 × total lymphocyte count (109/L)] and albumin-to-globulin ratio (AGR). As expected, most of the known studies proved preoperative AGR[9,10,14–16] and PNI[10,12,18,19] were independent prognostic factors of OS for patients with glioma. Unfortunately, there were still occasional studies to indicate PNI was not associated with prognosis.[13,14,20] Hereby, their prognostic significance also needs further assessment.[21]
In present study, we aimed to re-evaluate the prognostic significance of preoperative serum albumin, AGR, and PNI for patients with glioma by performing a meta-analysis.
2. Materials and methods
This meta-analysis was conducted under the guidelines developed in The Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA). Ethical approval and patient consent were not required since this study was a meta-analysis based on published studies.
2.1. Search strategy
All relevant literatures published up to December 31, 2019 were retrieved by searching the databases of PubMed, EMBASE, and Cochrane Library. The search keywords and terms included: (“albumin” OR “hyperalbuminemia” OR “hypoalbuminemia” OR “albumin-to-globulin ratio” OR “albumin/globulin ratio” OR “prognostic nutritional index”) AND (“glioblastoma” OR “gliomas”). Meanwhile, the references listed in all included articles and reviews were also manually screened to identify additional related articles.
2.2. Inclusion and exclusion criteria
The study selection process was performed by 2 independent investigators, with any disagreements being discussed. Publications that met the following inclusion criteria were included:
-
1.
the prognostic value of serum albumin, AGR, or PNI was investigated for glioma patients;
-
2.
the related clinical indicators (level of serum albumin, globulin, and lymphocyte count) were collected preoperatively;
-
3.
the hazard ratio (HR) with 95% confidence interval (CI) were provided or can be estimated from Kaplan–Meier curve;
-
4.
published in English; and
-
5.
had full text.
The studies were excluded according to exclusion criteria:
-
1.
duplicated or with overlapped data;
-
2.
observational study;
-
3.
abstracts, letters, case reports, reviews, and comments;
-
4.
performed on animals;
-
5.
focusing on the association between postoperative serum albumin and prognosis; and
-
6.
no sufficient data for calculating HRs and 95%CIs.
2.3. Data extraction
Two authors extracted the following data independently: the surname of the first author, year of publication, country, case number, study design, follow-up, cancer type, cut-off value, HR source and HR with 95%CI for prognosis outcomes. HRs and 95%CIs were preferentially extracted from the multivariable analysis if both univariate and multivariate analyses were available. The survival data were estimated using a digitizing software-Engauge Digitizer (version 4.1; http://digitizer.sourceforge.net/) if only Kaplan–Meier curve was included in studies.
2.4. Quality assessment
The quality of enrolled studies was evaluated by 2 independent researchers using the Newcastle-Ottawa Scale (NOS) criteria.[22] NOS score ranged from 0 to 9. A study was regarded as high quality if its NOS was higher than 7.
2.5. Statistical analysis
The association between serum albumin/AGR/PNI and prognostic outcomes (such as OS) of glioma patients was assessed by pooled HR and 95% CI. HR < 1 indicated a protective effect for prognosis in patients with high albumin, AGR, or PNI; otherwise, it indicated a risk effect. Statistical difference was determined by using z test (P < .05) with 95%CI (not overlapping 1). Heterogeneity among studies was evaluated using Cochranes Q (Chi-Squared) and I2 tests. The random-effects model was used to calculate the pooled HR and 95%CI when P was less than.10 and I2 was more than 50% (that is, significant heterogeneity among studies); otherwise, a fixed-effects model was chosen. Subgroup analyses were performed according to country (Asian or Non-Asian), sample size (<200 or >200), cut-off (Yes or No), cancer type (GBM, all gliomas, high-grade glioma, or low-grade gliomas) and source of HR (univariate or multivariate). Egger linear regression test was used to detect the publication bias, with P < .05 indicating the presence of publication bias.[23] All analyses were performed or figures were generated using STATA 13.0 (STATA Corporation, College Station, TX, USA).
3. Results
3.1. Study search
Figure 1 provides detailed strategy of search and selection for relevant studies used in this meta-analysis. The initial literature search in available databases yielded 623 records. A total of 156 articles were found to be duplicated and then removed. After reading the titles and abstracts, 453 studies were excluded because they did not meet the eligibility criteria, including animal experiment (n = 153); irrelevant topic (n = 156); without prognosis outcome (n = 114); not glioma (n = 14); case report (n = 3); meta-analysis (n = 4); and observational studies (n = 9). The full text of the remaining 14 studies were downloaded and then read. Of them, 3 studies were further discarded because no sufficient data could be obtained (n = 2) and serum albumin was collected postoperatively (n = 1). Finally, 11 studies with 2928 cases were included in this meta-analysis.[9–17,19,20]
Figure 1.
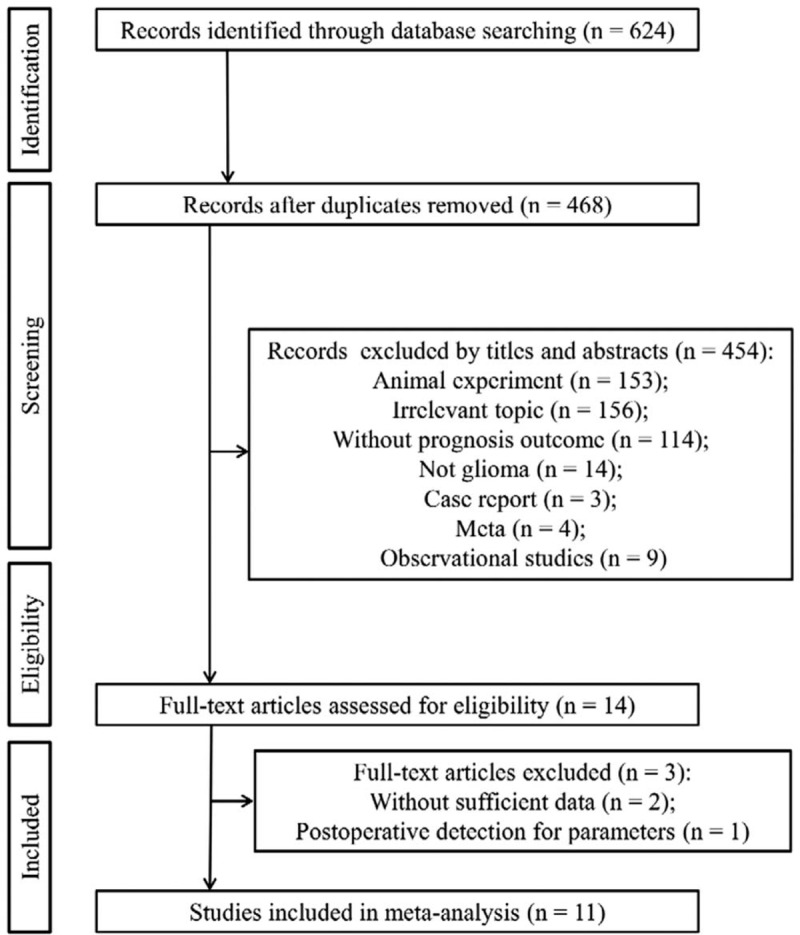
Flow diagram of included studies.
3.2. Study characteristics and quality assessment
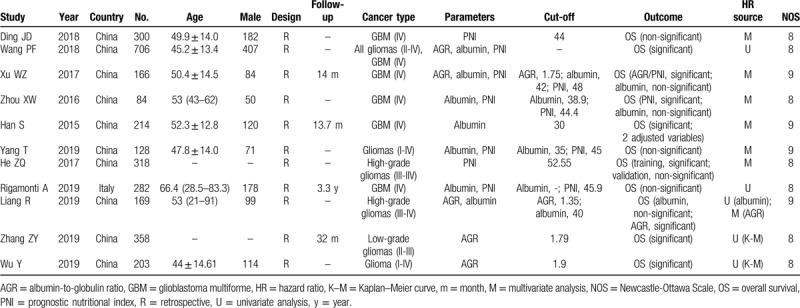
The main characteristics of eligible studies are summarized in Table 1. Among these 11 studies, the study of Han et al [9] contained 2 multivariate analysis results due to different adjusted variables; the study of He et al[19] included the training and validation cohorts for prognosis analysis; the study of Wang e al[10] independently analyzed all the gliomas and GBM samples; thus, in fact, 14 datasets were used for this meta-analysis. Seven studies with 9 datasets[9–15] assessed the prognostic value of serum albumin on OS; 5 studies with 6 datasets[10,11,15–17] investigated the association between AGR and OS; 7 studies with 9 datasets[10,12–14,18–20] focused the predictive ability of PNI for OS; and 1 study with 2 datasets reported the relationship between PNI and PFS.[19] Six studies evaluated patients belonging to GBM (IV), 2 evaluated high-grade patients (III-IV), 1 focused on low-grade cases (II-III), and 3 studies evaluated all glioma types (I-IV). All these studies were retrospectively conducted in population from China (n = 10) and Italy (n = 1). HRs and 95%CIs were directly obtained from 9 studies; there were 2 articles[16,17] only providing Kaplan–Meier curves and their HRs were estimated by Engauge Digitizer. Cut-off values of albumin ranged from 30 to 42; AGR ranged from 1.35 to 1.79; PNI ranged from 44.4 to 52.55. The NOS was 9 for 4 articles and 8 for 7 studies, indicating the quality of all eligible studies was high (Table 1).
Table 1.
Characteristics of included studies.
3.3. Impact of preoperative serum albumin on postoperative OS
Since an obvious heterogeneity was found between included studies (I2 = 69.7%, P = .001), a random-effect model was used for meta-analysis. Pooled results demonstrated that there was no significant difference in OS between the patients with high- and low-level of serum albumin (HR = 0.96, 95% CI: 0.89–1.04, P = .276) (Table 2). To explore the source of heterogeneity, the subgroup analysis was performed. The results showed that a high preoperative serum albumin was significantly related with excellent OS in subgroups with sample size less than 200 (HR = 0.77, 95% CI: 0.59–0.99, P = .047), having clear cut-off (HR = 0.77, 95% CI: 0.59–0.99, P = .047), undergoing multivariate analysis (HR = 0.96, 95% CI: 0.94–0.98, P < .001; Fig. 2) and belonging to GBM grade (HR = 0.95, 95% CI: 0.91–0.99, P = .018; Fig. 3) (Table 2).
Table 2.
Meta-analyses for the prediction ability of albumin for OS.
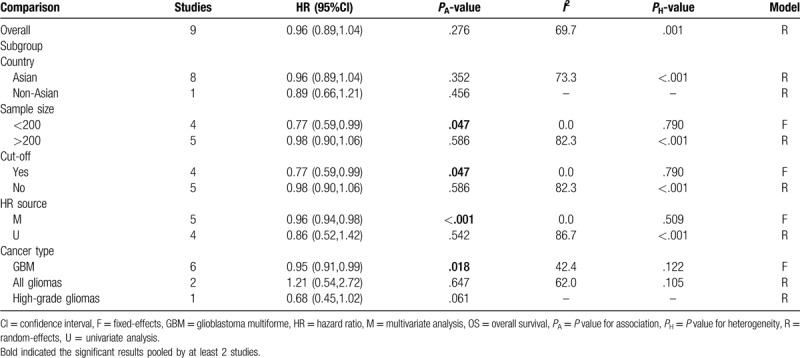
Figure 2.
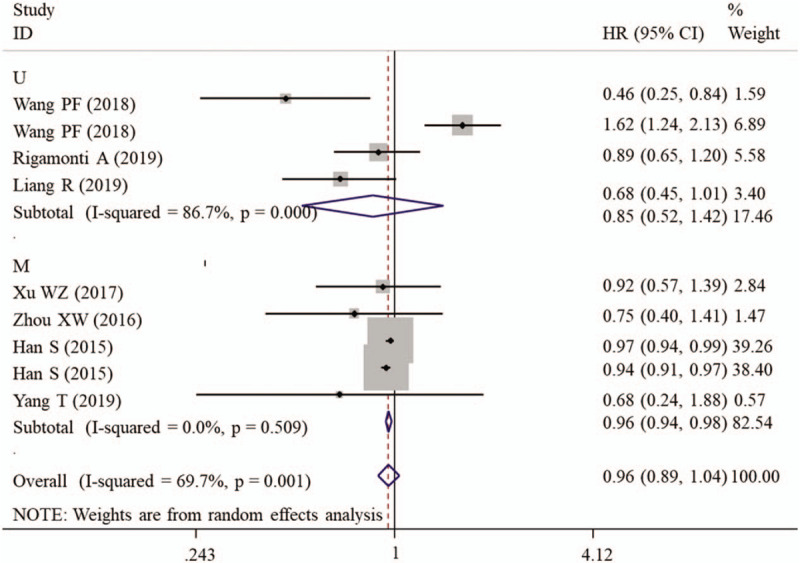
Forest plots to investigate the correlation between albumin and overall survival based on HR source subgroup analyses. CI = confidence interval, HR = hazard ratio, M = multivariate analysis, U = univariate analysis.
Figure 3.
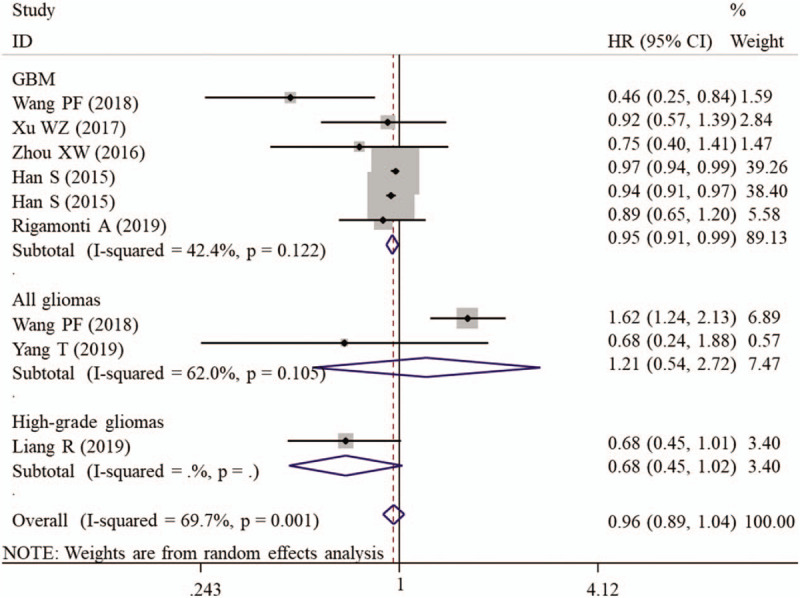
Forest plots to investigate the correlation between albumin and overall survival based on cancer type subgroup analyses. CI = confidence interval, GBM = glioblastoma multiforme, HR = hazard ratio.
3.4. Impact of preoperative AGR on postoperative OS
It was noticeable that a large heterogeneity was present between included studies (I2 = 88.4%, P < .001), thus a random-effect model was used for the pooled analysis. The meta-analysis result showed that AGR was not associated with OS (HR = 0.90, 95% CI: 0.56–1.46, P = .670) (Table 3). Subsequently, subgroup analysis was further conducted to explore the sources of heterogeneity. Subgroup analysis of studies with sample size less than 200 (HR = 0.68, 95% CI: 0.47–0.97, P = .033), having clear cut-off (HR = 0.64, 95% CI: 0.51–0.79, P < .001) and undergoing multivariate analysis (HR = 0.96, 95% CI: 0.94–0.98, P < .001; Fig. 4) revealed that a low preoperative AGR predicted poor OS (Table 3).
Table 3.
Meta-analyses for the prediction ability of AGR for OS.
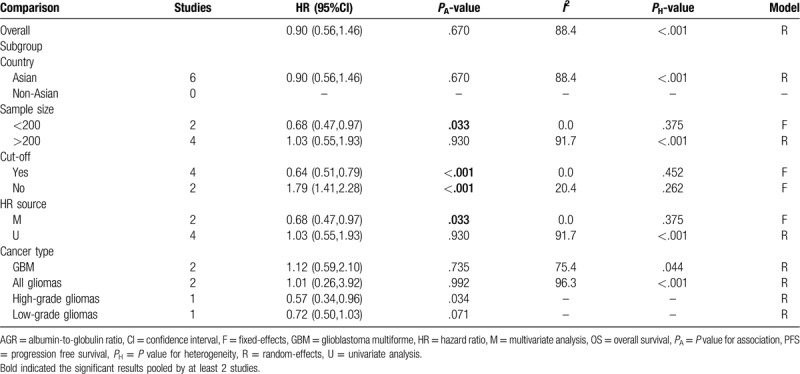
Figure 4.
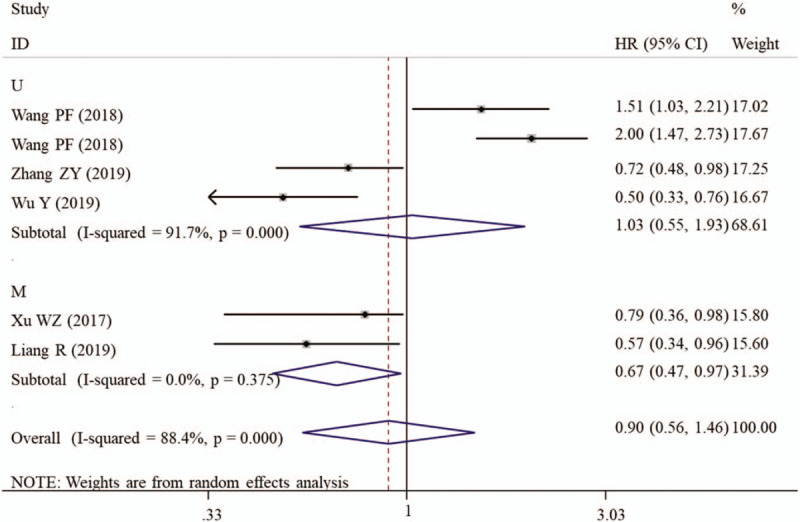
Forest plots to investigate the correlation between albumin-to-globulin ratio and overall survival based on HR source subgroup analyses. CI = confidence interval, HR = hazard ratio, M = multivariate analysis, U = univariate analysis.
3.5. Impact of preoperative PNI on postoperative OS
A random-effect model was used to calculate the HR and 95% CI because a significant heterogeneity existed between included studies (I2 = 84.4%, P < .001). Overall meta-analysis showed that the OS was not significantly different between the patients with high PNI and those with low PNI (Table 4). However, the subgroup study proved that high PNI may be a protective factor of favorable OS for patients with high-grade gliomas (HR = 0.56, 95% CI: 0.43–0.73, P < .001; Fig. 5). Also, the prognostic value of PNI was significant if the studies enrolled less than 200 samples (HR = 0.60, 95% CI: 0.49–0.74, P < .001), had defined cut-off (HR = 0.63, 95% CI: 0.52–0.77, P < .001) and performed multivariate analysis (HR = 0.63, 95% CI: 0.52–0.77, P < .001; Fig. 6) (Table 4).
Table 4.
Meta-analyses for the prediction ability of PNI for OS.
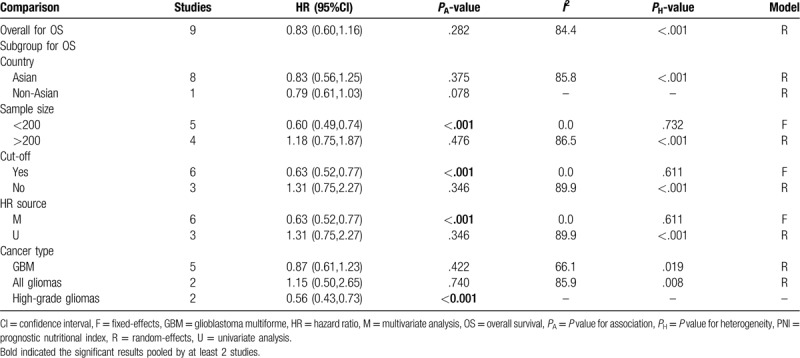
Figure 5.
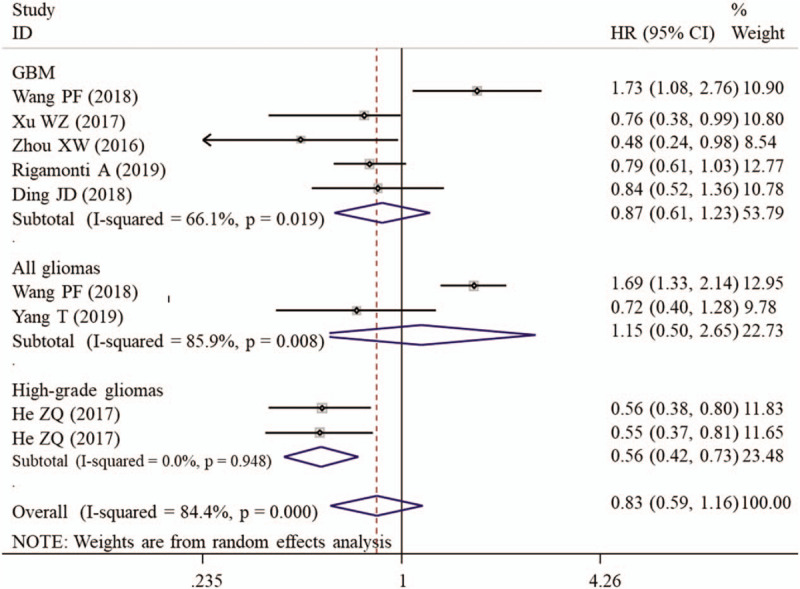
Forest plots to investigate the correlation between prognostic nutritional index and overall survival based on cancer type subgroup analyses. CI = confidence interval, GBM = glioblastoma multiforme, HR = hazard ratio.
Figure 6.
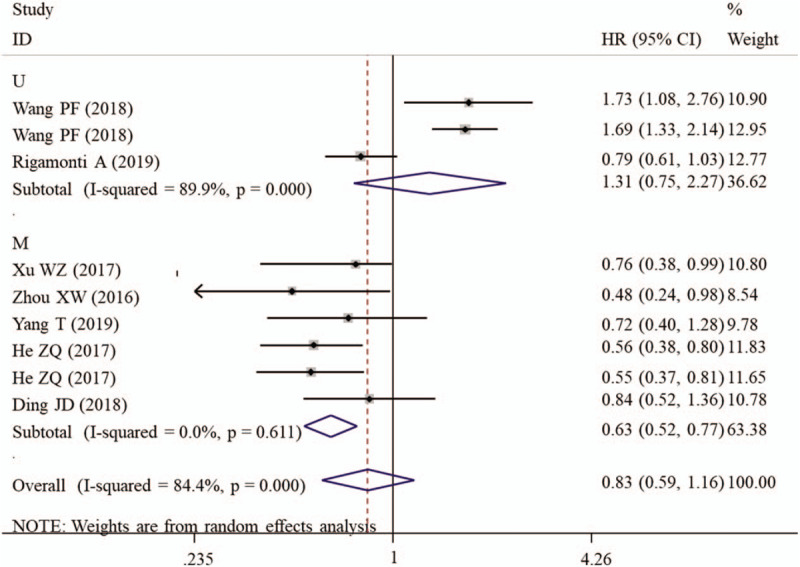
Forest plots to investigate the correlation between prognostic nutritional index and overall survival based on HR source subgroup analyses. CI = confidence interval, HR = hazard ratio, M = multivariate analysis, U = univariate analysis.
3.6. Publication bias
Although heterogeneities were observed among studies for investigating the prognostic values of serum albumin, AGR, and PNI, Egger test results showed there was no obvious evidence of publication bias (albumin, P = .596; AGR, P = .134; PNI, P = .206).
4. Discussion
There have numerous articles to use the meta-analysis to confirm the prognostic significance of preoperative serum albumin for patients with urothelial carcinoma,[24] epithelial ovarian cancer,[25] bladder cancer[26]; AGR for digestive system cancers,[27] or combined all cancer types[28,29]; and PNI for lung cancer,[30,31] hepatocellular carcinoma,[32] esophageal cancer,[33] and pancreatic cancer.[34] All these studies revealed the cancer patients with decreased albumin, AGR, and PNI held a worse OS compared with those who carried a higher albumin, AGR, and PNI. In line with these reports, our analysis of studies with multivariate association tests and clear cut-off also found a higher preoperative serum albumin, AGR, and PNI indicated a better OS, especially for patients with GBM or high-grade gliomas.
Although the exact protective mechanisms of albumin for gliomas remain unclear, we speculated its association with malnutrition and inflammation may be possible contributors. Clinically, weight loss of >10% of normal body weight is considered to represent protein-energy malnutrition.[35] Albumin protein concentrations were reported to be positively correlated with the percent ideal body weight (r = 0.390, P < .05) and negatively associated with extent of reported weight loss (r = −0.492, P < .01).[36] Multivariate analysis in the study of Ehrsson et al [37] revealed tumor stage was the only independent factor of maximum weight loss. Weight gain increased the survival and quality of life of patients with cancer.[38] Furthermore, cytokine tumor necrosis factor α concentrations were also observed to be significantly higher in cancer patients with severe weight loss than in those with moderate or light weight loss (26.3 ± 8.3 vs 8.9 ± 4.2 or 3.8 ± 2.1).[39] Serum C-reactive protein (CRP) was also proved to be an independent variable in determining degree of weight loss (estimate of effect, 34%).[40] Therefore, low albumin-weight loss-inflammation may be the possible mechanism for the development and progression of gliomas. This hypothesis can be indirectly reflected by the negative correlation between albumin protein level and C-reactive protein concentration in cancer.[36,41]
In addition to albumin, the protective effects of PNI and AGR may also result from the secretion of more lymphocytes and production of less globulin. Lymphocytes and globulin were also reported to respectively exert anti-tumor or proto-oncogenic roles in previous studies on cancer. For example, Quattrocchi et al demonstrated that local infusion of autologous tumor infiltrating lymphocytes was effective for treatment of some patients with recurrent malignant gliomas, with the partial response of 50% after a long term follow-up.[42] Misao et al observed that treatment of an endometrial cancer cell line with oestradiol-17 beta (E2) significantly increased corticosteroid-binding globulin mRNA expression in a dose-dependent manner. E2 was implied to promote endometrial carcinoma progression via activating the IL-6 pathway.[43]
There were some limitations that should be acknowledged. First, the number of eligible studies remained relatively small, which may result in low statistical power for some of the subgroup analyses. Thus, the effect size may be overestimated or underestimated. Second, the HRs and 95%CIs were obtained from univariate analysis in some included studies in which the effect size may also be overestimated. Third, some HRs and 95%CI were estimated from Kaplan–Meier curve, which may be different from the actual data and may introduce potential errors. Fourth, all these studies were retrospectively performed, which may introduce unavoidable bias in patient selection and data collection. Fifth, the populations in a majority of the studies were Chinese and thus its applicability to other ethnicities remained discussed. Sixth, the cut-off value was not unified in different articles, which influenced its generalization in clinical practice. Seventh, significant heterogeneity was observed in the overall analysis which may affect the robustness of the pooled analysis. However, Egger test results showed there was no obvious evidence of publication bias and most of subgroup analyses with significant results were obtained from studies without heterogeneity. So our conclusion still may be believable. Accordingly, more studies with prospective design, larger sample size and cases from other ethnicities are needed to further confirm the prognostic values of albumin, AGR, and PNI for glioma in the future.
5. Conclusion
This meta-analysis indicated that preoperative serum albumin, AGR, and PNI may represent promising biomarkers to predict the prognosis in patients with glioma, especially for high-grade. Decreased serum albumin, AGR, and PNI were correlated with worse survival in patients with glioma.
Author contributions
Conceptualization: Mingchang Liu, Liwen Wang.
Data curation: Mingchang Liu.
Formal analysis: Mingchang Liu, Liwen Wang.
Methodology: Mingchang Liu.
Writing – original draft: Mingchang Liu.
Writing – review & editing: Liwen Wang.
Footnotes
Abbreviations: AGR = albumin-to-globulin ratio, CI = confidence interval, GBM = glioblastoma multiforme, HR = hazard ratio, IL = interleukin, NOS = Newcastle-Ottawa Scale, OS = overall survival, PNI = prognostic nutritional index, PRISMA = Preferred Reporting Items for Systematic Review and Meta-analysis.
How to cite this article: Liu M, Wang L. Prognostic significance of preoperative serum albumin, albumin-to-globulin ratio and prognostic nutritional index for patients with glioma: a meta-analysis. Medicine. 2020;99:27(e20927).
The authors report no conflicts of interest in this work.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Rasmussen BK, Hansen, Laursen RJ, et al. Epidemiology of glioma: clinical characteristics, symptoms, and predictors of glioma patients grade I–IV in the Danish Neuro-Oncology Registry. J Neurooncol 2017;135:571–9. [DOI] [PubMed] [Google Scholar]
- [2].Kumar A, Torres ML, Cliby WA, et al. Inflammatory and nutritional serum markers as predictors of peri-operative morbidity and survival in ovarian cancer. Anticancer Res 2017;37:3673–7. [DOI] [PubMed] [Google Scholar]
- [3].Seretis C, Kaisari P, Wanigasooriya K, et al. Malnutrition is associated with adverse postoperative outcome in patients undergoing elective colorectal cancer resections. J BUON 2018;23:36–41. [PubMed] [Google Scholar]
- [4].Read JA, Boris Choy ST, Beale PJ, et al. Evaluation of nutritional and inflammatory status of advanced colorectal cancer patients and its correlation with survival. Nutr Cancer 2006;55:78–85. [DOI] [PubMed] [Google Scholar]
- [5].Brock F, Bettinelli LA, Dobner T, et al. Prevalence of hypoalbuminemia and nutritional issues in hospitalized elders. Rev Lat Am Enfermagem 2016;24:e2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fernández J, Clària J, Amorós A, et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology 2019;157:149–62. [DOI] [PubMed] [Google Scholar]
- [7].Pfensig C, Dominik A, Borufka L, et al. A new application for albumin dialysis in extracorporeal organ support: characterization of a putative interaction between human albumin and proinflammatory cytokines il-6 and tnf(. Art Organs 2016;40:397–402. [DOI] [PubMed] [Google Scholar]
- [8].Andersson CE, Lönnroth IC, Gelin LJ, et al. Pretranslational regulation of albumin synthesis in tumor-bearing mice. The role of anorexia and undernutrition. Gastroenterology 1991;100:938–45. [DOI] [PubMed] [Google Scholar]
- [9].Han S, Huang Y, Li Z, et al. The prognostic role of preoperative serum albumin levels in glioblastoma patients. BMC Cancer 2015;15:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang PF, Meng Z, Song HW, et al. Preoperative changes in hematological markers and predictors of glioma grade and survival. Front Pharmacol 2018;9:886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xu WZ, Li F, Xu ZK, et al. Preoperative albumin-to-globulin ratio and prognostic nutrition index predict prognosis for glioblastoma. Onco Targets Ther 2017;10:725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhou XW, Dong H, Yang Y, et al. Significance of the prognostic nutritional index in patients with glioblastoma: a retrospective study. Clin Neurol Neurosurg 2016;151:86–91. [DOI] [PubMed] [Google Scholar]
- [13].Yang T, Mao P, Chen X, et al. Inflammatory biomarkers in prognostic analysis for patients with glioma and the establishment of a nomogram. Oncol Lett 2019;17:2516–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rigamonti A, Imbesi F, Silvani A, et al. Prognostic nutritional index as a prognostic marker in glioblastoma: data from a cohort of 282 Italian patients. J Neurol Sci 2019;400:175–9. [DOI] [PubMed] [Google Scholar]
- [15].Liang R, Li J, Tang X, et al. The prognostic role of preoperative systemic immune-in fl ammation index and albumin/globulin ratio in patients with newly diagnosed high-grade glioma. Clin Neurol Neurosurg 2019;184:105397. [DOI] [PubMed] [Google Scholar]
- [16].Zhang ZY, Zhan YB, Zhang FJ, et al. Prognostic value of preoperative hematological markers combined with molecular pathology in patients with diffuse gliomas. Aging 2019;11:6252–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wu Y, Song Z, Sun K, et al. A novel scoring system based on peripheral blood test in predicting grade and prognosis of patients with glioma. Onco Targets Ther 2019;12:11413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang B, Huang Y, Lin T. Prognostic impact of elevated pre-treatment systemic immune-inflammation index (SII) in hepatocellular carcinoma: a meta-analysis. Medicine 2020;99:e18571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].He ZQ, Ke C, Al-Nahari F, et al. Low preoperative prognostic nutritional index predicts poor survival in patients with newly diagnosed high-grade gliomas. J Neurooncol 2017;132:239–47. [DOI] [PubMed] [Google Scholar]
- [20].Ding JD, Yao K, Wang PF, et al. Clinical significance of prognostic nutritional index in patients with glioblastomas. Medicine 2018;97:e13218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang DP, Kang K, Lin Q, et al. Prognostic significance of preoperative systemic cellular inflammatory markers in gliomas: a systematic review and meta-analysis. Clin Transl Sci 2020;13:179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Andreas S. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [23].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liu J, Wang F, Li S, et al. The prognostic significance of preoperative serum albumin in urothelial carcinoma: a systematic review and meta-analysis. Biosci Rep 2018;38:BSR20180214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ge LN, Wang F. Prognostic significance of preoperative serum albumin in epithelial ovarian cancer patients: a systematic review and dose–response meta-analysis of observational studies. Cancer Manag Res 2018;10:815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li J, Cheng Y, Liu G, et al. The association of pretreatment serum albumin with outcomes in bladder cancer: a meta-analysis. Onco Targets Ther 2018;11:3449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Guo HW, Yuan TZ, Chen JX, et al. Prognostic value of pretreatment albumin/globulin ratio in digestive system cancers: a meta-analysis. Plos One 2018;13:e0189839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lv GY, An L, Sun XD, et al. Pretreatment albumin to globulin ratio can serve as a prognostic marker in human cancers: a meta-analysis. Clin Chim Acta 2018;476:81–91. [DOI] [PubMed] [Google Scholar]
- [29].He J, Pan H, Liang W, et al. Prognostic effect of albumin-to-globulin ratio in patients with solid tumors: a systematic review and meta-analysis. J Cancer 2017;8:4002–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang Z, Wang Y, Zhang X, et al. Pretreatment prognostic nutritional index as a prognostic factor in lung cancer: review and meta-analysis. Clin Chim Acta 2018;486:303–10. [DOI] [PubMed] [Google Scholar]
- [31].Hu, Shen J, Liu R, et al. Prognostic value of pretreatment prognostic nutritional index in non-small cell lung cancer: a systematic review and meta-analysis. Int J Biol Markers 2018;33:372–8. [DOI] [PubMed] [Google Scholar]
- [32].Man Z, Pang Q, Zhou L, et al. Prognostic significance of preoperative prognostic nutritional index in hepatocellular carcinoma: a meta-analysis. HPB 2018;20:888–95. [DOI] [PubMed] [Google Scholar]
- [33].Xue Y, Zhou X, Xue L, et al. The role of pretreatment prognostic nutritional index in esophageal cancer: A meta-analysis. J J Cell Physiol 2019;234:19655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Li S, Tian G, Chen Z, et al. Prognostic role of the prognostic nutritional index in pancreatic cancer: a meta-analysis. Nutr Cancer 2019;71:207–13. [DOI] [PubMed] [Google Scholar]
- [35].Collins N. Protein-energy malnutrition and involuntary weight loss: nutritional and pharmacological strategies to enhance wound healing. Expert Opin Pharmacother 2003;4:1121–40. [DOI] [PubMed] [Google Scholar]
- [36].McMillan DC, Watson WS, O’Gorman P, et al. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer 2001;39:210–3. [DOI] [PubMed] [Google Scholar]
- [37].Ehrsson YT, Langius-Eklöf A, Laurell G. Nutritional surveillance and weight loss in head and neck cancer patients. Support Care Cancer 2012;20:757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tozer RG, Tai P, Falconer W, et al. Cysteine-rich protein reverses weight loss in lung cancer patients receiving chemotherapy or radiotherapy. Antioxid Redox Signal 2008;10:395–402. [DOI] [PubMed] [Google Scholar]
- [39].Bossola M, Muscaritoli M, Bellantone R, et al. Serum tumour necrosis factor-alpha levels in cancer patients are discontinuous and correlate with weight loss. Eur J Clin Invest 2000;30:1107–12. [DOI] [PubMed] [Google Scholar]
- [40].Deans DAC, Tan BH, Wigmore SJ, et al. The influence of systemic inflammation, dietary intake and stage of disease on rate of weight loss in patients with gastro-oesophageal cancer. Br J Cancer 2009;100:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wang X, Han H, Duan Q, et al. Changes of serum albumin level and systemic inflammatory response in inoperable non-small cell lung cancer patients after chemotherapy. J Cancer Res Ther 2013;10:1019–23. [DOI] [PubMed] [Google Scholar]
- [42].Quattrocchi KB, Miller CH, Cush S, et al. Pilot study of local autologous tumor infiltrating lymphocytes for the treatment of recurrent malignant gliomas. J Neurooncol 1999;45:141–57. [DOI] [PubMed] [Google Scholar]
- [43].Che Q, Xiao X, Jun X, et al. 17β-estradiol promotes endometrial cancer proliferation and invasion through IL-6 pathway. Endocr Connect 2019;8:961–87. [DOI] [PMC free article] [PubMed] [Google Scholar]


