Supplemental Digital Content is available in the text
Keywords: cardiovascular risk score, HIV, Kenya, metabolic syndrome
Abstract
To determine the prevalence and correlates of metabolic syndrome (MetS) and compare 10-year cardiovascular disease (CVD) risk among Kenyan adults with and without HIV infection.
We conducted a cross-sectional study among adults ≥30 years of age with and without HIV infection seeking care at Kisumu County Hospital. Participants completed a health questionnaire and vital signs, anthropomorphic measurements, and fasting blood were obtained. MetS was defined using 2009 Consensus Criteria and 10-year Atherosclerotic CVD (ASCVD) risk score was calculated. Chi-square, independent t tests, Wilcoxon ranksum test and multivariable logistic regression were used to determine differences and associations between HIV and MetS, CVD risk factors and ASCVD risk score.
A total of 300 people living with HIV (PLWHIV) and 298 HIV-negative participants with median age 44 years enrolled, 50% of whom were female. The prevalence of MetS was 8.9% overall, but lower among PLWHIV than HIV-negative participants (6.3% vs 11.6%, respectively; P = .001). The most prevalent MetS components were elevated blood pressure, decreased high density lipoprotein, and abdominal obesity. Adjusting for covariates, PLWHIV were 66% less likely to have MetS compared to HIV-negative participants (adjusted odds ratio [aOR] 0.34; 95% confidence interval [95%CI] 0.18, 0.65; P = .005). Median ASCVD risk score was also lower among PLWHIV compared to HIV-negative participants (1.7% vs 3.0%, P = .002).
MetS was more common among HIV-negative than HIV-positive adults, and HIV-negative adults were at greater risk for CVD compared to PLWHIV. These data support integration of routine CVD screening and management into health programs in resource-limited settings, regardless of HIV status.
1. Introduction
Globally, 38 million people were living with HIV (PLWHIV) in 2018, with over half residing in Eastern and Southern Africa.[1] One of the greatest successes in HIV has been the introduction of highly effective antiretroviral therapy (ART) which has resulted in a decrease in opportunistic infections, a decline in HIV-related mortality and increased survival among PLWHIV.[2–4] The life expectancy of PLWHIV who are virally suppressed on ART is nearly that of those who are HIV-negative.[5] Consequently, an estimated 16% of PLWHIV were aged 50 and above in 2016 and is projected to increase to 21% by 2020.[6] In 2016 approximately 80% of PLWHIV over the age of 50 lived in low-and middle-income countries and sub-Saharan Africa (SSA) will continue to bear the greatest burden of aging PLWHIV in the future.[6] As PLWHIV live longer, risk of chronic non-communicable diseases such as cardiovascular disease (CVD) increases, posing new challenges to health care systems.[7,8] In several studies in the US and Europe, PLWHIV had up to a two-fold increased risk of incident atherosclerotic CVD (ASCVD) compared to those without HIV infection.[9–15] Based on this, HIV may account for nearly 25% of the CVD burden in parts of SSA, the highest population attributable fraction globally.[9]
HIV has been associated with increased risk of traditional CVD risk factors such as smoking, harmful alcohol use, reduced physical activity, unhealthy diets, hypertension and diabetes in high income countries.[16–20] In addition to these traditional risk factors, PLWHIV may be at increased CVD risk due to HIV-related inflammation and immune activation, or due to use of ART-induced dyslipidemia, insulin resistance, and glucose intolerance.[10–12,21] Metabolic syndrome (MetS), is defined as a cluster of CVD risk factors including raised blood pressure, dyslipidemia (raised triglycerides and lowered high-density lipoprotein cholesterol [HDL-C]), hyperglycemia, and central obesity.[22] Individuals with MetS are at approximately two-fold increased risk of CVD compared to their controls.[23,24] While some studies in SSA have demonstrated increased diagnosis of MetS among PLWHIV[25–28] results have been inconsistent.[29]
Since MetS is a significant predictor of CVD, it can be used to identify patients who may benefit from interventions to reduce CVD risk.[30–34] Risk scores can also be used to predict future CVD events. The Atherosclerotic CVD (ASCVD) risk score, is a multivariable race and sex-specific risk factor algorithm incorporating age, total and HDL-C, systolic blood pressure, current smoking status and history of diabetes and antihypertensive treatment.[35] The ASCVD risk score is a well validated tool, incorporating longitudinal data from across ethnic groups and continents.[36]
Despite increasing concerns of elevated risk of CVD among PLWHIV, there is limited data on CVD risk factors, MetS and CVD risk scores in SSA, a region with considerable HIV burden. We sought to define the prevalence and correlates of MetS, and its individual components, and to compare the 10- year ASCVD risk among Kenyan adults with and without HIV infection. Based upon data from Western countries, we hypothesized that PLWHIV would have a higher prevalence of MetS and a higher ASCVD risk score as compared to HIV-negative individuals.
2. Methods
2.1. Study design and setting
Between September 2017 and May 2018, we conducted a cross-sectional study among HIV-positive and HIV-negative women and men from Kisumu County Hospital, a tertiary, public county referral facility located in Western Kenya where HIV prevalence is high at 16.3%.[37] CVD risk assessment is not part of the routine screening among PLWHIV and HIV-negative individuals at Kisumu County Hospital.
2.2. Study procedures
Eligible participants had to be at least 30 years of age and live within a 50 km radius of the hospital. In addition, PLWHIV had to be engaged in care at the HIV Comprehensive Care Clinic (CCC) and taking ART for at least 6 months. PLWHIV who met the inclusion criteria and provided consent were consecutively enrolled by a study nurse from the CCC while HIV-negative participants were recruited from the HIV testing points until the sample size was reached. Human subject approval was obtained from the University of Washington Institutional Review Board and locally from the Kenyatta National Hospital (KNH)/University of Nairobi (UoN) Ethical and Scientific Review Committee. All participants provided written informed consent prior to any study procedures or data collection.
Clinical procedures: Using tablets, study nurse counselors interviewed all participants at the time of enrollment to collect data on socio-demographics, HIV disease status if HIV-positive, and CVD risk factors using the validated World Health Organization (WHO) STEPwise approach to chronic disease risk factor surveillance (STEPS) questionnaires modified to fit the Kenyan context.[38] Waist and hip circumference, weight and height were measured to determine body mass index (BMI) and waist hip ratio. Two blood pressure readings on each arm and pulse were measured and averaged. Participants were asked to return the following day after fasting 8 hours for blood draw if not already fasting.
Laboratory procedures: Fasting blood samples were collected for quantification of lipids (total cholesterol, HDL-C, low density lipoprotein cholesterol [LDL-C], triglycerides) and glucose. All samples were processed to serum, frozen and stored at the Kenya Medical Research Institute (KEMRI) Lab at −80°C. Serum lipids and glucose tests were performed at the University of Washington Research Testing Services using an automated Beckman Coulter AU5812. CD4 count and viral load testing was performed at the KEMRI laboratory in Kisumu.
2.3. Primary outcomes and dependent variables
The primary outcomes were MetS and ASCVD risk score. MetS was defined by the Consensus Criteria 2009 as any three of the following:
-
(1)
abdominal obesity (waist circumference of >88 cm for women and >94 cm for men);
-
(2)
triglycerides ≥150 mg/dL;
-
(3)
HDL-C <50 mg/dL for women and <40 mg/dL for men;
-
(4)
blood pressure >130/85 mmHg;
-
(5)
fasting plasma glucose ≥ 100 mg/dL.[22]
For each participant without prior history of ASCVD (myocardial infarction or stroke), we calculated their 10-year ASCVD risk score using the Pooled Cohort Equation as outlined in the 2019 American College of Cardiology (ACC) / American Heart Association (AHA) Guideline on the Primary Prevention of Cardiovascular Disease.[35] We used the race and sex specific ASCVD risk score which includes sex, age, race, total cholesterol, HDL-C, systolic blood pressure and history of smoking, diabetes and treatment for hypertension. 10-year CVD risk was classified as low (<5%), borderline (5 to <7.5%), intermediate (7.5 to <20%) or high (≥20%). Subjects were considered to be at elevated risk if their predicted risk was ≥7.5%.[36]
2.4. Statistical analysis
We summarized continuous variables using mean and standard deviation for normally distributed variables and median and inter-quartile range (IQR) for non-normally distributed variables. We tested for differences in patient baseline characteristics using chi-square tests for categorical subgroups and a 2-group independent means t test for continuous variables. We calculated the prevalence of MetS and its individual components, and determined ASCVD 10-year risk using the Pooled Cohort Equations for participants without prior atherosclerotic CVD. We used multivariable logistic regression to estimate the association between HIV and MetS and the CVD risk factors. Unadjusted and adjusted models were fit to evaluate associations with or without potential confounders including age, sex, education, current smoking, alcohol use, healthy diet (at least 5 daily servings of fruits and vegetables) and exercise (at least 150 minutes/week of moderate activity or 75 minutes/week of vigorous physical activity as per WHO recommendations). Differences in the ASCVD risk score by HIV status were assessed by the chi-square test and Wilcoxon rank sum test. We also calculated 95% confidence intervals (95%CI) and used a significance (α) level of 0.05. All analyses were conducted using Stata version 14.0 (StataCorp, College Station, TX).
3. Results
3.1. Baseline characteristics
Between September 2017 and May 2018, we screened 610 participants, of whom 600 were eligible and participated. We excluded two participants whose data was not available during analysis. The study enrolled 300 PLWHIV and 298 HIV-negative participants, with similar numbers of males and females in each group (Table 1). Fasting blood samples were available for 564 (94%) of the 598 participants. Median age was 45 years (IQR 39.5, 53.0) and 40 years (IQR 31, 55) for the PLWHIV and HIV-negative participants, respectively. The majority of participants was married (74%) and employed (77%). Compared to the HIV-negative group, PLWHIV were older (P < .001), less educated (P < .001), and more likely to be separated, divorced or widowed (P = .002) (Table 1).
Table 1.
Baseline characteristics of 598 study participants by HIV status.
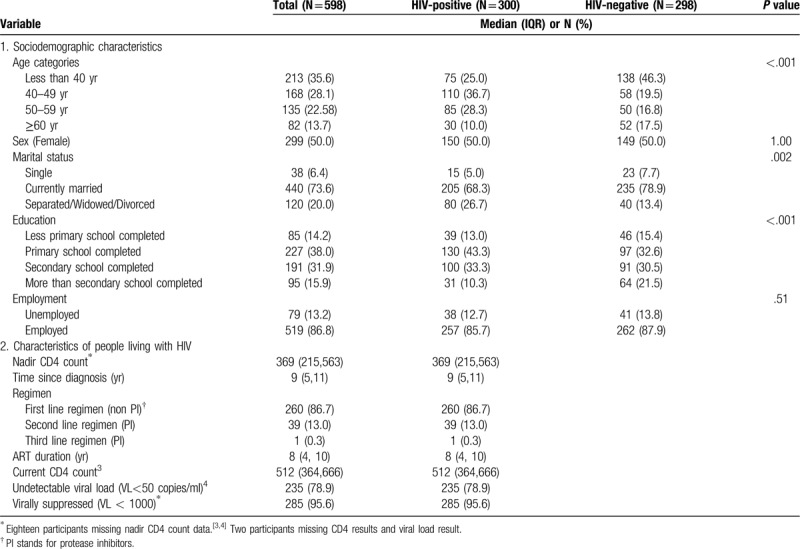
Among PLWHIV, the median duration on ART was 8 years (IQR 4, 10) with a median CD4 count of 512 cells/mm3 (IQR 364, 666) (Table 1), and 96% were virally suppressed (viral load <1000 copies/ ml), with 79% having a viral load <50 copies/ml (lower detection limit), which is considered undetectable by Kenyan national guidelines.[39] PLHIV were mostly on first line therapy (87%) (non-protease inhibitor [PI] regimen) with only 13% (39/300) on a second line PI-based regimen.
Out of the 598 participants, 71 (12%) self-reported prior history of hypertension (Table 2) with only 26 (37%) being on treatment. There were 10 participants who self-reported diabetes and only 6 (60%) of them were on oral hypoglycemic agents or insulin treatment. Eight out of the 10 participants who had history of stroke and heart attack were PLWHIV.
Table 2.
History of cardiovascular disease diagnosis and traditional risk factors among 598 study participants.
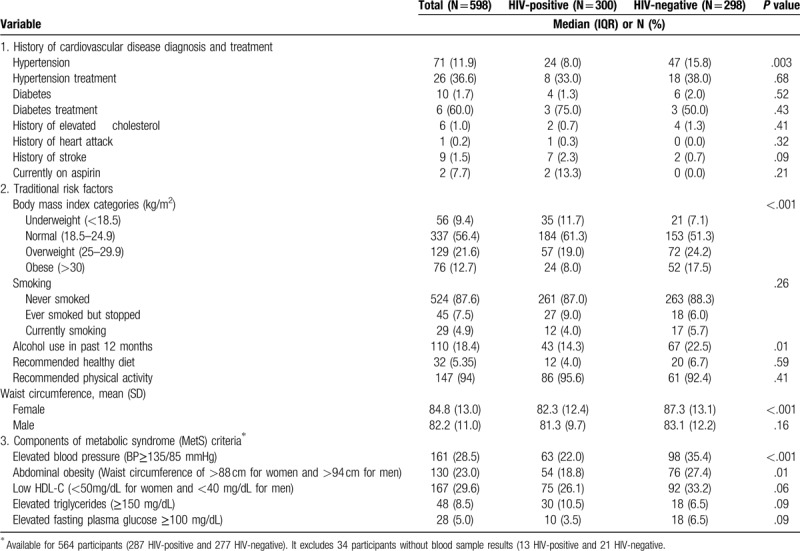
3.2. Traditional anthropomorphic and behavioral risk factors
A third of all participants were overweight or obese with higher prevalence of obesity among the HIV-negative participants compared to PLWHIV (42 vs 27%, respectively; P < .001). Abdominal obesity (assigned by waist circumference parameters) was also significantly higher among HIV-negative participants as compared to PLWHIV (P < .001). Only 5% of participants smoked in the past 30 days (Table 2), and there was no significant difference in smoking prevalence by HIV status. Alcohol consumption was also low across the entire cohort (18%) but was significantly higher among the HIV-negative participants (P = .010) (Table 2).
3.3. Prevalence and association of MetS and its individual components and HIV
MetS has 5 components and having 3 and more results in a diagnosis of MetS. Nearly 50% of participants presented with 1 or 2 components of MetS, while 50 participants (9%) had 3 or more individual components and were diagnosed with MetS (See Figure 1 Supplemental Digital Content 1 which illustrates the proportion of participants displaying various number of individual components of MetS stratified by HIV status). Of these 50, 42 (84%) presented with 3 components, 5 (10%) with 4, and 3 (6%) with all 5 components.
The prevalence of individual components of MetS was highest for low HDL-C (30%), followed by hypertension (29%), abdominal obesity (23%), hypertriglyceridemia (9%), and elevated fasting blood glucose (5%) (Table 2). The prevalence of hypertension was high in both PLWHIV (22%) and HIV-negative (36%) subjects. Of note, PLWHIV had a significantly lower prevalence of hypertension (P < .001) and abdominal obesity (P = .015) compared to HIV-negative participants (Table 2).
Overall, the prevalence of Mets was 8.9% (50/564). The prevalence of MetS was significantly lower among PLWHIV as compared to among HIV-negative participants (Table 3). Eighteen (6.3%) of 287 PLWHIV had MetS compared to 32 (11.6%) of 277 HIV-negative participants (P = .03) (Table 3).
Table 3.
The association between HIV status and metabolic syndrome and its components among 564 study participants∗.
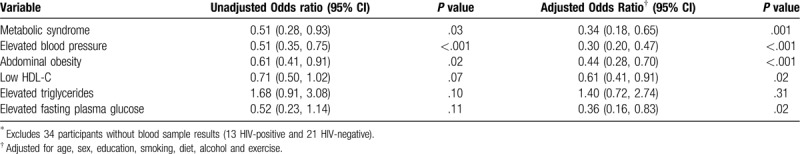
After adjusting for age, sex, education, smoking, alcohol use, diet and physical activity, PLWHIV were 66% less likely to have MetS compared to HIV-negative participants (adjusted odds ratio [aOR] 0.34; 95% confidence interval [95%CI] 0.18, 0.65; P = .001). PLWHIV were also less likely to have elevated blood pressure (aOR 0.30; 95%CI 0.20, 0.47; P < .001), abdominal obesity (aOR 0.44; 95%CI 0.28, 0.70; P < .001), low HDL-C (aOR 0.61; 95%CI 0.41, 0.91; P = .02), and elevated fasting glucose (aOR 0.36; 95%CI 0.1, 0.83; P = .02) when compared to the HIV-negative participants.
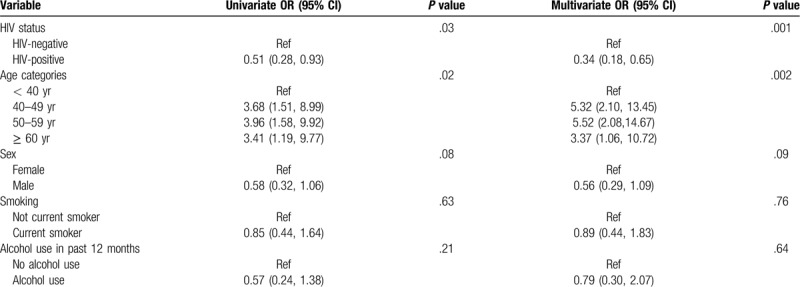
HIV status and age were independently associated with MetS (Table 4). Older participants (>40 years) compared to younger participants (30–40 years) were more likely to have MetS. There was a 5-fold increased likelihood of MetS among those aged 40 to 49 years and those 50–59 years compared to those < 40 years (aOR 5.32; 95%CI 2.10, 13.45; P = .002 and aOR 5.52 (95%CI 2.08, 14.67; P = .002, respectively). Among the PLWHIV in a model that in addition included CD4 count, viral load, ART duration and ART regimen, those with a CD4 count greater than 500 cells/mm3 were 4.5-fold more likely to be diagnosed with MetS (aOR 4.25 95% CI 1.11–16.28; P = .03) compared to those with CD4 count below this threshold. Other HIV-related factors such as WHO stage, viral load, duration on ART and ART regimen were not significantly associated with MetS.
Table 4.
Univariate and multivariate analysis of factors associated with metabolic syndrome.
3.4. Predicted 10-year ASCVD risk score
Out of 598 participants, we could not calculate the ASCVD risk score for 300 participants in accordance with the ASCVD risk score algorithm.[36] These included 34 participants without blood samples, 10 with prior history of stroke or myocardial infarction, and 202 participants aged <40 years or >79 years. Among those aged between 40 and 79 years, the data for 53 participants was not in the range accepted for risk calculation and included 6 participants who had HDL-C <20 mg/dL or >100 mg/dL, 41 had a total cholesterol <130 mg/dL or >320 mg/dL and 6 participants with a systolic blood pressure of <90 mmHg or >200 mmHg.
Among the 298 participants with complete data who had ASCVD risk scores computed, 182 (61%) were HIV-positive and 116 (39%) were HIV-negative. Of these, 137 (46%) were females and median age was 52 years (IQR 44, 58).
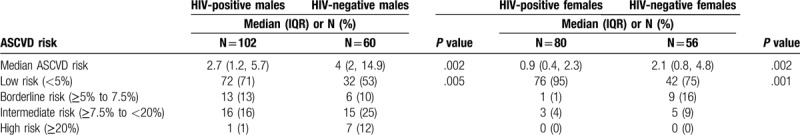
Overall, 3% of participants (8/298) had a high ASCVD risk score of which all were males and majority were HIV-negative (7/8). A quarter of participants (68/298) were classified as borderline and intermediate risk of ASCVD. The median ASCVD risk score was lower among PLWHIV as compared to HIV-negative participants (Median 1.7%; IQR 0.7%, 3.65% and 2.95%; IQR 1.1%, 7.15%, respectively; P = .002) (Table 5). PLWHIV had a lower median ASCVD score regardless of sex.
Table 5.
Atherosclerotic cardiovascular disease (ASCVD) risk score and risk categories stratified by HIV status and sex.
4. Discussion
We report findings of a large cross-sectional study examining prevalence and correlates of MetS and ASCVD risk scores among HIV-positive and HV-negative participants in Kenya. While studies in middle and high income countries have suggested that PLWHIV have higher CVD risk than those without HIV, we found that the prevalence of the MetS was higher among HIV-negative persons than PLWHIV; similarly, in a subset analysis of those participants >40 years of age, 10-year ASCVD risk was significantly greater among HIV-negative persons than PLWHIV.
Overall prevalence of MetS was high in this cohort (9%), as was prevalence of its individual components. There has been mixed evidence on the prevalence of MetS among PLWHIV in Africa. A recent metanalysis in SSA found an overall prevalence of MetS of 21.5% among PLWHIV compared to 12% among those without HIV infection, however there was great variation between studies that could be due to differences in populations and definitions of MetS criteria.[24] Other studies have reported results more consistent with those reported here, finding PLWHIV to have lower prevalence of MetS than HIV-negative adults. Jacobson et al found that PLWHIV in the Nutrition for Healthy Living study, were less likely to have MetS compared to National Health and Nutrition Examination Survey participants regardless of ART use,[40] and Nguyen et al reported no difference between the prevalence of MetS among PLWHIV and the general population globally in a meta-analysis including a total of 65 studies across the five continents.[41]
It is intriguing that MetS is less prevalent among PLWHIV than HIV-negative adults in this study based in rural Kenya. The fact that we included only those on ART >6 months is likely to be an important contributor; these results may reflect the success of ART, both in achieving viral suppression and reducing the contribution of HIV and HIV-related inflammation to MetS. Second, PLWHIV receive regular education on risk reduction and nutrition counseling in HIV clinics. PLWHIV in this cohort were required to be enrolled in an HIV clinic which mandates clinic visits every 3 months. HIV-negative individuals do not have similar opportunities for preventative healthcare and generally have less interaction with the health system. In addition, there is a potential of survival bias as successfully treated PLWHIV likely survive to an older age and hence may be different from HIV-negative individuals or untreated PLWHIV.
Elevated blood pressure, low HDL-C, and abdominal obesity were the most prevalent components of MetS in this cohort, findings similar to those reported by other studies in Kenya, as well as in other parts of SSA.[42,43] We also found that prevalence of these components was lower among PLWHIV compared to HIV-negative individuals. Similarly a recent meta-analysis including data from nearly 30,000 individuals in SSA reported a lower level of risk factors such as hypertension, diabetes, abdominal obesity, dyslipidemia and greater BMI among PLWHIV compared to HIV-negative adults.[29,44] Yet, despite PLWHIV having lower systolic blood pressure, total cholesterol, HDL-C and LDL-C, there was no significant difference in the prevalence of MetS by HIV status, according to Fourie et al.[29] It is also interesting to note that rates of smoking were lower in the HIV-positive than in the HIV-negative adults in our study, contrary to findings in Western countries where there are high rates of smoking and alcohol use among PLWHIV. While traditional risk factors clearly vary with local context, the fact that half of our participants had 1 to 2 CVD risk factors, precursors for developing MetS, suggests that a large proportion would likely benefit from interventions to deter progression to MetS.
Among PLWHIV, the relationship between CD4 and MetS remains unclear. We report here that a high CD4 count was associated with increased likelihood of MetS while other HIV-related factors, including viral suppression, were not significantly associated. Similar findings were reported by Mondy et al from the United States and Bonfanti et al from Italy.[45,46] One explanation for higher CD4 count being associated with MetS is that these participants with restored immune systems have been living with HIV and on ART longer and may be experiencing cumulative effects of the disease or drugs. Of note, we found no association with the ART drugs and MetS among PLWHIV. This may be due to adoption of drugs with fewer metabolic adverse effects as part of the Kenyan national ART regimen; only a small proportion of our participants were using PI-based regimens as seen in other studies in Kenya.[47] We did not find time since HIV diagnosis or duration of ART to be associated with MetS, however these merit further investigation. Future longitudinal studies with hard clinical endpoints, such as stroke and myocardial infarction, would help to confirm these trends in MetS and ASCVD risk scores for PLWHIV when compared to HIV-negative older adults in SSA.
Our study findings showed that PLWHIV had lower ASCVD risk scores than those who were HIV-negative. In addition, increasing age was associated with MetS, as has been noted in several African countries.[42] Because the criteria for MetS do not include age, smoking, or total cholesterol, a combination of MetS assessment and ASCVD risk scoring could help focus on those who are at highest risk for treatment, targeting prevention among those in borderline and intermediate risk groups and potentially reducing health care costs and improving treatment outcomes.
Our study has several strengths and limitations. This is one of the largest studies in the literature that directly compares the burden of MetS and CVD risk among people with and without HIV in SSA. Our study is also different from others in that it compared PLWHIV who on stable ART and mostly with undetectable HIV viral loads, to those who were HIV-negative, a comparison that is highly relevant in the current era of universal test and treat ART programs. The main study limitations are the cross-sectional study design and the fact that the study was conducted at a single facility; these may limit its generalizability. With respect to the ASCVD risk score, since the PLWHIV were older, we found a higher proportion of PLWHIV having prior cardiovascular events and these individuals were excluded from analysis of ASCVD risk score. Excluding high risk participants who are HIV-positive could have influenced our ASCVD results, as could the fact that the ASCVD risk score is not validated for African populations.
5. Conclusion
MetS prevalence was high in both HIV-positive and negative adults in western Kenya (1 in 20 and 1 in 10, respectively). Importantly, PLWHIV were less likely to have MetS and had lower ASCVD risk scores than HIV-negative participants. Together these findings emphasize the need for CVD risk assessment among people living with and without HIV infection and further longitudinal studies to determine those risk factors most highly predictive of CVD events. Early identification of persons with MetS and those at high ASCVD risk should guide decision-making regarding risk reduction strategies, such as targeting statin therapy, at the national level. This study also emphasizes the importance of advocating for additional support for integration of routine CVD screening and management into health programs in resource-limited settings, regardless of HIV status.
Author contributions
CF, STP, SJM developed and implemented the CVD study protocol. SJM, JH, and CF designed this analysis. JNM and SJM coordinated data collection. SJM, TT, JZ, AO analyzed the data, and SM drafted the manuscript. All authors contributed to editing of the manuscript and approved submission of the final draft for publication.
Conceptualization: Sarah Masyuko, Stephanie T. Page, John Kinuthia, Alfred O. Osoti, Stephen J. Polyak, Joseph M. Kibachio, Damalie Nakanjako, Carey Farquhar.
Data curation: Sarah Masyuko, Jerusha N. Mogaka.
Formal analysis: Sarah Masyuko, Alfred O. Osoti, Tecla M. Temu, Jerry S. Zifodya, James P. Hughes, Carey Farquhar.
Funding acquisition: Sarah Masyuko, Stephanie T. Page, John Kinuthia, Joseph M. Kibachio, Amos Otedo, Damalie Nakanjako, Carey Farquhar.
Investigation: Sarah Masyuko, Stephanie T. Page, Stephen J. Polyak, Jerusha N. Mogaka, Tecla M. Temu, Amos Otedo.
Methodology: Sarah Masyuko, Stephanie T. Page, John Kinuthia, Alfred O. Osoti, Stephen J. Polyak, Fredrick C. Otieno, Joseph M. Kibachio, Tecla M. Temu, Jerry S. Zifodya, Amos Otedo, Damalie Nakanjako, James P. Hughes, Carey Farquhar.
Project administration: Sarah Masyuko, John Kinuthia, Jerusha N. Mogaka, Carey Farquhar.
Resources: Sarah Masyuko, Stephanie T. Page, John Kinuthia, Stephen J. Polyak, Amos Otedo, Carey Farquhar.
Software: Sarah Masyuko.
Supervision: Sarah Masyuko, Stephanie T. Page, John Kinuthia, Alfred O. Osoti, Jerusha N. Mogaka, Tecla M. Temu, Jerry S. Zifodya, Carey Farquhar.
Validation: Sarah Masyuko, Stephanie T. Page, Alfred O. Osoti, Jerusha N. Mogaka, Tecla M. Temu, Jerry S. Zifodya, James P. Hughes, Carey Farquhar.
Visualization: Sarah Masyuko, Jerusha N. Mogaka, James P. Hughes, Carey Farquhar.
Writing – original draft: Sarah Masyuko.
Writing – review & editing: Sarah Masyuko, Stephanie T. Page, John Kinuthia, Alfred O. Osoti, Stephen J. Polyak, Fredrick C. Otieno, Joseph M. Kibachio, Jerusha N. Mogaka, Tecla M. Temu, Jerry S. Zifodya, Amos Otedo, Damalie Nakanjako, James P. Hughes, Carey Farquhar.
Supplementary Material
Footnotes
Abbreviations: 95%CI = 95% confidence interval, ACC = American College of Cardiology, AHA = American Heart Association, AOR = adjusted odds ratio, ART = antiretroviral therapy, ASCVD = atherosclerotic cardiovascular disease, BMI = body mass index, CCC = comprehensive care clinic, CVD = cardiovascular disease, HDL-C = High density lipoprotein cholesterol, IQR = inter-quartile range, KEMRI = Kenya Medical Research Institute, KNH = Kenyatta National Hospital, LDL-C = low density lipoprotein cholesterol, MetS = metabolic syndrome, PI = protease inhibitor, PLWHIV = people living with HIV, SSA = Sub-Saharan Africa, STEPS = STEPwise approach to chronic disease risk factor surveillance, UoN = University of Nairobi, WHO = World Health Organization.
How to cite this article: Masyuko SJ, Page ST, Kinuthia J, Osoti AO, Polyak SJ, Otieno FC, Kibachio JM, Mogaka JN, Temu TM, Zifodya JS, Otedo A, Nakanjako D, Hughes JP, Farquhar C. Metabolic syndrome and 10-year cardiovascular risk among HIV-positive and HIV-negative adults: a cross-sectional study. Medicine. 2020;99:27(e20845).
Human subjects approval was obtained from the University of Washington Institutional Review Board and locally from the Kenyatta National Hospital (KNH)/University of Nairobi (UoN) Ethical and Scientific Review Committee.
This study was funded byNational Institutes of Health (NIH) R21TW010459 and Fogarty International Center (FIC) D43 TW009580.
The authors have no conflicts of interest to disclose. Supplemental Digital Content 1. pdf (Figure 1: Proportion of participants displaying various number of individual components of MetS stratified by HIV status).
Supplemental Digital Content is available for this article.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].UNAIDS. Global HIV & AIDS statistics — 2019 Fact Sheet. 2019; Access date: 2/15/2020. https://www.unaids.org/en/resources/fact-sheet. [Google Scholar]
- [2].Boulle A, Schomaker M, May MT, et al. Mortality in patients with HIV-1 infection starting antiretroviral therapy in South Africa, Europe, or North America: a collaborative analysis of prospective studies. PLoS Med 2014;11:e1001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Danel C, Moh R, Gabillard D, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015;373:808–22. [DOI] [PubMed] [Google Scholar]
- [4].Lundgren JD, Babiker AG, Gordin F, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013;8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Autenrieth CS, Beck EJ, Stelzle D, et al. Global and regional trends of people living with HIV aged 50 and over: estimates and projections for 2000-2020. PLoS One 2018;13:e0207005–1207005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011;53:1120–6. [DOI] [PubMed] [Google Scholar]
- [8].Meir-Shafrir K, Pollack S. Accelerated aging in HIV patients. Rambam Maimonides Med J 2012;3:e0025–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shah ASV, Stelzle D, Lee KK, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV. Circulation 2018;138:1100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Currier JS, Taylor A, Boyd F, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr 2003;33:506–12. [DOI] [PubMed] [Google Scholar]
- [11].Durand M, Sheehy O, Baril JG, et al. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case-control study using Quebec's public health insurance database. J Acquir Immune Defic Syndr 2011;57:245–53. [DOI] [PubMed] [Google Scholar]
- [12].Klein D, Hurley LB, Quesenberry CP, Jr, et al. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection? J Acquir Immune Defic Syndr 2002;30:471–7. [DOI] [PubMed] [Google Scholar]
- [13].Lang S, Mary-Krause M, Cotte L, et al. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS (London, England) 2010;24:1228–30. [DOI] [PubMed] [Google Scholar]
- [14].Obel N, Thomsen HF, Kronborg G, et al. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clin Infect Dis 2007;44:1625–31. [DOI] [PubMed] [Google Scholar]
- [15].Triant VA, Lee H, Hadigan C, et al. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007;92:2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kaplan RC, Kingsley LA, Sharrett AR, et al. Ten-year predicted coronary heart disease risk in HIV-infected men and women. Clin Infect Dis 2007;45:1074–81. [DOI] [PubMed] [Google Scholar]
- [17].Nduka CU, Stranges S, Sarki AM, et al. Evidence of increased blood pressure and hypertension risk among people living with HIV on antiretroviral therapy: a systematic review with meta-analysis. J Hum Hypertens 2016;30:355–62. [DOI] [PubMed] [Google Scholar]
- [18].Nguyen NPT, Tran BX, Hwang LY, et al. Prevalence of cigarette smoking and associated factors in a large sample of HIV-positive patients receiving antiretroviral therapy in Vietnam. PLoS One 2015;10:e0118185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mdege ND, Shah S, Ayo-Yusuf OA, et al. Tobacco use among people living with HIV: analysis of data from Demographic and Health Surveys from 28 low-income and middle-income countries. Lancet Global Health 2017;5:e578–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yusuf S, Hawken S, Ôunpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–52. [DOI] [PubMed] [Google Scholar]
- [21].Nou E, Lo J, Grinspoon SK. Inflammation, immune activation, and cardiovascular disease in HIV. AIDS (London, England) 2016;30:1495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Alberti KGMM, Eckel Robert H, Grundy Scott M, et al. Harmonizing the metabolic syndrome. Circulation 2009;120:1640–5. [DOI] [PubMed] [Google Scholar]
- [23].Wilson PWF, D’Agostino RB, Parise H, et al. Metabolic syndrome as a precursor of cardiovascular disease and Type 2 diabetes mellitus. Circulation 2005;112:3066–72. [DOI] [PubMed] [Google Scholar]
- [24].Todowede OO, Mianda SZ, Sartorius B. Prevalence of metabolic syndrome among HIV-positive and HIV-negative populations in sub-Saharan Africa—a systematic review and meta-analysis. Syst Rev 2019;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Naidu S, Ponnampalvanar S, Kamaruzzaman SB, et al. Prevalence of metabolic syndrome among people living with HIV in developing countries: a systematic review. AIDS Patient Care STDS 2017;31:1–3. [DOI] [PubMed] [Google Scholar]
- [26].Mbunkah HA, Meriki HD, Kukwah AT, et al. Prevalence of metabolic syndrome in human immunodeficiency virus - infected patients from the South-West region of Cameroon, using the adult treatment panel III criteria. Diabetol Metab Syndr 2014;6:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ngatchou W, Lemogoum D, Ndobo P, et al. Increased burden and severity of metabolic syndrome and arterial stiffness in treatment-naive HIV+ patients from Cameroon. Vasc Health Risk Manag 2013;9:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Awotedu K, Ekpebegh C, Longo-Mbenza B, et al. Prevalence of metabolic syndrome assessed by IDF and NCEP ATP 111 criteria and determinants of insulin resistance among HIV patients in the Eastern Cape Province of South Africa. Diab Metabol Syndr 2010;4:210–4. [Google Scholar]
- [29].Fourie CM, Van Rooyen JM, Kruger A, et al. Lipid abnormalities in a never-treated HIV-1 subtype C-infected African population. Lipids 2010;45:73–80. [DOI] [PubMed] [Google Scholar]
- [30].Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol 2007;49:403–14. [DOI] [PubMed] [Google Scholar]
- [31].Grundy SM. Metabolic syndrome: a multiplex cardiovascular risk factor. J Clin Endocrinol Metab 2007;92:399–404. [DOI] [PubMed] [Google Scholar]
- [32].Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med 2006;119:812–9. [DOI] [PubMed] [Google Scholar]
- [33].Dekker Jacqueline M, Girman C, Rhodes T, et al. Metabolic syndrome and 10-year cardiovascular disease risk in the Hoorn study. Circulation 2005;112:666–73. [DOI] [PubMed] [Google Scholar]
- [34].D’Agostino Ralph B, Vasan Ramachandran S, Pencina Michael J, et al. General cardiovascular risk profile for use in primary care. Circulation 2008;117:743–53. [DOI] [PubMed] [Google Scholar]
- [35].Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice Guidelines. Circulation 2019;140:e596–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Arnett DK, Blumenthal RS, Albert MA, et al. ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. 2019;74(10):e177–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ministry of Health. Kenya HIV Estimates Report Nairobi, Kenya: MOH. 2018. [Google Scholar]
- [38].World Health Organization. The WHO STEPwise approach to chronic disease risk factor surveillance (STEPS). Access date: 12/15/2019. http://www.who.int/ncds/surveillance/steps/STEPS_Instrument_v2.1.pdf. [Google Scholar]
- [39].Ministry of Health NASCP. Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infection in Kenya 2018 Edition. Nairobi, Kenya: NASCOP. 2018. [Google Scholar]
- [40].Jacobson DL, Tang AM, Spiegelman D, et al. Incidence of metabolic syndrome in a cohort of HIV-infected adults and prevalence relative to the US population (National Health and Nutrition Examination Survey). J Acquir Immune Defic Syndr 2006;43:458–66. [DOI] [PubMed] [Google Scholar]
- [41].Husain NE, Noor SK, Elmadhoun WM, et al. Diabetes, metabolic syndrome and dyslipidemia in people living with HIV in Africa: re-emerging challenges not to be forgotten. HIV AIDS (Auckl) 2017;9:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Okafor CI. The metabolic syndrome in Africa: current trends. Indian J Endocrinol Metab 2012;16:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kiama CN, Wamicwe JN, Oyugi EO, et al. Prevalence and factors associated with metabolic syndrome in an urban population of adults living with HIV in Nairobi, Kenya. Pan Afr Med J 2018;29:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dillon DG, Gurdasani D, Riha J, et al. Association of HIV and ART with cardiometabolic traits in sub-Saharan Africa: a systematic review and meta-analysis. Int J Epidemiol 2013;42:1754–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mondy K, Overton ET, Grubb J, et al. Metabolic syndrome in HIV-infected patients from an urban, midwestern US outpatient population. Clin Infect Dis 2007;44:726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bonfanti P, De Socio GLV, Marconi P, et al. Is metabolic syndrome associated to HIV infection per se? Results from the HERMES Study. Curr HIV Res 2010;8:165–71. [DOI] [PubMed] [Google Scholar]
- [47].Osoti A, Temu TM, Kirui N, et al. Metabolic syndrome among antiretroviral therapy-naive versus experienced HIV-infected patients without preexisting cardiometabolic disorders in Western Kenya. AIDS Patient Care STDS 2018;32:215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


