Supplemental Digital Content is available in the text
Keywords: anticoagulation, atrial fibrillation, ischemic stroke, prediction model, stroke
Abstract
Atrial fibrillation (AF) is a major independent risk factor of stroke and anticoagulation therapy is needed in patients with AF after ischemic stroke. However, the detection rate of AF is low after ischemic stroke. Developing a prediction model for newly diagnosed AF after ischemic stroke will help to assess the subclinical AF.
We identified 98,103 patients with diabetes mellitus (DM) and 261,893 patients without DM, who were not AF history and admitted for newly ischemic stroke from the National Health Insurance Research Database in Taiwan. The prediction model for 3-year incidence of AF after ischemic stroke was derived from multivariate logistic regression and also the accuracy rate of the prediction model was compared with CHA2DS2-VASC and CHADS2 scores as a reference.
Four thousand nine hundred seventy six patients in the DM cohort and 16,127 patients in the non-DM cohort developed AF during 3 years of follow-up. The variables in the point-based prediction model for non-DM patients (range: -3–28), included age, heart failure, coronary artery disease, gout, obstructive pulmonary disease, hypertension, female, and statin use, while those for DM patients (range: -2–30) included age, heart failure, coronary artery disease, chronic kidney disease, hypertension, obstructive pulmonary disease, and statin use. Compared to the CHADS2 and CHA2DS2-VASc scoring systems, this scoring system was better at predicting 3-year risk of AF after ischemic stroke in both cohorts.
This model might be useful in evaluating the benefit of insertable cardiac monitor implantation and anticoagulation agents in individual patients after ischemic stroke.
1. Introduction
Atrial fibrillation (AF) is associated with an increased risk of stroke and about 20% to 30% of patients with ischemic stroke are diagnosed with AF before, during or after the event.[1] Patients with stroke due to AF have a worse clinical outcome than patients with stroke due to other factors.[2] Anticoagulation decreased the risk of new-onset and recurrent stroke in AF patients.[2] The choice of anticoagulation therapy is totally different for stroke patients with and without AF.[2,3] In addtion, rivaroxaban for stroke prevention after emoblic stroke with undetermined source was not superior and even more risk of bleeding than aspirin.[4] Therefore, the diagnosis and early detection of AF is important, especially in patients after stroke. Current clinical guidelines suggest prolonged electrocardiographic monitoring (e.g., a 72-hour Holter recording) for stroke patients to enhance the detection of undiagnosed AF.[5] However, even an implantable loop recorder and prolonged Holter recording could detect only 15% to 20% of AF in ischemic stroke patients within 6 months and 2 years.[3,6] Risk stratification for ischemic stroke patients may be helpful in determining high-risk AF patients, and also in identifying candidates for aggressive clinical monitoring. Previous studies showed the that prevalence of diabetes mellitus (DM) was lower in AF patients with stroke than in non-AF patients with stroke and that there were different stroke patterns in DM and non-DM patients,[7] so we analyzed risk stratification in both DM and non-DM cohorts, in an attempt to avoid the interaction of DM and AF occurrence in stroke patients. The aim of this study was to develop a risk stratification system to identify DM and non-DM patients at high risk of developing AF after ischemic stroke.
2. Methods
2.1. Data sources
This national cohort study utilized data on stroke patients retrieved from Taiwans National Health Insurance Research Database (NHIRD) from January 1, 2001 to December 31, 2013. The NHIRD is part of Taiwan's National Health Insurance program, which is a compulsory single-payer healthcare system covering more than 20 million Taiwanese, and contains health care information dating back to 1997. NHIRD includes data of any outpatient visits, hospitalization, drug prescriptions, comorbidities, and vital status. In addition, identifying data of all participants were encrypted to protect their privacy, but they can be longitudinally followed since the encrypting procedure was consistent. Diseases were identified using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Our study population was coded as ICD-9-CM: 433–435, those who had experienced an ischemic stroke event. This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital (201701617B0).
2.2. Study population in 2 prediction models
A total of 471,048 subjects admitted to the hospital with a principal diagnosis of ischemic stroke between January 1, 2001 and December 31, 2013 were identified for this study. Patients aged <40 years were excluded. In order to evaluate the post-stroke incidence of AF, patients who were diagnosed with AF before or at the time of hospitalization for index ischemic stroke were excluded. In order to limit the type of index ischemic stroke to unknown etiology, the following exclusion criteria were set in this study. Patients who were diagnosed with carotid disease, including stenting for carotid arteries, and those who were diagnosed with cerebral arterial aneurysm or arteriovenous fistula or stenosis of the cerebrovascular arteries, were excluded.
In addition, those who were diagnosed with valvular heart disease, rheumatic heart disease, and hyperthyroidism, and who had been given long-term anticoagulation agents, were also excluded. Patients who died during the index hospitalization were excluded from the analysis. In the end, 359,996 patients were eligible for analysis. Although DM was a strong predictor of AF, several studies showed the incidence of AF was inconsistent in DM patients admitted for ischemic stroke and the incidence of stroke associated with atherothrombotic disease was higher than stroke associated with cardioembolic disease in the DM population.[7,8] Therefore, we used 2 prediction models to predict the development of new-onset AF after ischemic stroke: 1 for DM patients and the other for non-DM patients. Finally, 98,103 DM patients and 261,893 non-DM patients who were admitted for ischemic stroke, respectively, were included in the study.
2.3. Ascertainment of ischemic stroke, AF, and comorbidities
The index event of ischemic stroke was based on the principal diagnosis (ICD-9-CM: 433–435), meaning that the index hospitalization was for ischemic stroke. The positive predictive value (PPV) of ischemic stroke in the AF population was close to 95% in our previous study.[9] AF was diagnosed according to the diagnostic code of AF (ICD-9-CM: 42731) in 1 admission or consecutive 2 outpatient visits and its PPV was around 90% in previous validation.[10] Besides, inpatient and outpatient diagnosis and prescription data during the 12-month period before the index date to ascertain the other comorbidities (ICD-9-CM codes proved in Supplemental Table S1). The definitions of comorbidities were the same criteria as AF was as listed in Supplemental Table S1. Similarly, the medication was ascertained. (Anatomical Therapeutic Chemical code provided in Supplemental Table S1) Data on patients who suffered from AF development 3 years after the index ischemic stroke event were censored. Data on patients who died within 3 years after the index date were also censored.
2.4. CHA2DS2-VASC and CHADS2 scores as a referent model
CHA2DS2-VASc and CHADS2 scores were used to predict the incidence of stroke in non-rheumatic AF, and they were also used to predict the incidence of AF in some studies.[11] Therefore, these 2 scoring systems were used for comparison (a referent model) with our proposed scoring system. The CHA2DS2-VASc score involves a point system in which 2 points were assigned for a history of stroke or transient ischemic attack or age ≥75 years, and 1 point each was assigned for those aged 65 to 74 years or with a history of hypertension (HTN), diabetes, heart failure (HF), vascular disease (myocardial infarction or peripheral artery disease), and female gender. In the CHADS2 scoring system, 2 points were assigned for a history of stroke or transient ischemic attack and 1 point each for the presence of HTN, diabetes, congestive HF, and age ≥75 years.
2.5. Statistical analyses
We compared the distribution of baseline characteristics between the AF and non-AF patients using the t test for continuous variables and Chi-Squared test for categorical variables. To investigate the factors associated with the risk of AF development, we performed Cox proportional hazard models in 3 steps. First, each variable (a total of 12) was studied as an independent variable in the univariate analyses. Second, the significant variables (P < .05) in the univariate analyses were further introduced into the multivariable Cox model. Third, to achieve a parsimonious and easy-to-use model, non-significant variables were dropped in a reduced model.
To examine the problem of over-fitting in the reduced model, internal validation was performed using the bootstrap method with 200 bootstrapped samples to compare the area under receiver operating characteristic curves (AUC) between the original model (optimistic) and the bootstrap-corrected model. To assess the extent of discriminating AF development, we compared the AUC between our proposed scoring system and the existing scoring systems (CHADS2 and CHA2DS2-VASc scores). Based on the results of the reduced multivariable model, we calculated a simplified point system to demonstrate the associations between explanatory variables (covariates) and incident AF during a 3-year follow-up. Briefly, the points system rounds off the regression coefficients obtained from the multivariable Cox model. In the first step, a continuous predictor (i.e., age) with a wide range of values was treated as the reference variable and categorized into clinically relevant categories (i.e., <55 yrs., 55–64 yrs., 65–74 yrs., and ≥75 yrs.). Next, the reference value of each category of each predictor was calculated according to the value of its regression coefficient (i.e., female gender) relative to that of the reference variable. Finally, as a form of sensitivity analysis, we categorized the patients into 4 groups according to their prediction scores and compared the risk of recurrent ischemic stroke during a 13-year follow-up using a trend test of the log-rank test. A P value of <.05 was considered statistically significant. Data analysis was conducted using commercial software (SAS 9.4, SAS Institute, Cary, NC).
3. Results
3.1. Baseline characteristics of DM and non-DM patients with ischemic stroke with and without the occurrence of AF
A total of 359,996 patients were included in the study. Among this group, 98,103 had DM and 261,893 did not; 4976 of the DM patients and 16,127 of the non-DM patients, respectively, developed AF within a 3-year follow-up after ischemic stroke (Supplemental Fig. 1). The differences in baseline characteristics among the patients with and without AF in the DM and non-DM cohort are shown (Supplemental Table S2). Briefly, AF patients were older than non-AF patients whether in the DM or non-DM cohort. The proportion of female patients was higher among the AF patients than the non-AF patients in both cohorts. Comorbidities (except for dialysis in both cohorts, and chronic kidney disease (CKD) and peripheral artery occlusive disease (PAOD) in the DM cohort) and medications were significantly different for AF and non-AF patients in both cohorts. Patients who developed AF during follow-up had old myocardial infarct, coronary artery disease (CAD), chronic obstructive pulmonary disease (COPD), gout, HTN, HF, dyslipidemia, and old stroke more frequently than patients who did not develop AF in both cohorts. Abnormal liver function tests were less frequently noted in patients who developed AF during follow-up than in patients who did not develop AF in both cohorts. Patients who developed AF had more CAD and PAOD than those who did not develop AF in the non-DM cohort. Regarding medication use, patients who developed AF received angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker, calcium channel blocker, and beta-blocker more frequently, and statin therapy less frequently than those who did not develop AF in both cohorts.
3.2. Clinical factors associated with risk of AF development after ischemic stroke
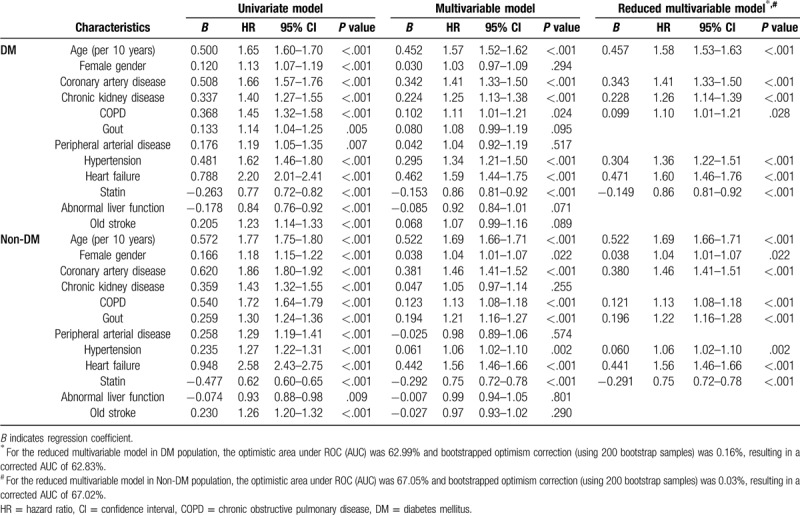
The univariate and multivariable analyses of clinical factors associated with the risk of developing AF after ischemic stroke are shown in Table 1. Twelve factors were significant in univariate analyses and were further introduced into the multivariable analyses of both the DM and non-DM cohorts. Seven independent factors (age, CAD, CKD, COPD, HTN, HF, and statin use) in the DM cohort and 8 independent factors (age, female, CAD, COPD, gout, HTN, HF, and statin use) in the non-DM cohort were significantly associated with the occurrence of AF after ischemic stroke during a 3-year follow-up. The predictors in the DM cohort, with the exception of statin, were positively associated with an increasing risk of developing AF during follow-up, after ischemic stroke. Statin was associated with a decreasing risk of AF after ischemic stroke in both the DM and non-DM cohorts.
Table 1.
Factors associated with risk of atrial fibrillation after ischemic stroke in patients with/without diabetes mellitus.
3.3. Bootstrapping validation of the proposed model
For the reduced multivariable model, the optimistic AUC was 62.99% and 67.05%, and the optimism correction using 200 bootstrapped samples was 0.16% and 0.03% in the DM and non-DM cohorts, respectively. This small discrepancy between the AUC obtained from the original model and that obtained using the bootstrap method indicated that over-fitting might be not an issue.
3.4. Comparisons of other scoring systems with the proposed scoring system
Figure 1 compare the AUCs for different risk scoring systems used to predict a 3-year AF risk after ischemic stroke in the DM and non-DM cohort. Figure 1A shows that, for predicting AF occurrence in DM patients with ischemic stroke, the c-indexes (AUC) were 0.576 (95% confidence interval [CI], 0.568–0.583) for CHADS2, 0.607 (95% CI, 0.600–0.615) for CHA2DS2-VASc, and 0.630 (95% CI, 0.623–0.638) for the proposed model. The ability of the proposed model to discriminate AF occurrence was superior to that of the CHADS2 and CHA2DS2-VASc scores (P of delta AUC < .001). Figure 1B shows similar results for non-DM patients, in that the proposed model (0.671; 95% CI, 0.667–0.675) had a higher AUC than the CHADS2 (0.600; 95% CI, 0.596–0.604) and CHA2DS2-VASc (0.636; 95% CI, 0.632–0.640) scoring systems.
Figure 1.
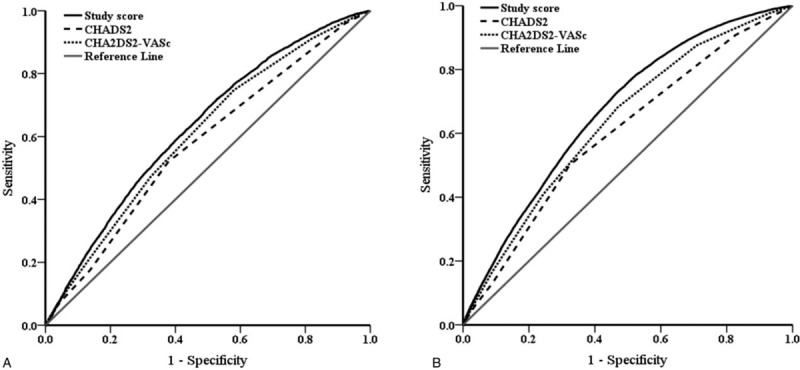
Receiver operating characteristic (ROC) curves of 3 different risk scoring systems in predicting a 3-year atrial fibrillation risk after ischemic stroke in the diabetes mellitus (DM) (A) and non-DM (B) cohort. 1A. ROC curves of the proposed model (area under the curve [AUC], 0.630; 95% confidence interval [CI], 0.623–0.638), CHADS2 (AUC, 0.576; 95% CI, 0.568–0.583) and CHA2DS2-VASc (AUC, 0.607; 95% CI, 0.600–0.615) in DM cohort. The AUC was significantly larger in the proposed model than in the CHADS2 and CHA2DS2-VASc (both P of delta AUC < .001). 1B. ROC curves of the proposed model (area under the curve [AUC], 0.671; 95% confidence interval [CI], 0.667–0.675), CHADS2 (AUC, 0.600; 95% CI, 0.596–0.604) and CHA2DS2-VASc (AUC, 0.636; 95% CI, 0.632–0.640) in non-DM cohort. The AUC was significantly larger in the proposed model than in the CHADS2 and CHA2DS2-VASc (both P of delta AUC < .001).
3.5. Simple point systems for the risk of AF development after ischemic stroke
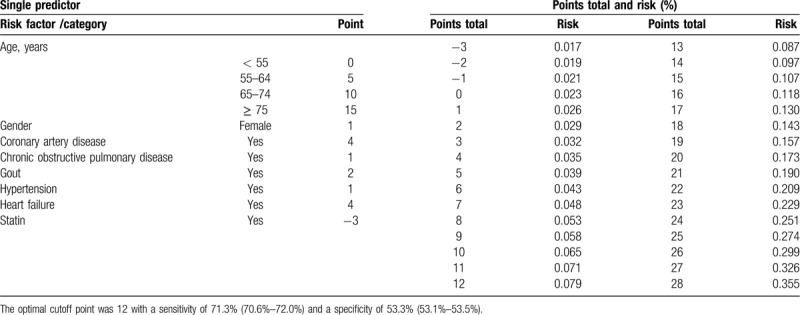
The simple point systems and risk of AF according to the reduced model of patients with and without DM are shown in Tables 2 and 3. Table 2 shows the points of a single predictor and the total points and risk of AF among patients with DM (total score: 30 points). Because of the reduced risk of AF with statin use, statin use was given -2 points. The total score ranged from −2 to 30. The risk of developing AF within 3 years ranged from 1.7% (score: −2) to 27.6% (score: 30). The optimal cutoff point was 15 with a sensitivity of 58.5% (95% CI: 57.2%–59.9%) and a specificity of 60.0% (95% CI: 59.7%–60.3%). Table 3 shows the points of a single predictor and the total points and risk of AF in patients without DM (total score: 28 points). The total score ranged from −3 to 28. The risk of developing AF within 3 years ranged from 1.7% (score: −3) to 35.5% (score: 28). The optimal cutoff point was 12 with a sensitivity of 71.3% (95% CI: 70.6%–72.0%) and a specificity of 53.3% (95% CI: 53.1%–53.5%).
Table 2.
Simple points system according to the reduced model of patients with diabetes mellitus (Total score 30 points).
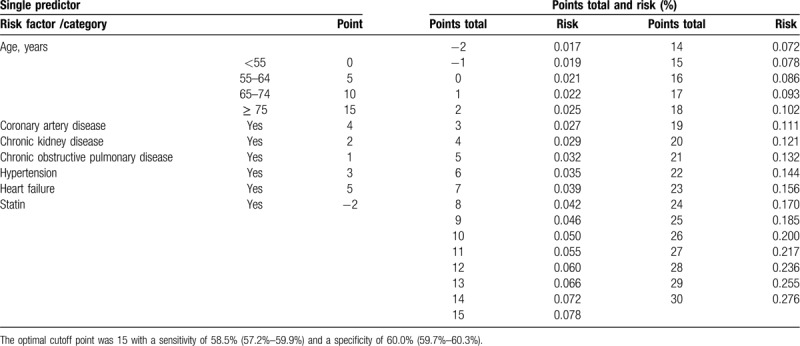
Table 3.
Simple points system according to the reduced model of patients without diabetes mellitus (Total score 28 points).
3.6. Simple points system for risk of recurrent stroke
The values of the simple points system were divided into 4 groups (quartiles), and the risk of recurrent stroke was compared among groups. Figure 2 shows that the risk of recurrent stroke was higher when the predicting scores increased in both the DM and non-DM cohorts (P trend < .001).
Figure 2.
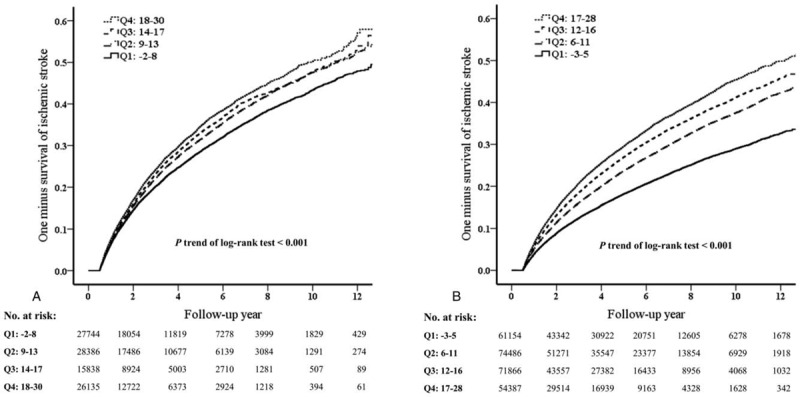
Survival after ischemic stroke stratified by the quartile of proposed study scores in the DM population (A) and non-DM population (B).
4. Discussion
There are several important findings in this study. First, patients with ischemic stroke who developed AF rhythm were older and had more comorbidity, whether in the DM or non-DM cohort. Second, patients with ischemic stroke who received statin therapy less frequently had AF rhythm during follow-up. Third, our scoring system was better at predicting AF occurrence after ischemic stroke than the CHADS2 score and CHA2DS2-VASc score. Finally, those patients who had a higher score had a higher risk of recurrent stroke than those patients who had a lower score.
4.1. Risk factors for patients in both cohorts with ischemic stroke developing AF during follow-up
Our study showed that patients with ischemic stroke shared the same risk factors for AF occurrence in both cohorts (age, HTN, HF, CAD). In clinical practice, these risk factors are common among patients at risk for silent AF.[12] A previous study reported the adjusted hazard ratio for AF among those with COPD was 2.23 compared to those without COPD, irrespective of DM.[13] The chronic inflammation status, hypoxemia and cardiac remodeling due to COPD may explain the increased risk of AF in COPD patients. In our study, COPD was an independent predictor of AF in patients with ischemic stroke. Our study also found that CKD was an independent factor for developing AF in ischemic stroke patients with DM. CKD is associated with insulin resistance and insulin resistance has been associated with increased risk of AF.[14] Although men have a higher incidence of AF,[15] women with AF have double the risk of stroke compared to men, after adjustment for other comorbidity.[16] This may explain the higher risk of female gender in predicting AF after ischemic stroke. The systemic inflammation and impact of left atrial remodeling of gout may explain its prediction of AF in our study.[17] Previous and current studies show statin use and adherence are associated with a reduced first or recurrent stroke risk in patients with and without AF.[18] Our study, using a large nationwide database, confirms previous findings, and reveals the independent predictors of AF occurrence in patients with ischemic stroke with and without DM.
4.2. A novel scoring system for predicting AF and recurrent stroke in patients with ischemic stroke, irrespective of DM
A previous small cohort study showed CHADS2 and CHA2DS2-VASc scores could predict the risk of AF in patients with ischemic stroke.[19] However, another large study reported no difference among patients with different CHA2DS2-VASc scores in the detection rate of AF using an implantable cardiac monitor.[20] In this study, our novel scoring system performed better than CHADS2 or CHA2DS2-VASc scores in predicting the occurrence of AF after ischemic stroke during traditional clinical follow-up of patients with and without DM. This means our scoring system is more useful in determining those ischemic stroke patients who are truly at high risk of AF incidence. In addition, the risk of recurrent stroke was higher in patients with higher scores in both cohorts, according to our study. Therefore, our scoring system is very helpful in defining patients at a higher risk of AF occurrence and recurrent stroke, which is beneficial to identifying those who need intensified cardiac monitoring or even more aggressive anticoagulation use.
The overall detection rate for any AF after ischemic stroke ranged from 9.5% to 43% in previous studies with different interventions, durations, monitoring methods, and diagnostic criteria for paroxysmal AF and cryptogenic stroke.[1] Prospective studies evaluating invasive cardiac monitoring in small groups of patients with cryptogenic stroke showed varying detection rates (from 4.2% to 40%) during long-term follow-up (14.5 to 36 months).[3,20,21] Detection rates tended to be higher with prolonged periods of monitoring in selected patient groups. However, insertable cardiac monitoring is more expensive and is inconvenient for patients in terms of quality of life. Our study also identified those patients with ischemic stroke who could obtain the most benefit from invasive cardiac monitoring to detect AF, which might influence the decision to initiate anticoagulation use.
4.3. Study limitations
There are some limitations in this retrospective insurance database study. First, there were no echocardiographic variables, including left atrial size, that might predict AF occurrence in stroke patients. However, 1 study showed that echocardiographic parameters did not improve the risk evaluation for predicting AF, and the accuracy of echocardiographic is highly technician-dependent, and measurement variability between observers is difficult to evaluate, even in a prospective trial with training and central analysis.[13] Therefore, echocardiographic measurement items may not be applicable for risk stratification in a clinical situation, especially with a large cohort population. Second, the subtype of AF was not recorded in the NHIRD database. However, most of the time, the AF type documented in patients with ischemic stroke is paroxysmal AF less than 2 days.[5] Third, neither the CHADS2 nor the CHA2DS2-VASC scores are designed to predict the occurrence of AF, they are intended to predict stroke risk related to AF. It is well known that atrial cardiopathy can induce embolism without the presence of AF.[22] This form of cardiopathy shares many of the described risk factors for AF, suggesting that there might be a pathophysiological continuum between it and AF. Further study is needed to evaluate the relationship and interaction of atrial cardiopathy and AF occurrence. Finally, although our study, using our novel risk scoring system, showed patients with a higher score had a higher risk of recurrent stroke, we did not evaluate the anticoagulation status before recurrent stroke; the use and adhesion of anticoagulation may influence the risk of recurrent stroke. We also did not evaluate the ablation status after occurrence of AF and before recurrent stroke. The different ablation strategies may influence the risk of recurrent AF and also recurrent stroke.[23–25]
5. Conclusions
This national cohort study showed that our novel risk scoring system might be used to predict AF and recurrent stroke effectively in ischemic stroke patients with or without DM. Patients in both cohorts shared the same risk factors for AF occurrence, including age, HTN, HF, CAD, and COPD. CKD was the independent predictive factor in the DM cohort, female gender, and gout were independent factors in the non-DM cohort. Our novel risk scoring system might be used for evaluation of the benefit of insertable cardiac monitoring and anticoagulation use.
Acknowledgments
We would like to thank Alfred Hsing-Fen Lin and Zoe Ya-Jhu Syu for the statistical assistance during the completion of this manuscript
Author contributions
Conceptualization: Yung-Lung Chen, Huang-Chung Chen, Wen-Jung Chung, Chi-Hung Liu, Yu-Sheng Lin.
Data curation: Yung-Lung Chen, Hui-Ting Wang, Huang-Chung Chen, Wen-Hao Liu, Shukai Hsueh, Chang-Ming Chung, Yu-Sheng Lin.
Formal analysis: Po-Jui Wu.
Investigation: Yung-Lung Chen, Hui-Ting Wang, Huang-Chung Chen, Shukai Hsueh, Po-Jui Wu, Yu-Sheng Lin.
Methodology: Hui-Ting Wang, Wen-Hao Liu, Po-Jui Wu.
Validation: Yung-Lung Chen, Wen-Hao Liu, Shukai Hsueh, Wen-Jung Chung, Chi-Hung Liu, Chang-Ming Chung, Yu-Sheng Lin.
Writing – original draft: Yung-Lung Chen.
Writing – review & editing: Hui-Ting Wang, Chi-Hung Liu, Yu-Sheng Lin.
Supplementary Material
Footnotes
Abbreviations: AF = atrial fibrillation, AUC = area under receiver operating characteristic curves, CAD = coronary artery disease, CKD = chronic kidney disease, COPD = chronic obstructive pulmonary disease, DM = diabetes mellitus, HF = heart failure, HTN = hypertension, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, NHIRD = National Health Insurance Research Database, PAOD = peripheral artery occlusive disease, PPV = positive predictive value.
How to cite this article: Chen YL, Wang HT, Chen HC, Liu WH, Hsueh S, Chung WJ, Wu PJ, Liu CH, Chung CM, Lin YS. A risk stratification scoring system for new-onset atrial fibrillation after ischemic stroke: a National cohort study. Medicine. 2020;99:27(e20881).
The authors report no conflicts of interest.
Supplemental Digital content is available for this article.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. This study has no funding source.
References
- [1].Kishore A, Vail A, Majid A, et al. Detection of atrial fibrillation after ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. Stroke 2014;45:520–6. [DOI] [PubMed] [Google Scholar]
- [2].Steffel J, Verhamme P, Potpara TS, et al. The 2018 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: executive summary. Europace 2018;20:1231–42. [DOI] [PubMed] [Google Scholar]
- [3].Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- [4].Hart RG, Sharma M, Mundl H, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med 2018;378:2191–201. [DOI] [PubMed] [Google Scholar]
- [5].Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation--developed with the special contribution of the European Heart Rhythm Association. Europace 2012;14:1385–413. [DOI] [PubMed] [Google Scholar]
- [6].Wachter R, Groschel K, Gelbrich G, et al. Holter-electrocardiogram-monitoring in patients with acute ischaemic stroke (Find-AFRANDOMISED): an open-label randomised controlled trial. Lancet Neurol 2017;16:282–90. [DOI] [PubMed] [Google Scholar]
- [7].Lamassa M, Di Carlo A, Pracucci G, et al. Characteristics, outcome, and care of stroke associated with atrial fibrillation in Europe: data from a multicenter multinational hospital-based registry (The European Community Stroke Project). Stroke 2001;32:392–8. [DOI] [PubMed] [Google Scholar]
- [8].Li L, Yiin GS, Geraghty OC, et al. Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population-based study. Lancet Neurol 2015;14:903–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lin YS, Chen TH, Chi CC, et al. Different implications of heart failure, ischemic stroke, and mortality between nonvalvular atrial fibrillation and atrial flutter-a view from a national cohort study. J Am Heart Assoc 2017;6:e006406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chang CH, Lee YC, Tsai CT, et al. Continuation of statin therapy and a decreased risk of atrial fibrillation/flutter in patients with and without chronic kidney disease. Atherosclerosis 2014;232:224–30. [DOI] [PubMed] [Google Scholar]
- [11].Barkas F, Elisaf M, Korantzopoulos P, et al. The CHADS2 and CHA2DS2-VASc scores predict atrial fibrillation in dyslipidemic individuals: Role of incorporating low high-density lipoprotein cholesterol levels. Int J Cardiol 2017;241:194–9. [DOI] [PubMed] [Google Scholar]
- [12].Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016;18:1609–78. [DOI] [PubMed] [Google Scholar]
- [13].Liao KM, Chen CY. Incidence and risk factors of atrial fibrillation in Asian COPD patients. Int J Chron Obstruct Pulmon Dis 2017;12:2523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nguyen JT, Benditt DG. Atrial fibrillation susceptibility in metabolic syndrome: simply the sum of its parts? Circulation 2008;117:1249–51. [DOI] [PubMed] [Google Scholar]
- [15].Humphries KH, Kerr CR, Connolly SJ, et al. New-onset atrial fibrillation: sex differences in presentation, treatment, and outcome. Circulation 2001;103:2365–70. [DOI] [PubMed] [Google Scholar]
- [16].Reeves MJ, Bushnell CD, Howard G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol 2008;7:915–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Watanabe H, Tanabe N, Watanabe T, et al. Metabolic syndrome and risk of development of atrial fibrillation: the Niigata preventive medicine study. Circulation 2008;117:1255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Amarenco P, Bogousslavsky J, Callahan A, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:549–59. [DOI] [PubMed] [Google Scholar]
- [19].Ziegler PD, Glotzer TV, Daoud EG, et al. Detection of previously undiagnosed atrial fibrillation in patients with stroke risk factors and usefulness of continuous monitoring in primary stroke prevention. Am J Cardiol 2012;110:1309–14. [DOI] [PubMed] [Google Scholar]
- [20].Reiffel JA, Verma A, Kowey PR, et al. Incidence of previously undiagnosed atrial fibrillation using insertable cardiac monitors in a high-risk population: the reveal AF study. JAMA Cardiol 2017;2:1120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lau CP, Siu CW, Yiu KH, et al. Subclinical atrial fibrillation and stroke: insights from continuous monitoring by implanted cardiac electronic devices. Europace 2015;17: Suppl 2: ii40–6. [DOI] [PubMed] [Google Scholar]
- [22].Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 2014;129:e28–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Guler TE, Aksu T, Yalin K, et al. Combined cryoballoon and radiofrequency ablation versus radiofrequency ablation alone for long-standing persistent atrial fibrillation. Am J Med Sci 2017;354:586–96. [DOI] [PubMed] [Google Scholar]
- [24].Yalin K, Abdin A, Lyan E, et al. Safety and efficacy of persistent atrial fibrillation ablation using the second-generation cryoballoon. Clin Res Cardiol 2018;107:570–7. [DOI] [PubMed] [Google Scholar]
- [25].Friberg L, Tabrizi F, Englund A. Catheter ablation for atrial fibrillation is associated with lower incidence of stroke and death: data from Swedish health registries. Eur Heart J 2016;37:2478–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


