Supplemental Digital Content is available in the text
Keywords: drug-related problems, medication adherence, parkinson's disease, pharmaceutical service, quality of life
Abstract
Background:
Medicines optimisation is important for the management of Parkinson's disease (PD). As many patients with PD have other long-term conditions, treatment is complex and risk of adverse events for these patients is high.
Objective:
To explore the role of pharmacists and impact of pharmacy interventions for PD patients.
Methods:
We comprehensively searched PubMed, Embase, the Cochrane Library and Chinese databases Sinomed, China National Knowledge Infrastructure to identify studies reporting pharmacist interventions and pharmacy services for PD patients using a predefined search strategy. The search period was from inception to March 2019. We also manually searched the reference list of included studies and ClinicalTrials.gov. We conducted meta-analyses to synthesize the evidence quantitatively.
Results:
A total of 1607 studies were identified by applying the search criteria. After screening, 19 cross-sectional and case-controlled studies with 1458 PD patients from 9 countries were included. Pharmacist interventions for PD patients most commonly related to adverse drug reactions (ADRs) (13 studies), adherence assessment (12 studies), medication review (12 studies), identification of drug interactions (11 studies), monitoring response to medication therapy (11 studies), identification of inappropriate medication (11 studies), and patient education (10 studies). Most pharmacy services were provided in outpatient settings (13 studies). Reported impact measures included adherence (8 studies), quality of life (7 studies), and identification of drug-related problems (6 studies) such as ADRs (393 times out of 1760 times, 22.33%, 6 studies), inappropriate drug choice (349 times, 19.83%, 6 studies), inappropriate dosage (335 times, 19.03%, 6 studies), inappropriate drug use (257 times, 14.60%, 3 studies) and drug-drug interactions (146 times, 8.3%, 4 studies). Pooled results from 3 studies indicated no statistically significant impact of pharmacy services on all subscales of PD Questionnaire-39.
Conclusion:
ADRs were the most widely reported drug-related problems for PD patients; pharmacy services may have a role to play in medication adherence but were not found to impact on quality of life.
1. Introduction
Parkinson's disease (PD) is a common neurodegenerative disease, characterized by progressive degeneration of dopaminergic neurons and pathological changes in the formation of Lewy bodies, and decreased dopamine transmitters in the striatum.[1] The estimated incidence of PD is 10 to 18 per 100,000 person-years globally[1] and 250 persons per 100,000 in the UK.[2] Epidemiological studies indicate that the prevalence of PD in the Chinese population aged 65 and over is 1.7%, and that this is rising as the population is aging, in common with global estimates of large increases in PD prevalence.[3] As a consequence, the treatment and management of PD patients represent a growing financial burden due to the high medication costs, hospitalization and productivity loss associated with the progression of PD.[1]
Choice of medication for PD patients is complicated, and is dependent on age of disease onset and stage of disease development.[4] Moreover, because PD patients often have a variety of comorbidities and require multiple therapeutic drugs, and are therefore likely to experience polypharmacy, PD patients are at an increased risk of drug interactions.[5] In addition, as the course of disease progresses, the likelihood of adverse events such as hypotension and hallucinations increases, which further complicates treatment decisions.[6] As a consequence, pharmacists can play an important role in improving medicines optimisation for PD patients, ensuring the safe and rational use of medication.
Pharmacists have a role in assessing patients’ previous medication history, drug allergy history, adverse events, and drug interactions, especially for multiple drugs and low safety index drugs. Martin et al conducted a cluster randomized trial on the effectiveness of a pharmacist-led educational intervention for older adults, and found greater discontinuation of prescriptions for inappropriate medication after 6 months compared with usual care.[7] Meanwhile, from an economic perspective, pharmacists’ participation in drug treatment management can reduce drug-related problems (DRPs) and by preventing medication errors and other associated costs pharmacists’ contributions to patient care are cost effective.[8]
Pharmacy services for PD patients are also becoming popular. Such services include pharmacists participating in outpatient clinics, reviewing patients’ previous medicines, providing drug information, patient education, evaluating medication adherence, and monitoring drug treatment effects.[9] The results of a number of pharmacy service evaluations have shown that patients’ adherence and quality of life (QoL) improved following pharmacist intervention.[10,11] However, a theory or logic model for how pharmacist interventions and pharmacy services produce these outcomes for PD patients and the likely causal mechanisms through which pharmacist interventions and pharmacy services work, and the influence of context on mechanisms and outcomes, have not been established. The first step in addressing this gap is to undertake a systematic review to identify what is already known about pharmacist interventions and pharmacy services for PD patients and the methods that have been used to evaluate them. In this study, we investigate the role of pharmacists and impact of pharmacy interventions for PD patients as a precursor to devising a logic model useful for informing future design and evaluation of a complex intervention for PD patients.
2. Materials and methods
2.1. Search strategy
We searched PubMed, Embase, the Cochrane Library, and 2 Chinese databases including Sinomed and China National Knowledge Infrastructure from inception to March 26th, 2019 to identify studies reporting findings related to pharmacy service for PD. The search terms included the following keywords
“Parkinson's disease”, “pharmacist”, “pharmaceutical care”, “pharmaceutical service”, “medication therapy management” and “drug-related problems” (Appendix 1, Available at:). We also manually searched the reference list of the included studies. We limited the language of articles to English and Chinese only, but had no limits on the type of pharmacy service or on the country in which the service was provided. We also manually searched the reference list of the included studies and ClinicalTrials.gov as a supplementary source for relevant literature. Experts in this field of study were consulted for advice regarding the search strategy. The systematic review with meta-analysis was registered on PROSPERO (No. CRD 42018107614). However, because it is a systematic review and meta-analysis it is exempt from requiring ethical approval, as the study involves analysis of previously published, anonymized data.
2.2. Study selection and outcome measures
The investigators worked in pairs (TTL and QYT; TTL and ZMY) to manually screen the references of all retrieved records for potentially relevant studies, beginning with title and abstract screening in the first stage, and full-text screening in the second. In the title and abstract screening stage, studies appearing to meet the inclusion criteria, or with insufficient information to make a clear judgment, were included in the full-text screening process. We obtained full texts of all these studies for the full-text screening. We included studies if they reported on pharmacy services for PD patients where either a description of the content or outcomes of a pharmacy service was given. Studies using randomized controlled trials or observational designs were included. All disagreements about study selection were resolved through discussion amongst the team.
Primary outcomes extracted for inclusion in the review were contents of pharmacy services and evidence of impact of the pharmacy service on patients. Secondary outcomes extracted included types and methods for measuring impact and outcomes of pharmacy service.
2.3. Data extraction and quality assessment
Data extraction was performed by the same 2 pairs (TTL and QYT; TTL and ZMY) using a pre-designed data collection tool. The following information was extracted from included studies: first author, publication year, country, patient source, number of patients, age, gender, duration of PD, number of medications/PD medications per patient, content and outcomes of pharmacy services.
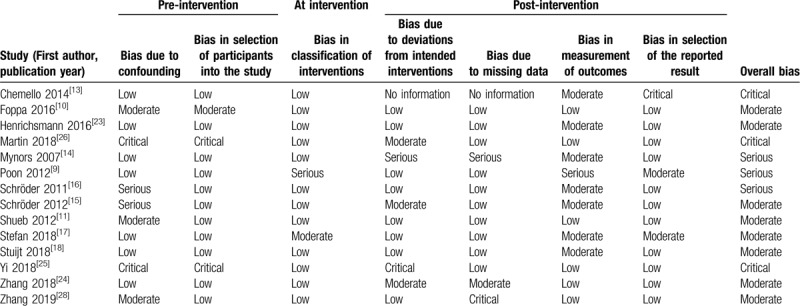
We assessed the risk of bias of randomized controlled trials included in this study using the Cochrane collaboration tool, and used the ROBINS-I tool for other study designs.[12] The ROBINS-I tool for assessing risk of bias considers bias due to confounding, classification of participants, classification of intervention, missing data, outcome measurements, and selective outcome reporting. The categories for risk of bias judgements are “low risk”, “moderate risk”, “serious risk” and “critical risk” of bias. The assessment was independently performed by TTL and QYT, and any discrepancies were resolved through discussion with ZMY.
2.4. Statistical analysis
We explored treatment effect through meta-analysis in an intention to treat manner (following the allocation of participants in studies). We pooled results of the same types of studies evaluating similar interventions in similar participants. We calculated the mean difference (MD) with 95% confidence intervals (CIs) for continuous outcomes and risk ratio for categorical outcomes. We performed meta-analyses with RevMan 5.3 software using the random-effect model. Statistical heterogeneity was assessed with the Mantel-Haenszel chi-square test and quantified with the I2 test (P-value of heterogeneity was .10). Sensitivity analysis was conducted by changing the random-effect methods to fixed-effect methods to pool the trials in terms of heterogeneity of findings. Finally, publication bias was examined by funnel plot if the number of included studies was over 10.
3. Results
3.1. Study selection
The initial search identified 1605 relevant records, with a further 2 additional records identified through other sources (consultation with PD experts). Of these, 1529 were excluded after duplicates were removed and title/abstract screening, leaving 78 papers eligible for full-text review. Of these 19 studies met the inclusion criteria[9–11,13–28] involving 1458 PD patients. Figure 1 provides details of the reasons for excluding studies from this review. The 2 included studies Schröder 2012 and Schröder 2011 included the same group of PD patients, thus the number of included PD patients were calculated once and similar information of the 2 studies were merged in Tables 1 and 3 and Table 5.
Figure 1.
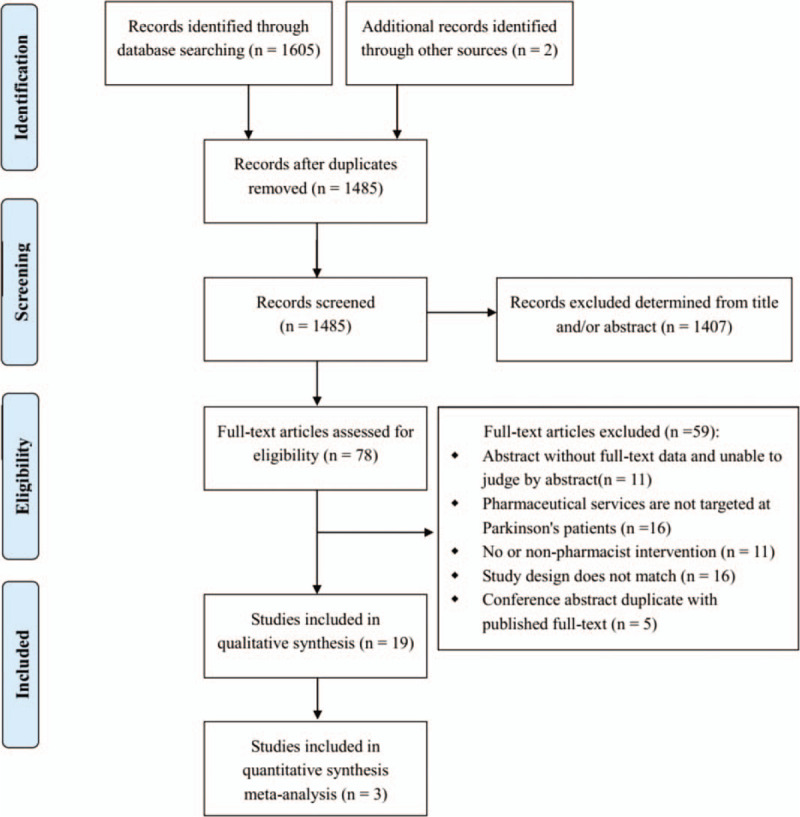
Flow diagram for literature search and study selection.
Table 1.
Characteristics of included studies.
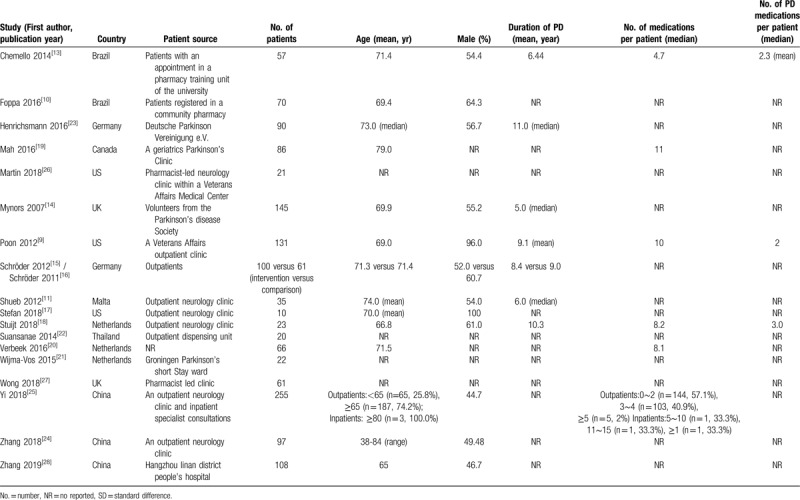
Table 3.
Content of pharmacy services for Parkinson's disease patients of included studies.
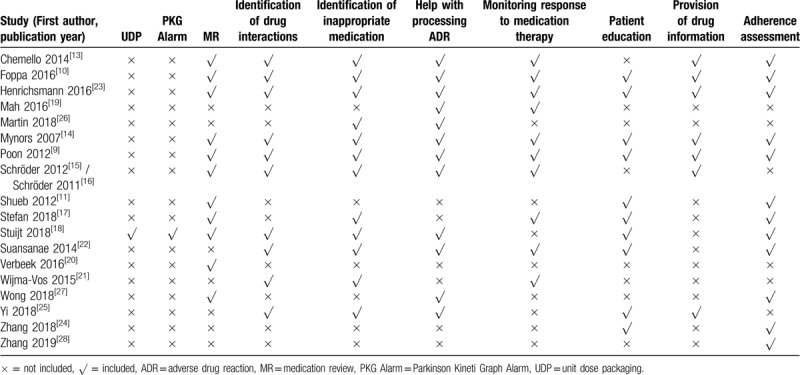
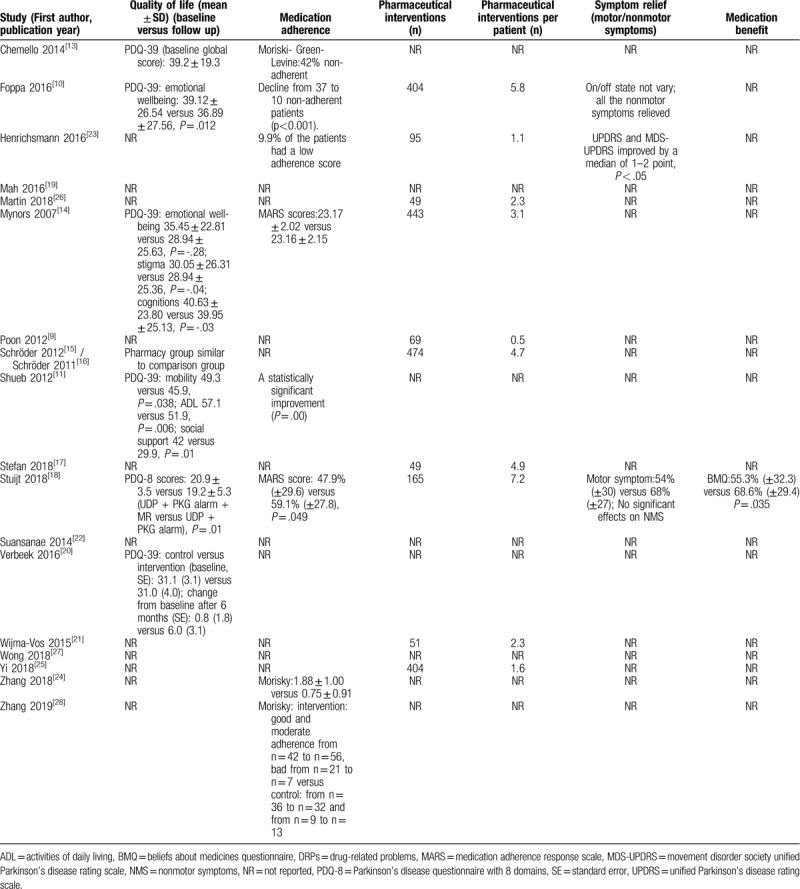
Table 5.
Outcomes of pharmacy services for Parkinson's disease patients of included studies.
3.2. Study characteristics and quality assessment
The 19 included studies were from 9 countries: three were conducted in the Netherlands,[18,20,21] Germany,[15,16,23] the United States,[9,17,26] China,[24,25,28] 2 were in Brazil[10,13] and in the UK.[14,27] Others were conducted in Canada, Malta and Thailand. The number of patients enrolled in a study ranged from 10 to 255, with patients aged, on average, over 65; the proportion of male patients ranged from 44.7% to 100%; duration of diagnosis of PD ranged from 5 to 11 years. Number of medicines per-patient ranged from 4 to over 15 and number of PD medicines ranged from 2 to 3 (see Table 1 for further details).
Follow-up time varied as follows: Chemello 2014: 1 month,[13] Foppa 2016: 6 months,[10] Henrichsmann 2016: 4 months,[23] Mynors 2007: 6 months,[14] Schröder 2012: 8 months,[15] Shueb 2012: 2 months,[11] Stuijt 2018: 10 weeks, 14 weeks, 26 weeks,[18] Zhang 2018: 1 month,[24] Zhang 2019: 6 months[28]; all other studies included did not provide details of follow-up time.
Applying the ROBINS-I tool to the 19 studies that used a cross-sectional studies or case-control design, 8 studies were categorised as moderate risk,[10–11,15,17–18,23–24,26] 3 were of serious risk,[9,14,16] and 3 were of critical risk of bias[13,25,26] (Table 2). Due to different risk of bias of the 2 included studies Schröder 2012 and Schröder 2011, the evaluation results were showed separately. We were unable to assess the risk of bias of 5 conference abstracts because of limited information provided.
Table 2.
Quality of included studies with the risk of bias in non-randomised studies of interventions tool.
3.3. Contents of pharmacy services
Content of the pharmacy services evaluated included helping with identifying, resolving or preventing adverse drug reactions (ADRs) (13 studies),[9–10,13–16,18–19,22–23,25–27] adherence assessment (12 studies),[9–11,13–14,17–18,22–24,27–28] medication review (12 studies),[9–11,13–18,20,23,27] identification of inappropriate medication (11 studies),[9–10,13–16,18,21–23,25] monitoring response to medication therapy (11 studies),[9–10,13–17,19,21–23] identification of drug interaction (11 studies),[9–10,13–16,18,21–23,25] patient education (10 studies),[9–11,14,17–18,22–25] provision of drug information (8 studies),[9–10,13–16,23,25] unit dose packaging (1 study)[18] and Parkionson KinetiGraph Alarm (1 study)[18] (Table 3).
3.4. Setting of pharmacy services
Most pharmacy services were provided in outpatient clinic (13 studies),[9,11,15–19,22–27] although some were located in both outpatient and inpatient settings[25] (see Table 1).
3.5. Reported outcome measures of pharmacy services
3.5.1. Drug-related problems
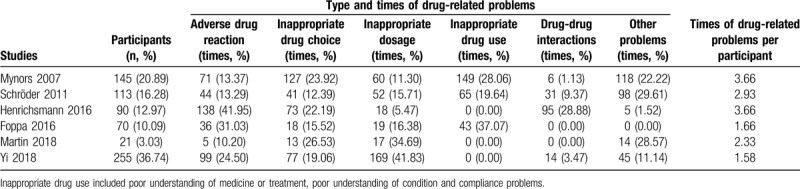
The studies included in this review used a number of different measures to determine outcomes or impact of the pharmacy services being investigated. The most widely reported outcomes were DRPs with times of drug-related problems per participant ranging from 1.58 to 3.66; of these, the most frequently occurring were ADRs (393 out of 1760 times, 22.33%, 6 studies),[10,14,16,23,25–26] inappropriate drug choice (349 times, 19.83%, 6 studies),[10,14,16,23,25–26] inappropriate dosage (335 times, 19.03%, 6 studies),[10,14,16,23,25–26] inappropriate drug use (257 times, 14.60%, 3 studies)[10,14,16] and drug-drug interactions (146 times, 8.3%, 4 studies)[14,16,23,25] (Table 4). The included studies only reported DRPs found by pharmacists during the evaluation period and hence no DRPs that occurred before this were included for comparison to establish change or difference in number of DRPs following introduction of a service; furthermore, no studies involved a control group for comparison of number and type of DRPs, preventing comparison between intervention and non-intervention groups that would allow for measurement of intervention effect.
Table 4.
Drug-related problems in included studies.
3.5.2. Medication adherence and QoL
In addition to DRPs and pharmacist interventions, a number of studies used patient-reported outcome measures of medication adherence (8 studies)[10–11,13–14,18,23–24,28] and QoL (7 studies).[10–11,13–15,18,20] Six case-control studies of the 8 studies showed that pharmacy services improved patient adherence comparing with baseline data.[10–11,18,23–24,28] One study showed no statistically significant difference in adherence.[14] One study only reported the percentage of non-adherent patients.[13] However, due to inconsistency in adherence scales and methods for reporting adherence used by different studies, it was not possible to undertake a meta-analysis of medication adherence.
QoL was evaluated using either the PD questionnaire (PDQ)-8 or the PDQ-39. Two of the 7 studies showed no statistically significant difference in QoL after the intervention. One study only reported patients’ baseline QoL, 1 study reported results of the PDQ-8 scale, and 3 studies provided evidence of some subscales of PDQ-39 improving, although results were inconsistent when findings of different studies were compared. The pooled PDQ-39 data of 3 studies [10,14,20] indicated no statistically significant change in the mobility subscale (MD = 2.67, 95% CI -2.40 to 7.74), activities of daily living subscale (MD = 2.08, 95% CI -2.56 to 6.72), emotional well-being subscale (MD = -1.10, 95% CI -8.86 to 6.67), stigma subscale (MD = 0.19, 95% CI -4.62 to 4.99), social support subscale (MD = 2.39, 95% CI -2.79 to 7.56), cognition subscale (MD = 1.21, 95% CI -3.11 to 5.53), communication subscale (MD = 0.60, 95% CI -4.08 to 5.27) and bodily pain subscale (MD = 3.09, 95% CI -1.85 to 8.03) (see Fig. 2 for details). However, further analysis of PDQ-8 data could not be undertaken due to only 1 study presenting relevant data.[18]
Figure 2.
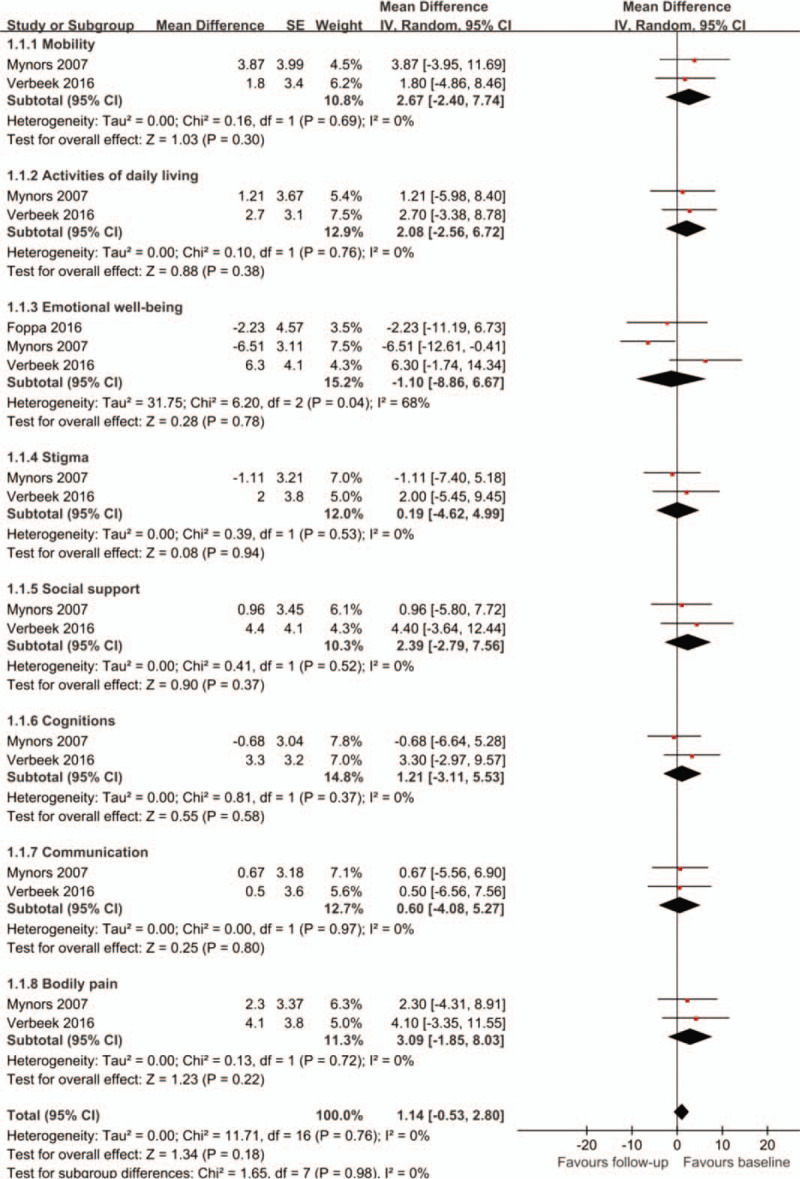
Changes of PDQ-39 from baseline. PDQ-39 = Parkinson's disease questionnaire with 39 domains.
Eleven studies[9–10,23,14–18,21,25–26] reported impact in relation to the number of pharmacist interventions made – with the number of interventions recorded ranging from 49 to 474 and frequency of interventions per patient ranging from 0.5 to 7.2. Improved results in PDQ-39 and adherence were shown in patients receiving more interventions; for example, improvements in QoL and adherence were reported by Foppa 2016 and Stuijt 2018 where an average of 5.8 and 7.2 interventions were made per patient, respectively (Table 5).
3.5.3. Other outcome measures
Change in motor/ non-motor symptoms[10,18,23] and surveys capturing beliefs about medicines[18] were also used to establish impact, with considerable variation in results reported. One study indicated that clinic providers and patients were satisfied with pharmacist's services (4.79 points using a 5-point Likert scale).[9] Four studies reported findings related to medication therapy management service for PD patients[10,17,23,25] (Table 5).
3.6. Sensitivity analysis and publication bias
The I2 of pooled results of emotional well-being subscale of PDQ-39 was 68% indicating heterogeneity among pooled studies (Fig. 2). As a result, we conducted a sensitivity analysis by changing the random-effect methods to fixed-effect methods to pool the trials and the results remained unchanged. Due to a limited number of included studies used to provide data related to the pooled outcome, we could not assess risk of publication bias.
4. Discussion
We conducted a systematic review to identify the role of pharmacists and impact of pharmacy interventions for PD patients with a view to using the evidence produced by this review to design a complex intervention following the guidance produced by the Medical Research Council.[29] Findings from this review indicate that most pharmacy services for PD include help with identifying, resolving or preventing ADRs, adherence assessment, medication review, identification of drug interactions, monitoring response to medication therapy, identification of inappropriate medication, and patient education. This review has also found that the most widely reported DRPs of PD patients were ADRs and that pharmacy services may have an impact on medication adherence but not on self-reported QoL.
To the best of our knowledge, this study is the first systematic review to analyse pharmacist services for PD patients. We included studies from all over the world to ensure the comprehensiveness of the systematic review and the relevance of results. In addition, we sought to identify the setting of pharmacy services and the impact of these services to begin to describe the contexts in which pharmacists are most likely to have an influence on patient outcomes and the likely mechanisms through which the work of pharmacists and pharmacy services work and produce patient-reported outcomes. Yet while the results of this review provide valuable evidence that pharmacy services are effective and has identified validated measures used by studies to investigate the outcomes and impact of these services, it is apparent from the findings of this review that although hospitals around the world are developing pharmacy services for patients, most of these pharmacy services have not designed using a theoretical framework or logic model to inform the development and evaluation of such complex interventions.
Our study has some limitations. First, the number of studies included limited further analysis, such as comparison between treatments and services designed for PD patients at country level,comparison between services provided in different (hospital, outpatient) settings or analysis to identify differences occurring as a result of frequency and/or duration of an intervention. Moreover, only the PDQ-39 data from 3 studies could be combined due to different outcome measures used by studies included in this review, limiting sample size used in the analysis and may explain our finding that pharmacy services overall have no impact on QoL, despite some previously published studies[10,11] indicating improvement in QoL following the implementation of pharmacy services for PD patients. Furthermore, only English-language and Chinese-language studies were included, although studies were conducted in many different countries and health systems. We tried to include conference abstracts in databases searching, but we failed to find those reporting findings in sufficient detail to be included in the review. Fourth, only cross-sectional studies and case-control studies with moderate to critical risk of bias were found, which limited comparability of the studies and the quality of evidence.
Although PD guidelines for pharmacists were published by the Parkinson Society Canada in 2014,[30] there is still no consensus regarding best practice or a gold standard for providing pharmacy services for PD patients. Our review provides evidence to inform this, and further provides a synthesis of the types of pharmacist interventions and outcome measures that have been used previously in evaluations of pharmacy services for PD patients. What is currently missing, is a process evaluation or theory-based evaluation supported by an underpinning theory of how a pharmacy service works that has clearly defined mechanisms of impact linking the content of the service to outcomes through a process of change. Having established what has been previously been successfully implemented our findings can be used to design an evidence-based pharmacy service intervention that can be tested with a large samples and long follow-up time, using patient-reported and pharmacist interventions as outcome measures. Such an approach has previously been successful in developing and evaluating complex healthcare interventions in geriatrics[31] and nursing,[32] and is needed for PD patients
5. Conclusion
This review provides evidence that the most frequently reported DRPs of PD patients were ADRs and that pharmacy services may have an impact on medication adherence but not QoL. However, evidence is limited due to study limitations and poor generalizability. As a result, there are unanswered questions related to the mechanisms or process of change producing reported impact and outcomes of pharmacy services for PD patients.
Acknowledgments
We extend special thanks to DR Li-Chia Chen and DR Douglas Steinke from University of Manchester for expert consultation. We also thank Xiao-Feng Ni from Ji-Lin University for help with searching results updated.
Author contributions
All the authors contributed extensively to the work presented in this paper. ZMY led the study design and model selection. ZMY, TTL and QYT conducted the literature review, designed and data analysis. YZ, SW and SDZ reviewed data analysis. All authors jointly contributed to result in interpretations and manuscript writing. All authors read and approved the final manuscript.
Supplementary Material
Footnotes
How to cite this article: Yi ZM, Li TT, Tang QY, Zhang Y, Willis S, Zhai SD. Content and impact of pharmacy services for patients with Parkinson's disease: a systematic review and meta-analysis. Medicine. 2020;99:27(e20758).
Abbreviations: ADRs = adverse drug reactions, CIs = confidence intervals, DRPs = drug-related problems, MD = mean difference, PD = Parkinson's disease, PDQ = Parkinson's disease questionnaire, QoL = quality of life, RCTs = randomized controlled trials.
This study was funded by the Beijing Pharmaceutical Association (2018–01–04). Funding was not contingent upon publication of the manuscript.
Supplemental Digital Content is available for this article.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Kalia LV, Lang AE. Parkinson's disease. Lancet 2015;386:896–912. [DOI] [PubMed] [Google Scholar]
- [2].Rogers G, Davies D, Pink J, et al. Parkinson's disease: summary of updated NICE guidance. BMJ 2017;358:j1951. [DOI] [PubMed] [Google Scholar]
- [3].McLean G, Hindle JV, Guthrie B3, et al. Co-morbidity and polypharmacy in Parkinson's disease: insights from a large Scottish primary care database. BMC Neurol 2017;17:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fox SH, Katzenschlager R, Lim SY, et al. Movement disorder society evidence-based medicine committee. International Parkinson and movement disorder society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson's disease. Mov Disord 2018;33:1248–66. [DOI] [PubMed] [Google Scholar]
- [5].Vrdoljak D, Borovac JA. Medication in the elderly - considerations and therapy prescription guidelines. Acta Med Acad 2015;44:159–68. [DOI] [PubMed] [Google Scholar]
- [6].Mantri S, Fullard M, Gray SL, et al. Patterns of dementia treatment and frank prescribing errors in older adults with Parkinson's disease. JAMA Neurol 2019;76:41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Martin P, Tamblyn R, Benedetti A, et al. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. JAMA 2018;320:1889–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Avery AJ, Rodgers S, Cantrill JA, et al. A pharmacist-led information technology intervention for medication errors (PINCER): a multicentre, cluster randomised, controlled trial and cost-eff ectiveness analysis. Lancet 2012;379:1310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Poon LH, Lee AJ, Chiao TB, et al. Pharmacist's role in a Parkinson's disease and movement disorders clinic. Am J Health Syst Pharm 2012;69:518–20. [DOI] [PubMed] [Google Scholar]
- [10].Foppa AA, Chemello C, Vargas-Pelaez CM, et al. Medication therapy management service for patients with Parkinson's Disease: a before-and-after study. Neurol Ther 2016;5:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shueb AS. Pharmacist intervention in the management of Parkinson's disease: evaluating the pharmacist's intervention at a movement disorders outpatient clinic. Eur Geriatr Med 2012;5:S127. [Google Scholar]
- [12].Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chemello C, Souza DF, Patricio ES, et al. Pharmaceutical care as a strategy to improve the safety and effectiveness of patients’ pharmacotherapy at a pharmacy school: a practical proposal. Brazilian J Pharm Sci 2014;50:185–93. [Google Scholar]
- [14].Mynors G, Jenkinson C, MacNeill V, et al. A pilot evaluation of specialist community pharmacy services for patients with Parkinson's disease. Pharm J 2007;278:709–12. [Google Scholar]
- [15].Schröder S, Martu P, Odin P, et al. Impact of community pharmaceutical care on patient health and quality of drug treatment in Parkinson's disease. Int J Clin Pharm 2012;34:746–56. [DOI] [PubMed] [Google Scholar]
- [16].Schröder S, Martu P, Odin P, et al. Drug-related problems in Parkinson's disease: the role of community pharmacists in primary care. Int J Clin Pharm 2011;33:674–82. [DOI] [PubMed] [Google Scholar]
- [17].Stefan TC, Elharar N, Garcia G. Implementation and evaluation of Parkinson's disease management in an outpatient clinical pharmacist-run neurology telephone clinic. Ment Health Clin 2018;8:159–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Stuijt C, Karapinar-Carkit F, van den Bemt B, et al. Effect of pharmacist-led interventions on (non)motor symptoms, medication-related problems, and quality of life in Parkinson's disease patients: a pilot study. Clin Neuropharmacol 2018;41:14–9. [DOI] [PubMed] [Google Scholar]
- [19].Mah G, Lee J, Lin M, et al. Impact of telephone intervention service provided at a community-based geriatrics clinic for Parkinson's: an observational study. J Parkinsons Dis 2016;6:155. [Google Scholar]
- [20].Verbeek A, Oonk N, Munster E, et al. The effect of a structured medication review on quality of life in patients with Parkinson's disease. An interim analysis. Mov Disord 2016;31: suppl 2: S170–1. [Google Scholar]
- [21].Wijma-Vos L. Contribution of a pharmacist to the multidisciplinary consultation on Parkinson's short-stay ward. Mov Disord 2015;30: suppl 1: S136–7. [Google Scholar]
- [22].Suansanae T, Wongpraprutdee P, Duangdee M, et al. Drug-related problems in patients with Parkinson's disease identified by pharmacists at the outpatient pharmacy unit. Mov Disord 2014;29:S19–20. [Google Scholar]
- [23].Henrichsmann M, Hempel G. Impact of medication therapy management in patients with Parkinson's disease. Int J Clin Pharm 2016;38:54–60. [DOI] [PubMed] [Google Scholar]
- [24].Zhang C, Luo W, Han JY. Application of clinical model which consisted of clinician and clinical pharmacists in compliance of patients with Parkinson's disease. Chinese J Clin Pharm 2018;27:43–6. [Google Scholar]
- [25].Yi ZM, Ni XF, Li TT, et al. Practice and experience of pharmaceutical service in medication therapy management for Parkinson's disease. Clin Med J 2018;16:61–4. [Google Scholar]
- [26].Martin AW, Heberle AP, Knight JM. Interventions associated with implementation of a pharmacist-led neurology pharmacotherapy clinic in an ambulatory care setting. J Am Coll Clin Pharm 2018;1:1–7. [Google Scholar]
- [27].Wong E. Exploring the role of a prescribing pharmacist in the management of Parkinson's disease. Movement Disorders 2018;33: supplement 2: S403. [Google Scholar]
- [28].Zhang C, Han JY, Bao HR, et al. Effect of new model of pharmaceutical service on patient compliance in “Parkinson's Disease Patient Association”. Chin J Mod Appl Pharm 2019;36:349–52. [Google Scholar]
- [29].Developing and evaluating complex interventions Following considerable development in the field since 2006, MRC and NIHR have jointly commissioned an update of this guidance to be published in 2019. Available at: www.mrc.ac.uk/complexinterventionsguidance. Accessed 29th May 2019. [Google Scholar]
- [30].Patel T, Chang F. Parkinson Society Canada. Parkinson's disease guidelines for pharmacists. Can Pharm J (Ott) 2014;147:161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Faes MC, Reelick MF, Esselink RA, et al. Developing and evaluating complex healthcare interventions in geriatrics: the use of the medical research council framework exemplified on a complex fall prevention intervention. J Am Geriatr Soc 2010;58:2212–21. [DOI] [PubMed] [Google Scholar]
- [32].Pinto S, Caldeira S, Martins J. The use of the Medical Research Council framework in the study of complex interventions in nursing: a literature review. Nurse Res 2018;May 15. doi: 10.7748/nr.2018.e1530. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


