Supplemental Digital Content is available in the text
Keywords: factor, multiple sclerosis, relapse, risk, systematic review
Abstract
Background:
The relapse is character of relapsing-remitting multiple sclerosis. The therapeutic goal is to reduce the risk of relapse. Factors associated with relapses can help to manage and prevent relapses. In addition, patients and doctors all pay attention to it. However, there are differences between studies. Our aim is to summarize factors associated with relapses in relapsing-remitting multiple sclerosis (RRMS).
Methods:
PubMed, EMBASE, Web of science, Cochrane library, CNKI, Wanfang, SinoMed, and VIP were searched to identify risk factors about relapses in RRMS, which should be in cohort or case-control studies. This article was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The quality of studies was evaluated by the Newcastle-Ottawa Scale (NOS). Meta-analysis, subgroup and sensitivity analyses, and publication bias were all performed with Stata. This research has been registered on the international prospective register of systematic reviews (PROSPERO, CRD42019120502).
Results:
43 articles were included. Infection, postpartum period, risk gene, stress, and vitamin D were risk factors for relapses in RRMS. Pregnancy period was the protective factor. Among those, infection increased the risk of relapses in infection period (relative risk [RR], 2.07 [confidence interval (CI), 1.64 to 2.60]). Women in the postpartum period increased the risk of relapses compared with women before pregnancy (RR, 1.43 [CI, 1.19 to 1.72]), or women in pregnancy period (RR, 2.07 [CI, 1.49 to 2.88]). Women in the pregnancy period decreased the risk of relapses (RR, 0.56 [CI, 0.37 to 0.84]) compared with women before pregnancy. However, fewer studies, heterogeneity, and sample size were the limitations.
Conclusion:
It is reliable to adopt results about infection, pregnancy period, and postpartum period.
1. Introduction
Multiple sclerosis (MS) is the most prevalent chronic inflammatory disease of the central nervous system (CNS).[1] Approximately 85% of patients have a relapsing-remitting course from onset. Although a large expansion treatment options for MS have occurred in recent 20 years,[2] there is no curative treatment available, and the aim of current therapeutic strategy is to reduce the risk of relapse.[3] It is of great significance to pathogenesis and prevention of MS by studying the factor associated with relapses. Such as risk factors in Framingham research, which changed clinical practice and evidence-based guidelines in cardiovascular disease.[4] Although many factors associated with relapses in MS have been reported, the results vary substantially between studies, and few systematic reviews consider more than 1 risk factor at a time.[5]
The aim of this article is to summarize factors associated with relapses in relapsing-remitting multiple sclerosis (RRMS), which were reported in observational studies, and examine the effect of these factors on relative risk of relapses in adult RRMS patients. Finally, it will help to manage and prevent relapses more effectively.
2. Materials and methods
This systematic review and meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).[6] As recommended by the PRISMA guidelines, this systematic review and meta-analysis was registered on the international prospective register of systematic reviews (PROSPERO, CRD42019120502).
2.1. Literature search
The following databases were systematically screened for literature: PubMed, EMBASE, Web of Science, Cochrane library, Chinese National Knowledge Infrastructure Databases (CNKI), Wanfang, SinoMed, and Chongqing Chinese Science and Technology Periodical Database (VIP), from January 1, 1983, to December 1, 2018. The search strategy used the terms “multiple sclerosis” or “MS” or “RRMS” or “relapsing multiple sclerosis”, and “relapse” or “attack” or “recurrence” in combination with “risk” or “risk factor” or “factor” or “effect” or “influence” or “impact” or “prediction” or “predictor” or “predisposition”.
2.2. Inclusion and exclusion criteria
This article included cohort and case-control studies that selected RRMS patients aged over 18 years, and their diagnosis met the Poster[7] or McDonald criteria.[8–10] This article also included studies, sufficient data in which were available to provide risk estimate (odds ratio [OR] or relative risk [RR] or hazards ratio [HR]), or construct 2 × 2 tables. Factors in literatures were clearly defined. Exclusion criteria were: patients with progressive multiple sclerosis in studies, or factors in studies which were drugs or treatment, or the literature is a review.
2.3. Study selection
One researcher (YX) screened titles of articles to exclude the obviously irrelevant studies, and obtained the full text of relevant studies. All abstracts and full-text articles of potentially relevant articles were independently screened by 2 researchers (YX, ZYT). Any disagreements were resolved by discussion or decided by a third researcher (SBL). When there was no available data, investigators of studies should be contacted by email.
2.4. Data extraction
The following data were extracted: publication, general information, study design, participant characteristics, diagnostic criteria, factors, the statistical method, adjusted parameters, outcome, duration of follow-up, effect value, etc. The data were evaluated and extracted independently by 2 researchers (YX and ZYT).
2.5. Quality assessment
Newcastle-Ottawa Scale (NOS) was used to evaluate the quality of studies.[11] Cohort studies and case-control studies were assessed by 8 items, mainly including selection, comparability, exposure, and outcome. The full score of scale is 9 stars. This article graded quality as good (≥7 stars), fair (4–6 stars), poor (<4 stars).[12]
2.6. Statistical analysis
This meta-analysis calculated risk ratio (RR) and 95% confidence interval (95% CI) for factors in relation to relapses. The risk estimate (OR or RR or HR), or 2 × 2 tables data were extracted from included studies, then different data types were converted into risk ratio. If the odds ratio was described, the data was converted to a relative risk for meta-analysis (RR=OR/([1 − pRef] + [pRef × OR])), where pRef is the prevalence of relapses in the reference group.[13] The following subgroups were planned to analyze: cohort studies versus case-control studies; corrected immunomodulatory therapy versus non-corrected immunomodulatory therapy; length of follow-up (<1 year versus ≥1 year). The random effects model (DerSimonian and Laird method) was applied in all meta-analysis, because the clinical and methodological condition differed to some extent in included studies. A random effects model is more conservative.[14]
Q-test has poor power to analyze heterogeneity in the situation of few studies.[15] Heterogeneity among the included studies were assessed by I2. According to Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0), the degree of heterogeneity was defined by I2 value: might not be important (0%–40%), moderate heterogeneity (30%–60%), substantial heterogeneity (50%–90%), considerable heterogeneity (75%–100%).
Publication bias was assessed by the funnel plot and Egger test. The p value less than 0.05 was considered statistically significant. Sensitivity analyses were also undertook to analyze the stability of results in meta-analysis. All analyses were performed with Stata version 15.1.
3. Results
3.1. Characteristics of the included studies
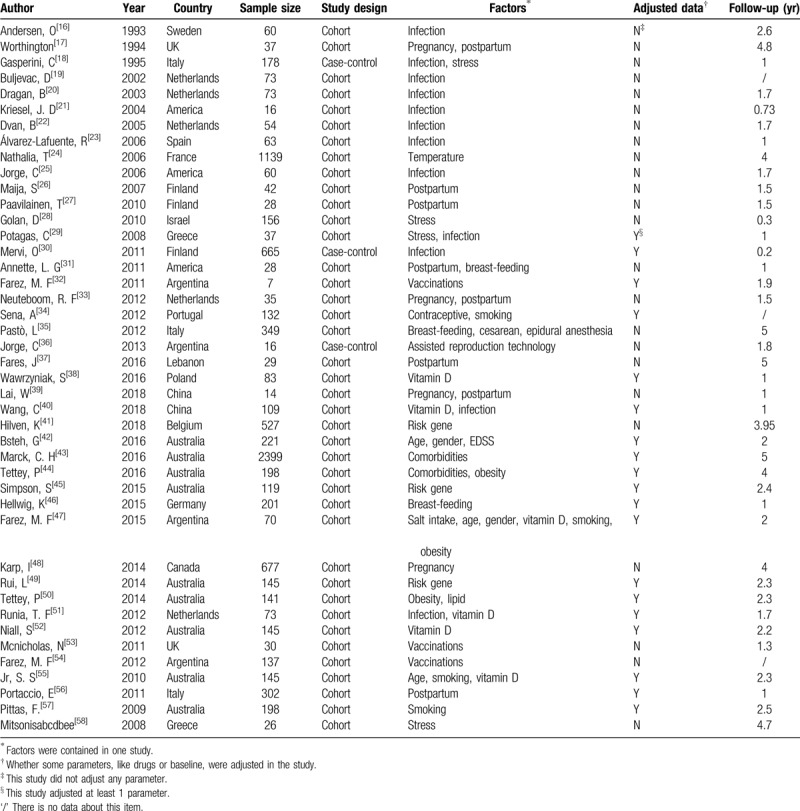
A total of 21,965 records had been identified in the literature search, from which 318 full-text articles were assessed (Fig. 1). Of these, 43 articles were included (Table 1). This review included 9229 RRMS patients, 40 cohort studies, and 3 case-control studies.[16–58] Nineteen studies had adjusted the immunomodulatory therapy. The mean length of follow-up ranged from 2 months to 5 years. Factors included infection, pregnancy, postpartum, stress, vaccine, vitamin D, comorbidity, genes, smoking, obesity, age, lipid, gender, salt intake, temperature, the Kurtzke Expanded Disability Status Scale (EDSS). In these studies, 16 good quality studies, 25 fair quality studies, and 2 poor quality studies were identified by Newcastle-Ottawa Scale.
Figure 1.
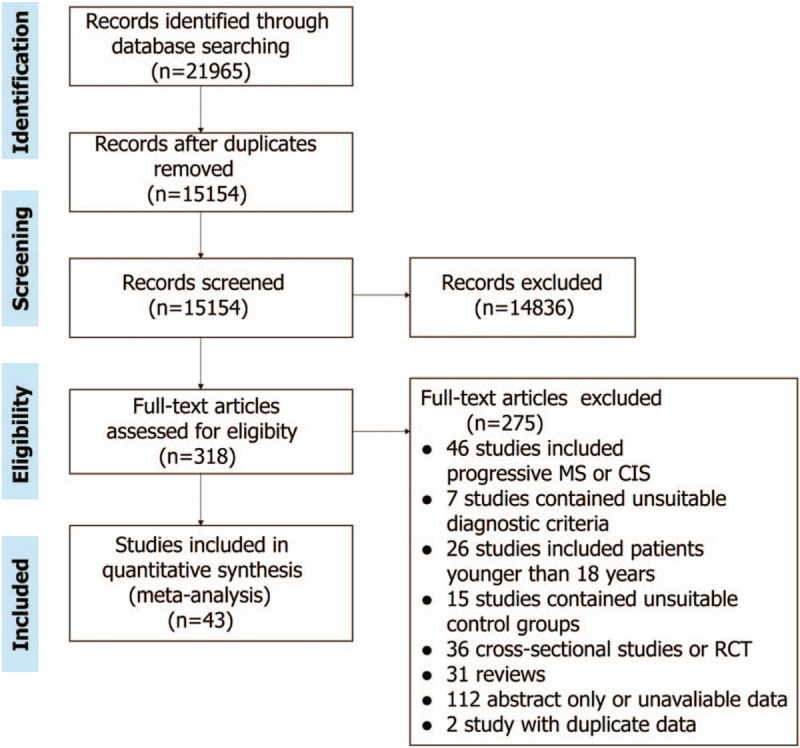
Flow diagram of publication searches and study selection.
Table 1.
Characteristics of 43 included studies.
3.2. Results of the overall meta-analysis
Figure 2 presents the integrated meta-analysis results for factors associated with relapses in RRMS. Infection, vaccinations, pregnancy and postpartum, bread-feeding, stress, vitamin D, genetic risk factors, age, smoking, obesity were involved. Strong risk factors were infection, postpartum period, risk gene. Pregnancy period was the protective factor. Forest plots of each factor are displayed in supplementary figure.
Figure 2.
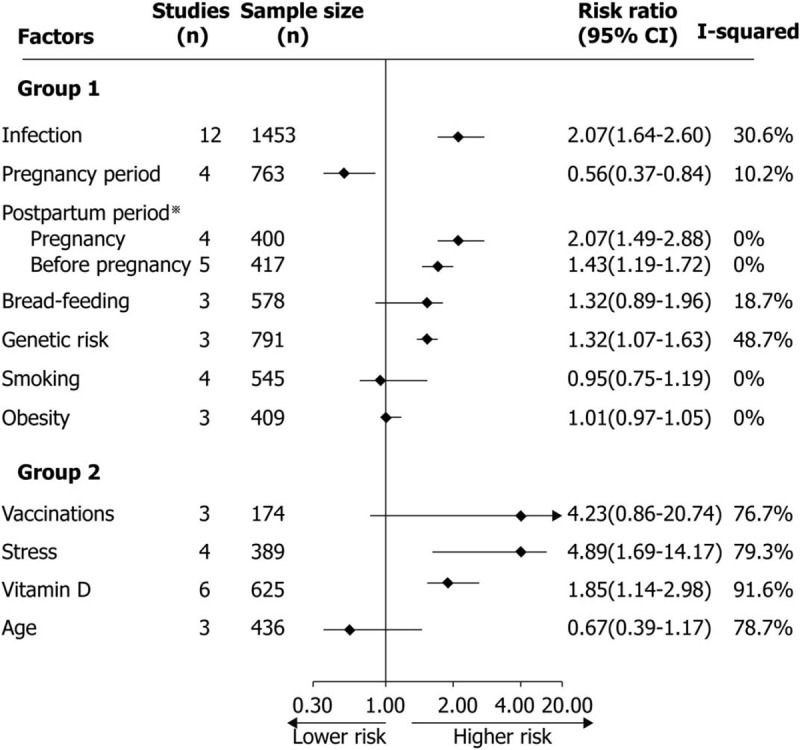
Meta-analysis results for factors associated with relapses in RRMS. Group 1: heterogeneity in risk factors were mild, or moderate. Group 2: heterogeneity in risk factors were significant. For postpartum period, studies respectively selected pregnancy period or period before pregnancy as the control group, and meta-analysis was performed according to different control groups.
3.2.1. Infection
For virus or bacterial infection, 10 cohort studies and 2 case-control study had been included. Only 4 studies adjusted the immunomodulatory therapy, and 9 studies were followed over 1 year. The result of meta-analysis indicated it increased the risk of relapses in infection period (RR, 2.07 [confidence interval (CI), 1.64 to 2.60]) compared with RRMS patients in uninfected period. The heterogeneity showed small (I2 = 30.6%). A total of 7 studies defined the type of virus or bacteria, including influenza virus, chlamydia pneumoniae, Epstein–Barr virus, Human Herpes Virus-6 (HHV-6), adenovirus. The influenza virus and Epstein-Barr virus had 2 studies, the others only had 1 study. Only chlamydia pneumoniae increased the risk of relapses (RR, 2.15 [CI, 1.26 to 4.95]), and the meta-analysis of these 7 studies also showed it increased the risk of relapses (RR, 1.59 [CI, 1.01 to 2.50]) without any significant heterogeneity (I2 = 18.9%).
3.2.2. Vaccinations
Three cohort studies had been included. Only 1 study adjusted the immunomodulatory therapy, and all studies were followed more than 1 year. The result of meta-analysis indicated vaccinations had on effect on the risk of relapses (RR, 4.23 [CI, 0.86 to 20.74]). But the heterogeneity showed considerable (I2 = 76.7%). Of these studies, 2 studies were about H1N1 vaccine, and one study was about yellow fever vaccine. Although the yellow fever vaccine increased the risk of relapses (RR, 12.79 [CI, 4.29 to 38.18]), but the sample size was small.
3.2.3. Pregnancy and postpartum
3.2.3.1. Pregnancy period
There were 4 cohort studies for pregnancy period. All these were followed more than 1 year, and it did not adjust the treatment. In the meta-analysis, results showed women in the pregnancy period decreased the risk of relapses (RR, 0.56 [CI, 0.37 to 0.84]) compared with women before pregnancy. There was a tiny heterogeneity with I2 = 10.2%.
3.2.3.2. Postpartum period
Eight cohort studies were included. All of these were followed over 1 year, but only 1 study adjusted the treatment. The meta-analysis showed women in the postpartum period increased the risk of relapses compared with women before pregnancy (RR, 1.43 [CI, 1.19 to 1.72]), or women in pregnancy period (RR, 2.07 [CI, 1.49 to 2.88]). The heterogeneity of these studies was none (I2 = 0%).
3.2.3.3. Breast-feeding
Three cohort studies were included. The studies were followed more than 1 year, but only 1 study adjusted the treatment. In the meta-analysis, results showed breast-feeding had no effect on the risk of relapses (RR, 1.32 [CI, 0.89 to 1.96]). The heterogeneity showed small (I2 = 18.7%).
3.2.3.4. Other factors about pregnancy
Other factors were about assisted reproduction technology, oral contraceptive use, cesarean delivery, and epidural anesthesia, which respectively had 1 study associated with relapses. Only assisted reproduction technology (RR, 6.93 [CI, 3.36 to 14.27]) and oral contraceptive use (RR, 2.97 [CI, 1.24 to 6.54]) increased the risk of relapses. However, the sample size about assisted reproduction technology was small. The cohort study about oral contraceptive use included 137 RRMS patients, and it was assessed with good quality.
3.2.4. Stress
For stress, 3 cohort studies and 1 case-control study had been included. Three studies were followed more than 1 year, and 1 study adjusted the treatment. In the meta-analysis, results showed RRMS patients in the stress events increased the risk of relapses (RR, 4.89 [CI, 1.69 to 14.17]). However, the heterogeneity showed considerable (I2 = 79.3%).
3.2.5. Vitamin D
There were 6 cohort studies about vitamin D. All studies had adjusted the immunomodulatory therapy, and the length of follow-up were over 1 year. In the meta-analysis, the pooled data showed RRMS patients with lower vitamin D level increased the risk of relapses (RR, 1.85 [CI, 1.14 to 2.98]), but the heterogeneity was considerable (I2 = 91.6%), which showed considerable heterogeneity.
3.2.6. Genetic risk factors
Only 3 cohort studies were about gene. All studies were followed for more than 1 year, and 2 studies had adjusted the treatment. The pooled data of meta-analysis indicated risk genes brought the risk of relapses (RR, 1.32 [CI, 1.07 to 1.63]), and the heterogeneity was moderate (I2 = 48.7%).
3.2.7. Age
Old age as a risk factor, 3 cohort studies were included. All studies were followed over 1 year, and it had adjusted the immunomodulatory treatment. The result of meta-analysis indicated older age had no effect on relapses (RR, 0.67 [CI, 0.39 to 1.17]). However, the heterogeneity was considerable (I2 = 78.7%).
3.2.8. Smoking
Four cohort studies were included on smoking. Similarly, all studies were followed over 1 year, and these treatments were also adjusted. From merged data in meta-analysis, it showed smoking did not influence the risk of relapses (RR, 0.95 [CI, 0.75 to 1.19]). And there was no heterogeneity (I2 = 0%).
3.2.9. Obesity
There were 3 cohort studies on obesity. All studies were followed more than 1 year, and the treatment were also corrected. In the meta-analysis, obesity did not influence the risk of relapses (RR, 1.01 [CI, 0.97 to 1.05]). And it showed no heterogeneity (I2 = 0%).
3.2.10. Comorbidities
Only 2 cohort studies were included, and they were of fair quality according to Newcastle-Ottawa Scale. Two studies were followed over 1 year. Of these, 1 (Tettey, P 2016) included 11 defined comorbidities, in which rheumatoid arthritis and hay fever showed statistical significance after adjusting treatment, merged data of this study indicated comorbidities had no effect on relapse (RR, 1.26 [CI, 0.94 to 1.70]). Another study (Marck, C.H 2016) with 2399 RRMS patients showed that comorbidities can increase the risk (RR, 1.68 [CI, 1.30 to 2.18]) without adjusted parameter. In the meta-analysis of 2 studies, the result showed comorbidities increased the risk of relapses (RR, 1.33 [CI, 1.03 to 1.72]), and heterogeneity was moderate (I2 = 45.2%).
3.2.11. Other factors
Two studies were about gender, 1 study was respectively about lipid, salt intake, temperature, baseline EDSS. Only salt intake (RR, 3.37 [CI, 1.50 to 9.55]) increased the risk of relapses. Gender (RR, 0.92 [CI, 0.59 to 1.42]), lipid (RR, 1.06 [CI, 0.94 to 1.21]), temperature (RR, 1.14 [CI, 0.60 to 2.14]), and baseline EDSS (RR, 0.91 [CI, 0.78 to 1.06]) did not influence relapses. Those factors, except temperature, were good quality cohort studies with adjusted treatment.
3.3. Subgroup analyses
The heterogeneity of postpartum period, smoking, and obesity were none (I2 = 0%), and the heterogeneity of pregnancy period was tiny (I2 = 10.2%), so this article did not perform the subgroup analysis for these factors. Meanwhile, factors for vitamin D and age, all of these were cohort studies and had adjusted the treatment, the length of follow-up were also over 1 year. Therefore, subgroup analysis of those were not carried out as planned. For infection, vaccinations, stress, genetic risk factors, and breast-feeding, the subgroup analysis were generally consistent with main results of meta-analysis, except for infection. In the subgroup analysis of infection, study design may be an important part of heterogeneity.
3.4. Sensitivity analyses
All studies were performed with sensitivity analysis. The results of sensitivity analysis were also generally consistent with the main result of meta-analysis apart from the following 4 exceptions. For pregnancy and genetic risk factors, the quality of studies may consist of the main heterogeneity. For vaccinations and age, the sample size was noted a significant component of heterogeneity.
3.5. Publication bias
Only infection, postpartum, and vitamin D were performed the funnel plots and Egger test, because of enough studies for it. The other factors were performed Egger test. The funnel plots of vitamin D showed obvious asymmetry, and Egger test indicated significant publication bias (P = .019). Studies for age also had evidence of publication bias according to Egger test (P = .034).
4. Discussion and conclusions
4.1. Principal findings
RRMS patients in infection period increased 2.07-fold risk of relapses, compared with these in non-infection period. The study design may be the major component of small heterogeneity. For specific viruses or bacteria, including influenza virus, chlamydia pneumoniae, Epstein-Barr virus, HHV-6, or adenovirus, no enough evidence was identified to influence relapses. The risk of relapses in pregnancy period were 0.56 fold than the period before pregnancy. However, the risk of relapses in postpartum period were 1.43 fold than the period before pregnancy, and 2.07 fold than the pregnancy period. Breast-feeding did not impact the risk of relapses. These results were reliable due to tiny or no heterogeneity. Assisted reproduction technology and oral contraceptive use may increase the risk, but no enough studies support it. Patients in stress events increased 4.89-fold risk for relapses. However, Considerable heterogeneity, poor and fair quality of studies make this result should be taken careful consideration. Lower vitamin D lever brought 1.85-fold risk of relapses, compared with higher vitamin D level. This result must be interpreted with caution because of significant publication bias. Some risk gene increased 1.32-fold risk for relapses, but there were different genes between studies. Vaccinations, obesity, age did not influence the risk of relapses in this meta-analysis, only the result of obesity was reliable, vaccinations and age need further verification. Some other factors, like comorbidities and salt intake, also increased the risk of relapses in RRMS, but there were not enough studies.
4.2. Definitions of factors
Infection, pregnancy period, postpartum period, risk gene, stress, and lower vitamin D level were identified as factors associated with relapses in this meta-analysis. Table 2 compares different definitions of risk factors in included studies. For 1 factor in those, the definition varied in different studies, but some studies have the trend to use the same definitions. A clear definition can help popularize these conclusions and promote more further research.
Table 2.
The definition of factors.
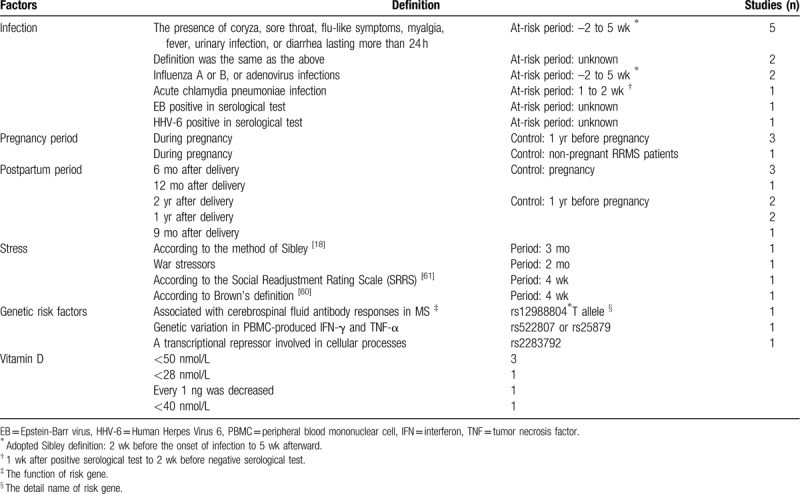
The definition of infection was based on symptoms or serological tests, which was consistent with clinical practice. At risk period mainly used Sibley's definition, extending from 2 weeks before the onset of infection to 5 weeks afterward.[59] Pregnancy period was during pregnancy, most studies chose 1 year before pregnancy as the control group, but 1 study selected the control group which different with others. For postpartum period, the control group was consisted of pregnancy or 1 year before pregnancy. And the length of postpartum period was from 6 months to 2 years. The criterion of stress[18,60–61] and risk genes varied between studies. And the time of stress life events was from 4 weeks to 3 months. The vitamin D level was all detected by serological test. The critical concentration was from 28 to 50 nmol/L, and less than 50 nmol/L was diagnosed with vitamin D deficiency.[62]
4.3. Strengths and limitations of study
An important strength of this article is to integrate all relevant factors in meta-analysis. To the best of our knowledge, this review is the first to quantitative assess all relevant factors associated with relapses in RRMS. Because relapses were the main outcome, this review only focused RRMS patients, and excluded the progressive MS. Study types at highest risk of bias for prognostic factor were also excluded, including cross-sectional and population-specific researches.[63] Therefore, doctors, patients, and medical decision makers can benefit from it. However, there were some limitations. Firstly, fewer studies were included after inclusion and exclusion, 6 factors only had 1 or 2 studies, the largest number of studies for 1 factor was 12. Only 3 or more studies can be used in meta-analysis.[64] Secondly, 5 factors in 11 factors indicated moderate or considerable heterogeneity, or publication bias. Finally, the sample size in most studies were not large.
4.4. Comparison with other studies
Although some previous reviews had reported factors about relapses. Several reviews or meta-analysis just focused on 1 factor. Or else, some reviews summarized many risk factors with qualitative description. D’hooghe et al[65] and McKay et al[66] respectively summarized factors about relapses without meta-analysis. The result of this article is consistent with those, but some factors not in this review, such as seasonal variation, menstruation, ethnicity, physical trauma, radiotherapy, and dietary habits. However, population, study design, diagnostic criteria, and unavailable data in above factors did not meet special criteria of this review. Meanwhile, some important studies[67,68] were also excluded. Vaccinations[69] and pregnancy[68] were researched by Confavreux et al, results showed vaccination did not appear to increase the short-term risk of relapse in multiple sclerosis, and the rate of relapse declined during pregnancy and increased during the first 3 months postpartum, which were also consistent with this article. But those were also excluded, because 2 studies all included progressive MS.
4.5. Future research
There are differences between RRMS and progressive MS in clinical course and pathology. Progressive MS is characterized by poor response to immunomodulatory treatments and an absence of new inflammatory demyelinating lesions, and hypocellular, gliotic core, and axonal swelling are contained in lesion.[69] So it is important to aim at RRMS patients for factor research. However, few studies and small sample size are current issues. Larger cohort studies with rigorous design are needed to verify and explore more factors. And registry cohort study will be the trend to explore it.[70,71] Meanwhile, verified factors will help to understand pathology and develop new treatment.
4.6. Conclusions
In conclusion, infection, postpartum period, genetic risk factors, stress, and vitamin D were all risk factors for relapses in RRMS. And pregnancy period was protective factor for relapses in RRMS women. Of those, it is reliable to adopt results about infection, pregnancy period, and postpartum period.
Acknowledgments
The authors thank all scholars for their participation. Special thanks to professor Qiang Liu for his guidance for this research. Meanwhile, special thanks are also extended to Yao Xie senior fellow apprentice Xingxing Lai in Tsinghua University.
Author contributions
Data analysis: Yao Xie.
Idea: Yao Xie, Ying Gao.
Manuscript drafting: Yao Xie, Fang Han.
Protocol: Yao Xie, Ziyu Tian, Shibing Liang.
Review & edit: Ying Gao, Dahua Wu.
Screen: Yao Xie, Ziyu Tian, Shibing Liang.
Search: Ziyu Tian.
Supplementary Material
Footnotes
Abbreviations: 95% CI = 95% confidence interval, CNKI = Chinese National Knowledge Infrastructure Databases, CNS = central nervous system, EDSS = the Kurtzke Expanded Disability Status Scale, HHV-6 =Human Herpes Virus-6, MS = multiple sclerosis, NOS = Newcastle-Ottawa Scale, OR = odds ratio, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RR = risk ratio, RRMS = relapsing-remitting multiple sclerosis, VIP = Chongqing Chinese Science and Technology Periodical Database.
How to cite this article: Xie Y, Tian Z, Han F, Liang S, Gao Y, Wu D. Factors associated with relapses in relapsing-remitting multiple sclerosis: a systematic review and meta-analysis. Medicine. 2020;99:27(e20885).
This study fulfils compliance with ethical standards.
The manuscript does not contain clinical studies or patient data.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med 2018;378:169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Doshi A, Chataway J. Multiple sclerosis, a treatable disease. Clin Med (Lond) 2017;17:530–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Eur J Neurol 2018;25:215–37. [DOI] [PubMed] [Google Scholar]
- [4].O’Donnell CJ, Elosua R. Cardiovascular risk factors. Insights from Framingham Heart Study. Rev Esp Cardiol 2008;61:299–310. [PubMed] [Google Scholar]
- [5].Mckay KA, Jahanfar S, Duggan T, et al. Factors associated with onset, relapses or progression in multiple sclerosis: a systematic review. Neurotoxicology 2007;61:189–212. [DOI] [PubMed] [Google Scholar]
- [6].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983;13:227–31. [DOI] [PubMed] [Google Scholar]
- [8].Mcdonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121–7. [DOI] [PubMed] [Google Scholar]
- [9].Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 2005;58:840–6. [DOI] [PubMed] [Google Scholar]
- [10].Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wells G. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. 2004; http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Access date:08/12/2018. [Google Scholar]
- [12].Huang Y, Cai X, Mai W, et al. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ 2016;355:i5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ziff OJ, Lane DA, Samra M, et al. Safety and efficacy of digoxin: systematic review and meta-analysis of observational and controlled trial data. BMJ 2015;351:h4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brunstrom M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ 2016;352:i717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [16].Andersen O, Lygner PE, Bergstrom T, et al. Viral infections trigger multiple sclerosis relapses: a prospective seroepidemiological study. J Neurol 1993;240:417–22. [DOI] [PubMed] [Google Scholar]
- [17].Worthington J, Jones R, Crawford M, et al. Pregnancy and multiple sclerosis: a 3-year prospective study. J Neurol 1994;241:228–33. [DOI] [PubMed] [Google Scholar]
- [18].Gasperini C, Grasso MG, Fiorelli M, et al. A controlled study of potential risk factors preceding exacerbation in multiple sclerosis. J Neurol Neurosurg Psychiatry 1995;59:303–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Buljevac D, Flach HZ, Hop WCJ, et al. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain 2002;125:952–60. [DOI] [PubMed] [Google Scholar]
- [20].Dragan B, Verkooyen RP, Jacobs BC, et al. Chlamydia pneumoniae and the risk for exacerbation in multiple sclerosis patients. Ann Neurol 2003;54:828–31. [DOI] [PubMed] [Google Scholar]
- [21].Kriesel JD, Andrea W, Hayden FG, et al. Multiple sclerosis attacks are associated with picornavirus infections. Mult Scler 2004;10:145–8. [DOI] [PubMed] [Google Scholar]
- [22].Dvan B. Epstein-Barr virus and disease activity in multiple sclerosis. J Neurol Neurosurg Psychiatry 2005;76:1377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].álvarez-Lafuente R, García-Montojo M, Heras VDL, et al. Clinical parameters and HHV-6 active replication in relapsing – remitting multiple sclerosis patients. J Clin Virol 2006;37:S24–6. [DOI] [PubMed] [Google Scholar]
- [24].Nathalia T, Chrystelle V, Pierre D, et al. Limited impact of the summer heat wave in France on hospital admissions and relapses for multiple sclerosis. Neuroepidemiology 2006;27:28–32. [DOI] [PubMed] [Google Scholar]
- [25].Jorge C, Marcela F, Wendy G. The risk of relapses in multiple sclerosis during systemic infections. Neurology 2006;67:652–9. [DOI] [PubMed] [Google Scholar]
- [26].Maija S, Saara VIN, Anna A, et al. Clinical and immunologic evaluation of women with multiple sclerosis during and after pregnancy. Gend Med 2007;4:45–55. [DOI] [PubMed] [Google Scholar]
- [27].Paavilainen T, Kurki T, Parkkola R, et al. Magnetic resonance imaging of the brain used to detect early post-partum activation of multiple sclerosis. Eur J Neurol 2010;14:1216–21. [DOI] [PubMed] [Google Scholar]
- [28].Golan D, Somer ES, Cuzin-Disegni L, et al. Impact of exposure to war stress on exacerbations of multiple sclerosis. Ann Neurol 2010;64:143–8. [DOI] [PubMed] [Google Scholar]
- [29].Potagas C, Mitsonis C, Watier L, et al. Influence of anxiety and reported stressful life events on relapses in multiple sclerosis: a prospective study. Mult Scler 2008;14:1262–8. [DOI] [PubMed] [Google Scholar]
- [30].Mervi O, Mikko L, Ville A, et al. Temporal relationship between environmental influenza A and Epstein-Barr viral infections and high multiple sclerosis relapse occurrence. Mult Scler 2011;17:672–80. [DOI] [PubMed] [Google Scholar]
- [31].Annette LG, Stella H, Eeden SKVD, et al. Vitamin D, pregnancy, breastfeeding, and postpartum multiple sclerosis relapses. Arch Neurol 2011;68:310–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Farez MF, Jorge C. Yellow fever vaccination and increased relapse rate in travelers with multiple sclerosis. Arch Neurol 2011;68:1267–71. [DOI] [PubMed] [Google Scholar]
- [33].Neuteboom RF, Janssens ACJW, Siepman TAM, et al. Pregnancy in multiple sclerosis: clinical and self-report scales. J Neurol 2012;259:311–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sena A, Couderc R, Vasconcelos JC, et al. Oral contraceptive use and clinical outcomes in patients with multiple sclerosis. J Neurol Sci 2012;317:47–51. [DOI] [PubMed] [Google Scholar]
- [35].Pastò L, Portaccio E, Ghezzi A, et al. Epidural analgesia and cesarean delivery in multiple sclerosis post-partum relapses: the Italian cohort study. BMC Neurol 2012;12:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jorge C, Farez MF, Ysrraelit MC. Increase in multiple sclerosis activity after assisted reproduction technology. Ann Neurol 2013;72:682–94. [DOI] [PubMed] [Google Scholar]
- [37].Fares J, Nassar AH, Gebeily S, et al. Pregnancy outcomes in Lebanese women with multiple sclerosis (the LeMS study): a prospective multicentre study. BMJ Open 2016;6:e11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wawrzyniak S, Mikołajewska E, Kuczko-Piekarska E, et al. Association of vitamin D status and clinical and radiological outcomes in a treated MS population in Poland. Brain & Behavior 2016;7:e609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lai W, Kinoshita M, Peng A, et al. Does pregnancy affect women with multiple sclerosis? A prospective study in Western China. J Neuroimmunol 2018;321:24–8. [DOI] [PubMed] [Google Scholar]
- [40].Wang C, Zeng Z, Wang B, et al. Lower 25-hydroxyvitamin D is associated with higher relapse risk in patients with relapsing-remitting multiple sclerosis. J Nutr Health Aging 2018;22:1–6. [DOI] [PubMed] [Google Scholar]
- [41].Hilven K, Vandebergh M, Smets I, et al. Genetic basis for relapse rate in multiple sclerosis: association with LRP2 genetic variation. Mult Scler 2018;24:1773–5. [DOI] [PubMed] [Google Scholar]
- [42].Bsteh G, Feige J, Ehling R, et al. Discontinuation of disease-modifying therapies in multiple sclerosis – clinical outcome and prognostic factors. Mult Scler 2017;23:1241–8. [DOI] [PubMed] [Google Scholar]
- [43].Marck CH, Neate SL, Taylor KL, et al. Prevalence of comorbidities, overweight and obesity in an international sample of people with multiple sclerosis and associations with modifiable lifestyle factors. PLoS One 2016;11:e148573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tettey P, Siejka D, Simpson SJ, et al. Frequency of comorbidities and their association with clinical disability and relapse in multiple sclerosis. Neuroepidemiology 2016;46:106–13. [DOI] [PubMed] [Google Scholar]
- [45].Simpson S, Stewart N, Van IMD, et al. Stimulated PBMC-produced IFN-γ and TNF-α are associated with altered relapse risk in multiple sclerosis: results from a prospective cohort study. J Neurol Neurosurg Psychiatry 2015;86:200–7. [DOI] [PubMed] [Google Scholar]
- [46].Hellwig K, Rockhoff M, Herbstritt S, et al. Exclusive breastfeeding and the effect on postpartum multiple sclerosis relapses. JAMA Neurol 2015;72:1132–8. [DOI] [PubMed] [Google Scholar]
- [47].Farez MF, Fiol MP, Gaitán MI, et al. Sodium intake is associated with increased disease activity in multiple sclerosis. J Neurol Neurosurg Psychiatry 2015;86:26–31. [DOI] [PubMed] [Google Scholar]
- [48].Karp I, Manganas A, Sylvestre MP, et al. Does pregnancy alter the long-term course of multiple sclerosis? Ann Epidemiol 2014;24:504–8. [DOI] [PubMed] [Google Scholar]
- [49].Rui L, Taylor BV, Steve S, et al. Association between multiple sclerosis risk-associated SNPs and relapse and disability – a prospective cohort study. Mult Scler 2014;20:313–21. [DOI] [PubMed] [Google Scholar]
- [50].Tettey P, Jr, Taylor SS. Adverse lipid profile is not associated with relapse risk in MS: results from an observational cohort study. J Neurol Sci 2014;340:230–2. [DOI] [PubMed] [Google Scholar]
- [51].Runia TF, Hop WC, de Rijke YB, et al. Lower serum vitamin D levels are associated with a higher relapse risk in multiple sclerosis. Neurology 2012;79:261–6. [DOI] [PubMed] [Google Scholar]
- [52].Niall S, Steve S, Ingrid VDM, et al. Interferon-β and serum 25-hydroxyvitamin D interact to modulate relapse risk in MS. Neurology 2012;79:254–60. [DOI] [PubMed] [Google Scholar]
- [53].Mcnicholas N, Chataway J. Relapse risk in patients with multiple sclerosis after H1N1 vaccination, with or without seasonal influenza vaccination. J Neurol 2011;258:1545–7. [DOI] [PubMed] [Google Scholar]
- [54].Farez MF, Ysrraelit MC, Fiol M, et al. H1N1 vaccination does not increase risk of relapse in multiple sclerosis: a self-controlled case-series study. Mult Scler 2012;18:254–6. [DOI] [PubMed] [Google Scholar]
- [55].Simpson SS, Jr, Taylor BL, Ponsonby AL, et al. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol 2010;68:193–203. [DOI] [PubMed] [Google Scholar]
- [56].Portaccio E, Ghezzi A, Hakiki B, et al. Breastfeeding is not related to postpartum relapses in multiple sclerosis. Neurology 2011;77:145–50. [DOI] [PubMed] [Google Scholar]
- [57].Pittas F, Ponsonby AL, van der Mei IA, et al. Smoking is associated with progressive disease course and increased progression in clinical disability in a prospective cohort of people with multiple sclerosis. J Neurol 2009;256:577–85. [DOI] [PubMed] [Google Scholar]
- [58].Mitsonisabcdbee CI. The impact of stressful life events on risk of relapse in women with multiple sclerosis: a prospective study. Eur Psychiatry 2008;23:497–504. [DOI] [PubMed] [Google Scholar]
- [59].Sibley WA, Bamford CR, Clark K. Clinical viral infections and multiple sclerosis. Lancet 1985;1:1313–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Brown GW, Harris TO. Life Events and Illness. New York: Guilford Press; 1989. [Google Scholar]
- [61].Masuda M, Holmes TH. The Social Readjustment Rating Scale: a cross-cultural study of Japanese and Americans. J Psychosom Res 1967;11:227–37. [DOI] [PubMed] [Google Scholar]
- [62].Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30. [DOI] [PubMed] [Google Scholar]
- [63].Riley RD, Hayden JA, Steyerberg EW, et al. Prognosis Research Strategy (PROGRESS) 2: prognostic factor research. PLoS Med 2013;10:e1001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Gill PJ, Ashdown HF, Wang K, et al. Identification of children at risk of influenza-related complications in primary and ambulatory care: a systematic review and meta-analysis. Lancet Respir Med 2015;3:139–49. [DOI] [PubMed] [Google Scholar]
- [65].D’Hooghe MB, Nagels G, Bissay V, et al. Modifiable factors influencing relapses and disability in multiple sclerosis. Mult Scler 2010;16:773–85. [DOI] [PubMed] [Google Scholar]
- [66].Mckay KA, Jahanfar S, Duggan T, et al. Factors associated with onset, relapses or progression in multiple sclerosis: a systematic review. Neurotoxicology 2017;61:189–212. [DOI] [PubMed] [Google Scholar]
- [67].Confavreux C, Suissa S, Saddier P, et al. Vaccinations and the risk of relapse in multiple sclerosis. Vaccines in Multiple Sclerosis Study Group. N Engl J Med 2001;344:319–26. [DOI] [PubMed] [Google Scholar]
- [68].Confavreux C, Hutchinson M, Hours MM, et al. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med 1998;339:285–91. [DOI] [PubMed] [Google Scholar]
- [69].Dutta R, Trapp BD. Relapsing and progressive forms of multiple sclerosis: insights from pathology. Curr Opin Neurol 2014;27:271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Mahmud SM, Bozat-Emre S, Mostaco-Guidolin LC, et al. Registry cohort study to determine risk for multiple sclerosis after vaccination for pandemic influenza A (H1nN1) with Arepanrix, Manitoba. Canada Emerg Infect Dis 2018;24:1267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Thalheim C. Pooling real-world multiple sclerosis patient data on a European level: a true story of success. Neurodegener Dis Manag 2015;5: Suppl 6: 55–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


