Abstract
The pathophysiological changes associated with hypoestrogenism of menopause, a condition known as genitourinary syndrome of menopause, are responsible for the hallmark symptoms of vulvovaginal atrophy (VVA), namely dyspareunia secondary to vaginal dryness. Many postmenopausal women with VVA find sexual relations to be challenging or impossible. Ospemifene has estrogen-like effects on the vaginal epithelium, and is indicated to treat moderate-to-severe symptomatic VVA (Europe) or moderate-to-severe symptomatic dyspareunia and vaginal dryness (United States) in postmenopausal women. The case studies presented in this article follow the progress of two women who began treatment with ospemifene for the main presenting symptom of dyspareunia. Both women had concerns about the impact of their symptomatology on new relationships. The patient in case 1 experienced relevant improvement within 3 months of treatment start and, by 1 year, dyspareunia was absent. Vaginal lubricants were no longer required. The patient in case 2 experienced relevant improvement within 4 weeks of starting ospemifene. At 15 months, with the use of a lubricant for vaginal penetration, she could enjoy sexual intercourse without pain. At the time of writing, she had been receiving ospemifene continuously for more than 2 years with effective symptom relief and good tolerability.
Keywords: ospemifene, sexual dysfunction, vulvar and vaginal atrophy
Introduction
Declining estrogen levels during menopause can adversely affect a range of genitourinary tissues,1 with associated loss of collagen and elastin, altered smooth muscle function, reduced vasculature, and increased connective tissue.2 A consistent finding in postmenopausal vaginal epithelium is a reduction in superficial and intermediate cells and an increase in parabasal cells.3 Vaginal epithelium becomes thin, pale, and less rugated, and the vagina becomes shortened and narrowed with less secretions.1,4 These pathophysiological changes are responsible for the hallmark symptoms of vulvovaginal atrophy (VVA), namely dyspareunia secondary to vaginal dryness.1,4 Many postmenopausal women with VVA find sexual relations to be challenging or impossible.
Although VVA symptoms manifest in 40–60% of postmenopausal women, most do not receive medical care.2 The European REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs (REVIVE) survey found that 32% of postmenopausal women with VVA were naïve to treatment and that 38% had never specifically discussed their symptoms with a healthcare professional.5 Among the reasons for underdiagnosis and undertreatment of VVA are women’s reluctance to seek help, inadequate questioning by medical practitioners, and the assumption by many women that their symptoms are a part of natural aging.1,5
Selective estrogen receptor modulators are a class of drugs that act on the estrogen receptor with differential ability to selectively inhibit or stimulate estrogen-like activity in various target tissues.6 Ospemifene has been shown to have beneficial estrogen-like effects on vaginal epithelium.7 In ovariectomized rats, ospemifene-induced increases in vaginal weight, epithelial thickness and mucification, and vacuolization were equivalent to those with 17α-ethylestradiol and greater than those with raloxifene.8 In phase 1 clinical trials in healthy postmenopausal women, ospemifene was shown to increase superficial and intermediate cells in the vaginal epithelium, while decreasing parabasal cells, confirming its estrogenic activity.9 Subsequent randomized controlled clinical trials in postmenopausal women confirmed that, compared with placebo, ospemifene 60 mg/day effectively reversed the morphological and physiological features of the vaginal mucosa that correlate with clinical symptoms of VVA, a major component of the genitourinary syndrome of menopause.10 In Europe, ospemifene is indicated for treatment of moderate-to-severe symptomatic VVA in postmenopausal women who are not candidates for local vaginal estrogen therapy.11 In the USA, ospemifene is indicated for the treatment of moderate-to-severe dyspareunia and vaginal dryness due to menopause.12
The case studies in this article report the experience of women who were able to recover their sexual interest and function, contributing to the establishment and maintenance of new relationships, during treatment with ospemifene for VVA. As patient-specific information was deidentified to ensure anonymity, patient consent was not required.
Case 1
Case 1 involves a woman (body mass index [BMI] 23.2 kg/m2) who presented in December 2017, at age 56 years, with complaints of vaginal dryness and dyspareunia. She had entered menopause at age 50 years with no vasomotor symptoms. Symptoms of vaginal dryness had manifested 1–2 years before menopause, but she could not recall the first appearance of dyspareunia as she had not had sexual relations for some years. Previous contraceptive practices included condoms, hormonal oral contraceptives for 6 years, and an intrauterine device until menopause. She had no history of surgery or concomitant conditions. She was a casual or limited alcohol drinker and social smoker (1–2 cigarettes per day). Occasionally, she would take supplements (collagen, magnesium, and vitamin complex), but otherwise was not receiving any pharmacological therapy.
The patient had previously been in a stable relationship for 27 years and had two children (both vaginal deliveries). Sexual relations with her long-term partner had been satisfactory until their relationship began to deteriorate, about 5 years before it ended. Subsequently, she was single for 5 years with only occasional relationships.
At presentation, the patient had been with a new partner for about a month. On resuming sexual activity, she experienced difficulty and pain during intercourse. Previously, she had used local low-dose estrogen twice weekly for about 6 months, as well as lubricants during sexual intercourse, but considered that neither of these treatments was sufficient to manage her current symptoms.
Gynecological examination of the vulva revealed reduction of the labium minus, a slightly pronounced clitoral hood, and a fissure in the left interlabial fold in the resolution phase. The posterior vulvar commissure was identified as the painful area. The vaginal mucosa was erythematous, friable, and showed mild retraction, and the patient reported discomfort during manual exploration. Speculoscopy was painful, vaginal secretions were absent, and the vaginal epithelium was pale and thin with decreased rugae. No lesions were visible on the cervix. A cervico-vaginal cytology sample and utero-vaginal examination revealed no pathology. A screening mammography performed 2 months earlier had been assessed as normal.
After being fully informed about the causes and implications of dyspareunia in terms of women’s sexuality and interpersonal relationships, and after reviewing existing treatment options, the patient decided on ospemifene, daily vaginal moisturizing, and continued use of vaginal lubricants until symptoms resolved. Ospemifene 60 mg/day was prescribed. In addition, she was trained to self-assess her symptom evolution during treatment using a vulvar chart to identify the painful area according to its extension and intensity, and to record its association with sexual intercourse. The purpose of this genital awareness tool is to actively involve the patient in the therapeutic process.
At follow-up in March 2018, after 3 months’ treatment with ospemifene, the patient reported improvement in dyspareunia, although with some persisting discomfort. The vulvar chart indicated a reduction in the extension and intensity of symptoms (Figure 1). Improvement in vulvar vestibular color and presence of vaginal secretions were noted during a gynecological examination. She had no pain on speculoscopy. She reported regular use of vaginal moisturizers for the first 7–10 days only of ospemifene treatment, although she continued to use vaginal lubricants as required. She asked to continue ospemifene treatment and was scheduled for a follow-up visit in 1 year.
Figure 1.
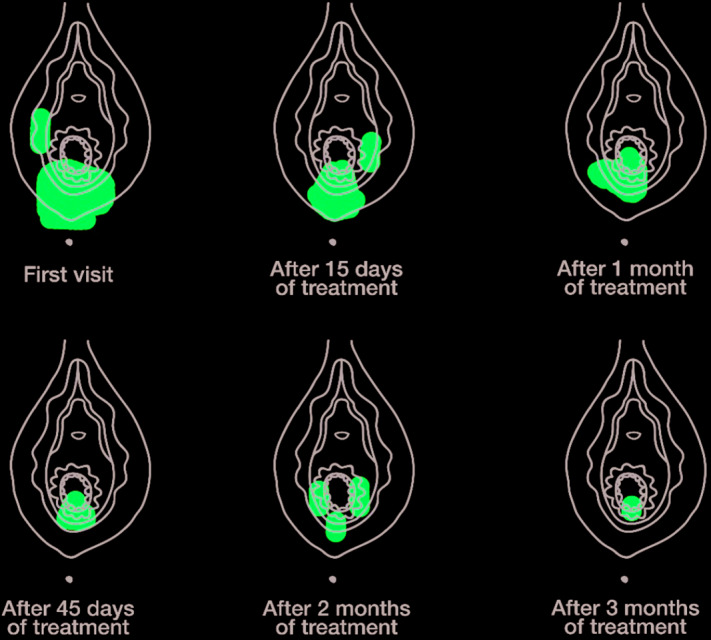
Vulvar chart showing dyspareunia evolution during treatment with ospemifene. The patient was trained in use of the genital awareness tool at her initial visit in order to self-assess the presence and location of painful areas on the vulva on a regular basis during treatment.
In January 2019, 2 months before her scheduled 1-year follow-up visit, the patient presented with condylomatous lesions in the right labium majora and complained of occasional hot flushes. She reported satisfactory sexual relations without dyspareunia. Vulvar examination confirmed the presence of condyloma acuminatum lesions, but otherwise good skin and mucosal color and adequate vaginal lubrication. Imiquimod cream was prescribed for the condylomas and phytotherapy for the hot flushes. She and her partner were advised to undergo screening for sexually transmitted diseases (STDs).
At follow-up in February 2019, the patient was asymptomatic. The condylomas had disappeared, vasomotor symptoms were controlled, and dyspareunia was absent. The STD screen was negative for both partners. Gynecological examination was normal. She had discontinued use of vaginal lubricants about 5 months after starting ospemifene treatment. She reported having satisfying sexual relations with her partner and asked to continue with ospemifene treatment.
Case 2
Case 2 describes a woman (body weight, 60 kg; BMI, 22 kg/m2) aged 58 years at presentation of dyspareunia in 2017. In 1995, at 36 years of age, she underwent total hysterectomy and adnexectomy following a diagnosis of ovarian cancer. She received hormone therapy (conjugated estrogen tablets, 0.625 mg, once daily) for 18 years (until 54 years), at which time treatment was discontinued based on medical recommendations current at the time and personal concerns about continued hormone use.
The patient first experienced dyspareunia in 2013, after discontinuing hormone therapy. The following year she divorced and, in the absence of a regular sexual partner, was not overly concerned. Initially, she managed her symptoms with estriol 0.5 mg pessaries twice weekly but, due to local irritation, switched to estriol 50 mg/g vaginal gel twice weekly. Over the next 2 years, she was poorly compliant with the vaginal gel.
At presentation in April 2017, the patient reported a vulvar burning sensation and dyspareunia, which had become a concern as she had started a new relationship. She reported that sexual intercourse was not possible most of the time. Although her partner was understanding, she was concerned that her dyspareunia would adversely affect the relationship.
In addition to vaginal symptoms, the patient had experienced 3 or 4 urinary tract infections (UTIs) requiring antibiotics during the previous year. Tenesmus was also present.
On physical examination, the external vulvae were sensitive to touch. The vulvar and vaginal mucosae were pale, with petechiae in the vaginal fundus, and decreased vaginal rugae. No pathological discharge was observed. The introit mucosae appeared erythemic all around and was fissured at 6. A urethral caruncle was observed. The labia minora was preserved. Vaginal elasticity was reduced, limiting examination to a small speculum. She reported pain during speculoscopy although not of the same intensity as that during sexual intercourse.
Vaginal cytology indicated intermediate and parabasal cells consistent with hypotrophy, along with a moderate inflammatory component, low levels of lactobacilli, and mixed vaginal microbiota. Her vaginal pH was 5. A urine test was negative for infection, although leukocytes were present.
Dyspareunia due to VVA was diagnosed. Taking into consideration the patient’s doubts about the efficacy of, and prior poor compliance with, local treatment, ospemifene 60 mg/day was prescribed. She was advised to use a vaginal gel moisturizer every night for 10 days and avoid vaginal penetration during sexual relations until reassessment in 4 weeks.
In May 2017, after 4 weeks’ treatment with ospemifene, the vulvar burning sensation had disappeared. Vulvar and vaginal examination showed improved color without petechia in the fundus. The introit mucosae were no longer erythemic and no fissures were observed. Improvement in vaginal elasticity permitted use of a normal speculum for examination. Her vaginal pH was 4. Vaginal cytology showed mainly superficial and intermediate cells (normal trophism). Lactobacillus acidophilus were observed.
In July 2018, after 15 months’ treatment with ospemifene, the vulvar burning sensation was mainly absent, other than occasionally after intercourse. The patient had not experienced any UTIs since beginning treatment. By using a lubricant for vaginal penetration, she could partake in sexual intercourse without pain. She and her partner were planning to live together.
The patient is currently 60 years old. She has been receiving ospemifene continuously for more than 2 years with effective symptom relief and no side effects.
Clinical overview
Dyspareunia associated with VVA can negatively impact on a woman’s quality of life, reducing self-esteem and undermining sexual confidence. Dyspareunia is a frequent reason for women to avoid or reject relationships and sexual activity.
The woman in case 1 had experienced a long phase without sexual activity. At the time of presentation, she was embarking on a new relationship and had concerns about the impact of dyspareunia on the relationship. Her wish was to enjoy normal sexual relations without the need for local treatments. In this patient, ospemifene improved vaginal and vulva functionality, allowing her to recommence sexual activity without pain and, eventually, without the need for moisturizers or lubricants. The woman in case 2 had a more complicated medical situation and was also disinclined to use topical therapies. Her symptoms were well managed with oral ospemifene, allowing her to resume sexual intercourse without pain and maintain a new relationship. At the time of writing, she had been receiving ospemifene for more than 2 years with effective symptom relief.
Outcomes in these women are consistent with a subanalysis of a pivotal randomized clinical trial of ospemifene in postmenopausal women with VVA, which assessed its effects on sexual function.13 Compared with placebo, ospemifene 60 mg/day was associated with significant improvements in the Female Sexual Function Index (FSFI) total score and in the FSFI domains of desire, arousal, lubrication, and pain reduction. Improvements were observed as early as week 4 of treatment and were maintained or further improved by week 12.
The vulvar vestibule is a key area of burning and dyspareunia, the main presenting symptoms of both women. A pilot study of 55 postmenopausal women with VVA investigated the effects of ospemifene 60 mg/day on the vulvar vestibule.14 The mean score for vulvar vestibular trophism (measured by severity of petechiae, pallor, friability, dryness, and mucosal redness on a 4-point scale from 0 = none to 3 = severe) decreased from 11.2 at baseline to 4.2 after 60 days’ treatment with ospemifene (p=0.02). Patient-reported severity scores for vaginal dryness, burning, and dyspareunia were significantly reduced. The mean score on the cotton swab test, which is used to assess pain locations on the vulva (on a 0–3 scale), decreased from 2.81 to 1.25 (p=0.01). The functional integrity of specific afferent nerve fibers from the periphery to the central nervous system was quantified using current perception threshold (CPT) testing. Higher CPT values after ospemifene treatment indicated less sensitivity (decrease of ~40%) and thus less pain in nerve endings.
The vaginal epithelial hypotrophy recorded in case 2 was normalized after treatment with ospemifene, as evidenced by a decrease in parabasal cells and an increase in superficial and intermediate cells, a finding consistent with the reported literature.9 Another notable finding in case 2 was the absence of UTIs during 15 months’ treatment with ospemifene, as compared with several UTIs in the year prior to treatment. There is clinical trial evidence to show that ospemifene may prevent recurrent UTIs in postmenopausal women.15
The case reports also illustrate that ospemifene effectively manages VVA symptoms in postmenopausal women during continuous long-term use, with good tolerability. Pooled safety data from six phase 2 and 3 randomized controlled trials of 6- to 52-weeks’ duration indicated good overall tolerability.16 Treatment-emergent adverse events (TEAEs) were reported by 67.6% of patients taking ospemifene (n=1242) and 54.1% of women taking placebo (n=958), but were mainly mild or moderate in intensity and tended to occur within the first 4–12 weeks of treatment. The most common treatment-related TEAE with ospemifene was hot flush (7.5 versus 2.6% for placebo), which rarely led to discontinuation (1.0 versus 0.3% for placebo). In case 2, the appearance of hot flushes after 1 year of ospemifene treatment suggested a menopausal origin and were readily controlled with phytotherapy. Interim (2-year) data from the Post-Approval Safety Study of ospemifene indicate a low incidence of side effects during real-life use,17 and suggest possible protection from venous thromboembolism (VTE),18 or at a minimum no excess risk of VTE compared with placebo.11 Nevertheless, current labelling recommends careful appraisal of the risk:benefit balance of ospemifene in candidate patients, and use is contraindicated in women with active thromboembolic or cerebrovascular disease or a history of these conditions.11,12
Conclusion
Both case studies involved relatively young postmenopausal women with VVA who were embarking on new relationships and had concerns about the impact of dyspareunia on their relationships. Neither woman was a candidate for vaginal therapy due to personal preference and previous poor compliance. In these patients, oral ospemifene 60 mg/day effectively managed symptoms of vaginal dryness and dyspareunia during up to 2 years of continuous treatment with a positive impact on sexual function and general life.
Acknowledgements
Medical writing assistance was provided by Jon Monk and Kerry Dechant on behalf of Content Ed Net (Madrid, Spain). This article forms part of a Special Issue. All authors contributed to developing this Special Issue by sharing their experience with the benefit of patients in mind. The publication is expected to benefit gynecologists in their daily clinical practice by increasing knowledge and expertise.
Footnotes
Contributions: All authors contributed equally to the preparation of this case report. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: Dr. Jurado López reports personal fees from Shionogi, outside the submitted work. Dr. Molero Rodríguez reports personal fees from Shionogi, outside the submitted work. The authors have also provided scientific support to Shionogi Spain by lecturing and/or taking part in Advisory Board meetings organized by Shionogi (Madrid, Spain). The authors’ time was compensated by Shionogi Spain according to local codes of practice. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2020/06/dic.2020-3-8-COI.pdf
Funding declaration: Medical writing assistance was funded by Shionogi (Madrid, Spain). This article forms part of a Special Issue funded by Shionogi (Madrid, Spain).
Correct attribution: Copyright © 2020 Jurado López AR, Molero Rodríguez F. https://doi.org/10.7573/dic.2020-3-8. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: submitted; externally peer reviewed.
Peer review comments to author: 16 April 2020
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Gandhi J, Chen A, Dagur G, et al. Genitourinary syndrome of menopause: an overview of clinical manifestations, pathophysiology, etiology, evaluation, and management. Am J Obstet Gynecol. 2016;215(6):704–711. doi: 10.1016/j.ajog.2016.07.045. [DOI] [PubMed] [Google Scholar]
- 2.Palacios S. Managing urogenital atrophy. Maturitas. 2009;63(4):315–318. doi: 10.1016/j.maturitas.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Pandit L, Ouslander JG. Postmenopausal vaginal atrophy and atrophic vaginitis. Am J Med Sci. 1997;314(4):228–231. doi: 10.1097/00000441-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Mac Bride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc. 2010;85(1):87–94. doi: 10.4065/mcp.2009.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nappi RE, Palacios S, Panay N, Particco M, Krychman ML. Vulvar and vaginal atrophy in four European countries: evidence from the European REVIVE Survey [published correction appears in Climacteric 2016 Apr;19(2):i] Climacteric. 2016;19(2):188–197. doi: 10.3109/13697137.2015.1107039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riggs BL, Hartmann LC. Selective estrogen-receptor modulators – mechanisms of action and application to clinical practice [published correction appears in N Engl J Med. 2003;348(12):1192] N Engl J Med. 2003;348(7):618–629. doi: 10.1056/NEJMra022219. [DOI] [PubMed] [Google Scholar]
- 7.Kangas L, Unkila M. Tissue selectivity of ospemifene: pharmacologic profile and clinical implications. Steroids. 2013;78(12–13):1273–1280. doi: 10.1016/j.steroids.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Unkila M, Kari S, Yatkin E, Lammintausta R. Vaginal effects of ospemifene in the ovariectomized rat preclinical model of menopause. J Steroid Biochem Mol Biol. 2013;138:107–115. doi: 10.1016/j.jsbmb.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Voipio SK, Komi J, Kangas L, Halonen K, DeGregorio MW, Erkkola RU. Effects of ospemifene (FC-1271a) on uterine endometrium, vaginal maturation index, and hormonal status in healthy postmenopausal women. Maturitas. 2002;43(3):207–214. doi: 10.1016/s0378-5122(02)00206-2. [DOI] [PubMed] [Google Scholar]
- 10.Di Donato V, Schiavi MC, Iacobelli V, et al. Ospemifene for the treatment of vulvar and vaginal atrophy: a meta-analysis of randomized trials. Part I: evaluation of efficacy. Maturitas. 2019;121:86–92. doi: 10.1016/j.maturitas.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Senshio®. Summary of product characteristics. [Accessed May 11, 2020]. Available at: https://www.medicines.org.uk/emc/product/9417/smpc.
- 12.Osphena (ospemifene) Prescribing information. 2019. [Accessed May 11, 2020]. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/203505s015lbl.pdf.
- 13.Constantine G, Graham S, Portman DJ, Rosen RC, Kingsberg SA. Female sexual function improved with ospemifene in postmenopausal women with vulvar and vaginal atrophy: results of a randomized, placebo-controlled trial. Climacteric. 2015;18(2):226–232. doi: 10.3109/13697137.2014.954996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murina F, Di Francesco S, Oneda S. Vulvar vestibular effects of ospemifene: a pilot study. Gynecol Endocrinol. 2018;34(7):631–635. doi: 10.1080/09513590.2018.1427717. [DOI] [PubMed] [Google Scholar]
- 15.Schiavi MC, Di Pinto A, Sciuga V, et al. Prevention of recurrent lower urinary tract infections in postmenopausal women with genitourinary syndrome: outcome after 6 months of treatment with ospemifene. Gynecol Endocrinol. 2018;34(2):140–143. doi: 10.1080/09513590.2017.1370645. [DOI] [PubMed] [Google Scholar]
- 16.Simon JA, Altomare C, Cort S, Jiang W, Pinkerton JV. Overall safety of ospemifene in postmenopausal women from placebo-controlled phase 2 and 3 trials. J Womens Health (Larchmt) 2018;27(1):14–23. doi: 10.1089/jwh.2017.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruyniks N, DeGregorio F, Gibbs T, Carrol R, Fraeman KH, Nordstrom BL. Safety of ospemifene during real-life use. J Gynecol Women’s Health. 2018;9(3):555762. doi: 10.19080/JGWH.2018.09.555762. [DOI] [Google Scholar]
- 18.Cai B, Nordstrom B, Yoshida Y, et al. Incidence of venous thromboembolism (VTE) among postmenopausal women prescribed ospemifene, selective oestrogen receptor modulators (SERM), or untreated vulvar and vaginal atrophy. P36. Maturitas. 2019;124:162. doi: 10.1016/j.maturitas.2019.04.142. [DOI] [PubMed] [Google Scholar]

