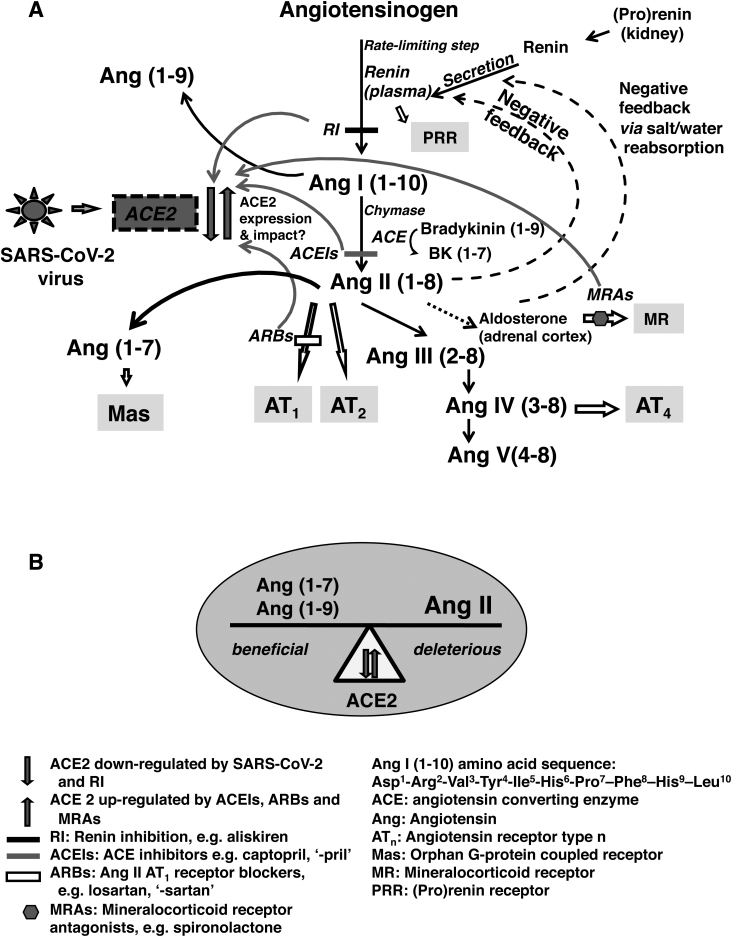
To the Editor—I further Hanff and colleagues’ [1] timely call for epidemiological and clinical investigations of coronavirus disease 2019 (COVID-19), including measurements of the renin-angiotensin-aldosterone system (RAAS) components, as substudies would be insightful of this pandemic. Angiotensin-converting enzyme 2 (ACE2) participates in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cell entry. This infection downregulates ACE2. Drugs that block RAAS also affect ACE2 expression; it is downregulated by renin inhibition and upregulated by ACE inhibitors, angiotensin receptor blockers [1], and mineralocorticoid receptor antagonists [2]. Other likely regulatory factors are age, type 2 diabetes, and sex difference [3]. These interactions would directly affect the balance between the beneficial and deleterious angiotensins (Angs), such as Ang (1–7) and Ang (1–9) vs excess Ang II. Such perturbations would also indirectly influence other RAAS components, and the coordination between circulating and local tissue expressions, as shown in Figure 1.
Figure 1.
Flow diagram showing the renin-angiotensin-aldosterone system (RAAS) with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and points of RAAS drug blockade (A) and implications of angiotensin-converting enzyme 2 (ACE2) perturbations on directly affected angiotensins (Angs) (B). Changes in ACE2 expression would impact the beneficial, deleterious, and other components of the RAAS. Physiologically, the RAAS maintains blood pressure and body water balance. ACE2 is downregulated by SARS-CoV-2 infection and renin inhibition (RI), and upregulated by angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), and mineralocorticoid receptor antagonists (MRAs). Renin is secreted from the kidney, which transforms angiotensinogen to Ang I, the rate-limiting step. Circulating renin can also bind to (pro)renin receptor (PRR) with likely activation of the local tissue renin-angiotensin system throughout the body. Ang I is converted to Ang II by ACE and by a second enzyme, chymase. ACE also converts bradykinin (1–9) to bradykinin (1–7) [Bk (1–7)]. Ang II is further transformed to Ang III, Ang IV, and Ang V. A negative feedback loop controls Ang II concentration changes with renin secretion that responds in the opposite direction. Ang II stimulates the release of aldosterone. However, excessive Ang II is deleterious and is associated with hypertension, congestive heart failure, and chronic kidney disease. ACE2 transforms Ang I and Ang II, that is, Ang I to Ang (1–9), and Ang II to Ang (1–7). Ang (1–9) and Ang (1–7) have protective effects balancing the deleterious Ang II, when in excess.
ACE2 is distributed throughout the body and is abundantly expressed in the lung, small intestine, and in blood vessels of many organs including the brain, heart, kidney, and testis [4]. These organs and blood vessels are potential sites of infection. The downregulation of ACE2 would reduce the production of Ang (1–7) and Ang (1–9), and concurrently prevent the reduction of Ang II, tilting the balance to Ang II accumulation that may lead to toxicity [1] (Figure 1B). Such dysregulation likely contributed to reported cases of acute respiratory distress syndrome [1], inflammation, myocardial injury [5], neurological incidences [6], and gastrointestinal manifestations [7].
Other components and the crosstalk between the systemic circulation and local tissue renin-angiotensin system would also be disrupted. Changes in circulating Ang II concentration alter renin secretion through a negative feedback loop (Figure 1A); as Ang II decreases, renin secretion increases and consequently affects renin concentration and plasma renin activity (PRA). Renin converts angiotensinogen to Ang I and PRA is a measure of this rate. Renin catalytic activity is enhanced when bound to its receptor (PRR) [8]. The inhibition of renin or ACE reduces circulating Ang II with an increase in renin concentration. It is conceivable that circulating renin could bind to PRR, where expressed, and activate the local tissue renin-angiotensin system. Ang II changes would also affect aldosterone stimulation and Ang IV production. Ang IV through its receptor AT4 has opposite biological effects to Ang II via receptor AT1. We therefore suggest the measurement [9] of potential affected RAAS components, Ang (1–7), Ang (1–9), Ang II, circulating ACE2, and PRA, from which Ang I can be derived. Such results would characterize the impact of the COVID-19 infection on the RAAS.
Notes
Acknowledgments. The author appreciates and thanks Dr Maryam Cassim for reading and making constructive suggestions to this letter.
Potential conflicts of interest. The author: No reported conflicts of interest. The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Hanff TC, Harhay MO, Brown TS, Cohen JB, Mohareb AM. Is there an association between COVID-19 mortality and the renin-angiotensin system—a call for epidemiologic investigations [manuscript published online ahead of print 26 March 2020]. Clin Infect Dis 2020. doi:10.1093/cid/ciaa329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keidar S, Gamliel-Lazarovich A, Kaplan M, et al. Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients. Circ Res 2005; 97:946–53. [DOI] [PubMed] [Google Scholar]
- 3. Chen J, Jiang Q, Xia X, et al. Individual variation of the SARS-CoV2 receptor ACE2 gene expression and regulation. Preprints [Preprint]. Posted 12 March 2020:2020030191 Available at: https://www.preprints.org/manuscript/202003.0191/v1. Accessed 23 June 2020. [Google Scholar]
- 4. Hamming I, Timens W, Bulthuis M, Lely A, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004; 203:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bonow RO, Fonarow GC, O’Gara PT, Yancy CW. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality [manuscript published online ahead of print 27 March 2020]. JAMA Cardiol 2020. doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- 6. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China [manuscript published online ahead of print 10 April 2020]. JAMA Neurol 2020. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. C, Han, C, Duan, S, Zhang, et al. Digestive symptoms in COVID-19 patients with mild disease severity: Clinical presentation, stool viral RNA testing, and outcomes. Am J Gastroenterol 2020; 115:916-923. doi: 10.14309/ajg.0000000000000664. Available at: https://pubmed.ncbi.nlm.nih.gov/32301761/. Accessed 23 June 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramkumar N, Kohan DE. The (pro)renin receptor: an emerging player in hypertension and metabolic syndrome. Kidney Int 2019; 95:1041–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Naik GO, Moe GW, Armstrong PW. Specific and non-specific measurements of tissue angiotensin II cascade members. J Pharm Biomed Anal 2001; 24:947–55. [DOI] [PubMed] [Google Scholar]