To the Editor—It was recently suggested that excess risk of respiratory failure due to coronavirus disease 2019 (COVID-19) may be lower than expected for people living with human immunodeficiency virus (HIV) [1]. We report the case of a 52-year-old man from our intensive care unit (ICU) who developed acute respiratory failure due to COVID-19, Pneumocystis jirovecii pneumonia (PJP), and newly diagnosed stage 3 HIV [2]. Diagnosis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disguised the presence of PJP.
On 25 April 2020, a 52-year-old man presented at the emergency unit of a nearby hospital with fever of 40°C, cough, and shortness of breath. He deteriorated soon after, requiring endotracheal intubation. SARS-CoV-2 was detected from tracheal aspirate. In addition, bronchial aspirate samples grew with Staphylococcus aureus, Pseudomonas aeruginosa, and Acinetobacter dijkshoorniae. Blood cultures grew vancomycin-resistant Enterococcus faecium and Staphylococcus epidermidis. Hence, a broad antibiotic regimen containing meropenem and linezolid was initiated, yet the patient continued to have daily fevers up to 40°C without responding to antipyretics or antibiotics. He deteriorated further despite escalated pressure-controlled invasive ventilation and was finally transferred to our intensive care unit on 13 May 2020, for possible initiation of extracorporeal membrane oxygenation (ECMO).
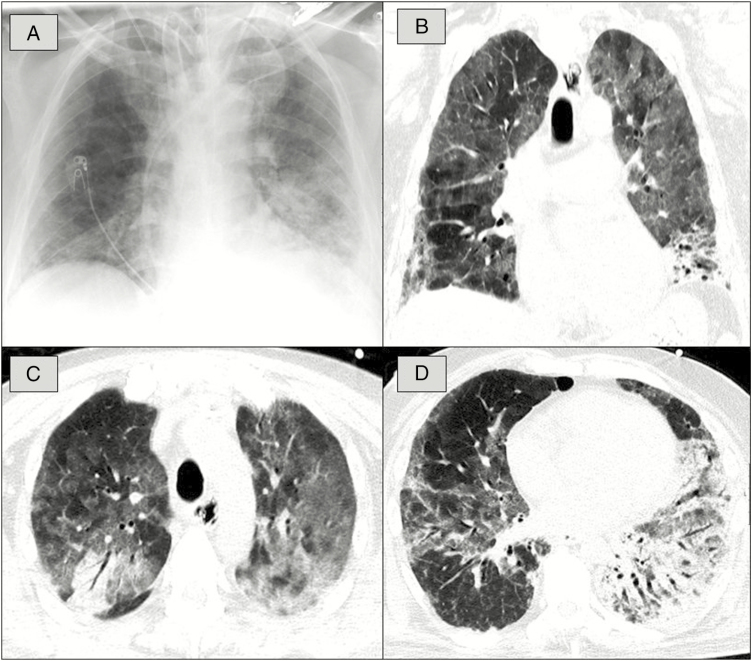
For evaluation of pulmonary COVID-19 manifestation and in preparation for possible ECMO, computed tomography (CT) was performed on 13 and 14 May. Chest CT showed bilateral ground-glass opacities, consolidations, and crazy-paving pattern typical for COVID-19 [3, 4]. As a potential sign for subacute manifestation, airway changes, pleural changes, fibrosis, and nodules were present (Figure 1). Careful changes of ventilator settings finally made ECMO unnecessary.
Figure 1.
A, Chest radiograph shows diffuse ground-glass opacification (GGO) of the lung on both sides and consolidation in the left lower lobe. B, Corresponding to the chest radiograph, computed tomography (CT) in coronal view depicts diffuse GGO and consolidation in the left lower lobe. C, Chest CT in transversal view illustrates diffuse GGO in both lungs and crazy-paving pattern combined with a distinct consolidation in the right upper lobe. D, Chest CT in transversal view reveals diffuse GGO in both lungs, large consolidation in the left lower lobe, and subpleural fibrosis combined with pleural changes in the right lower lobe.
Differential cytology (Supplementary Data) revealed a severe depletion of CD4+ cells (12 cells/µL, equaling 2%, and CD4+/CD8+ ratio of 0.08). Lack of CD4+ cells in combination with the fact that the patient is a man who has sex with men led us to the suspicion that he might be a HIV late presenter. Reverse-transcription polymerase chain reaction for HIV type 1 (HIV-1) was indeed positive, measuring a viral load of 360 000 HIV-1 RNA copies/mL. The presence of fine reticular changes in his chest CT together with an elevated level of lactate dehydrogenase prompted us to also consider PJP as a possible additional diagnosis. Bronchoalveolar lavage fluid was positive for P. jirovecii, and the patient was treated with intravenous trimethoprim-sulfamethoxazole (20 mg of trimethoprim component per kg body weight per day 4 times daily) together with 50 mg of prednisone daily to prevent adverse immune reactions in PCP and immune reconstitution inflammatory syndrome.
After the diagnosis of AIDS, rapid antiretroviral therapy (ART) was initiated consisting of orally administered darunavir (600 mg twice daily), ritonavir (100 mg twice daily), and tenofovir/emtricitabine (450/400 mg once daily). Concomitant cytomegalovirus (CMV) infection (170 000 U/mL blood) was treated with ganciclovir at 5 mg/kg body weight. Antibacterial treatment with meropenem and linezolid was continued for 7 days.
Coinfection with hepatitis B, hepatitis C, and syphilis was ruled out. On 15, 18, and 20 May, the patient tested negative for SARS-CoV-2 in bronchial aspirates. Cerebrospinal fluid testing showed 5600 copies/mL HIV-1 but no evidence of JC virus or bacterial meningitis or encephalitis.
Over the next 2 weeks, his state improved significantly. Noradrenaline administration could be terminated 3 days after initiation of trimethoprim-sulfamethoxazole. Antiretroviral therapy had no apparent side effects; creatinine clearance and liver function remained stable over the following weeks. CMV copies declined to <450 U/mL 2 weeks after ganciclovir was started on 14 May. Under ART, the viral load declined to 2800 copies/mL (equaling 2.11 log units) on 25 May. HIV-1 genotyping revealed no relevant drug resistance. After convalescence and ability to swallow reliably, ART was switched to a single-tablet regimen to maintain the patient’s compliance and to reduce the pill burden.
The patient is continuously improving. He is free from ventilator support and was discharged from ICU with 2 L/minute of supplementary oxygen on 4 June 2020.
The case is remarkable for several reasons: (1) SARS-CoV-2 infection initially prompted physicians to neglect other causes of respiratory failure; (2) the patient was immunosuppressed by HIV, which might have caused a mild course of COVID-19; and (3) administration of ART to analgosedated patients on the ICU is challenging since most currently approved therapeutics are pills that cannot be pulverized (see Supplementary Materials for a detailed discussion of these aspects).
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. All authors contributed to collection, review, and/or analysis of the data. “S.M. drafted the manuscript”. The manuscript does not contain statistical data. All authors have seen and approved the final version of the manuscript.
Acknowledgments. The authors are grateful to our patient for giving his consent for publication. The authors also acknowledge all colleagues from our department as well as other departments and laboratories at the University Hospital of Saarland for their individual contribution to diagnostics and treatment in the presented case.
Financial support. R.B. reports personal fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Grifols, Novartis, and CSL Behring as well as grants from AstraZeneca, Boehringer Ingelheim, Novartis, Wilhelm Sander-Stiftung, Dr. Rolf M. Schwiete Stiftung, Stiftung Deutsche Krebshilfe, Mukoviszidose e.V., and the German Federal Ministry of Education and Research ASCONET (Competence Network Asthma and Chronic Obstructive Pulmonary Disease) outside the submitted work.
Potential conflicts of interest. All authors report no potential conflicts of interest and have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Laurence J. Why aren’t people living with HIV at higher risk for developing severe coronavirus disease 2019 (COVID-19)? AIDS Patient Care STDS 2020; 34:247–8. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. HIV stage definitions Available at: https://www.cdc.gov/hiv/basics/whatishiv.html. Accessed 7 July 2020.
- 3. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020; 20:425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bao C, Liu X, Zhang H, Li Y, Liu J. Coronavirus disease 2019 (COVID-19) CT findings: a systematic review and meta-analysis. J Am Coll Radiol 2020; 17:701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.