Dear Editor:
The global health crisis triggered by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic presents puzzling individual and regional differences in the severity of patient outcomes. Nutritional status is a significant factor in immunity, and augmented virulence in humans has been linked to nutrient deficiencies, including that of the micronutrient selenium (1). As longstanding researchers in selenium biology, we were intrigued by the recent findings of Zhang et al. (2), detailing a positive association between reported coronavirus disease 2019 (COVID-19) cure rates and previously measured population selenium status in 17 cities across China. Regional selenium status varies considerably, with most European countries and certain provinces of China being more prone to suboptimal levels than the United States (3). Likewise, acknowledging that actual rates are unknown due to widespread undertesting, nations with the highest reported COVID-19 case-fatality rates (>13%) according to the Johns Hopkins Coronavirus Resource Center platform (4) correspond to regions where suboptimal selenium status was documented previously, such as Italy, France, and the United Kingdom (5). Meanwhile, in the United States, where most of the population is selenium adequate, case-fatality rates are substantially lower (∼6%) relative to Europe.
Among the proteins most impacted by selenium status is glutathione peroxidase 1 (GPX1), a cytosolic selenoenzyme with known antiviral properties. Viral infection increases reactive oxygen species (ROS) production in host cells, which, if not counterbalanced by antioxidant defense mechanisms, leads to oxidative stress. Excess oxidative stress, in turn, augments mutation of the viral genome, which can lead to the emergence of more virulent strains. GPX1 comprises a key defense against ROS, catalyzing the detoxification of hydrogen peroxide to water. In mice lacking the Gpx1 gene (Gpx1−/−), inoculation of a benign strain of coxsackievirus B3 (CVB3) led to viral genome mutations, increased virulence, and myocarditis, none of which were observed in wild-type mice (6). Moreover, the Gpx1−/− model recapitulated the effect of a selenium-deficient diet in wild-type mice, promoting mutagenesis of the benign CVB3 strain into a virulent one (6). In addition, both selenium-deficient wild-type mice (6) and Gpx1−/− mice (7) exhibit heightened inflammatory responses and more severe lung pathology in response to inoculation with influenza A, further indicating that GPX1 participates in molecular mechanisms protecting against viral infections of the respiratory tract. It is currently unknown whether SARS-CoV-2 is also modulated by GPX1 activity, but recent findings are highly suggestive.
Immediately prior to the aforementioned publication of Zhang et al., 2 separate articles provided evidence of a relation between GPX1 and Mpro, the main protease of SARS-CoV-2. Mpro promotes formation of the viral replication complex and hence is a target of great interest for therapeutic intervention. The 3-dimensional crystal structure of MPro was recently described (8), leading to the identification of potential antiviral compounds through structure-assisted drug design and virtual screening. Beginning from a pool of >10,000 compounds, the organoselenium compound, ebselen, emerged as the strongest inhibitor of Mpro. Ebselen is widely considered a GPX1 mimetic, as it reduces ROS via a mechanism similar to that of GPX1, whereby the selenol group is oxidized and subsequently reduced by glutathione (9). Correspondingly, the potential involvement of GPX1 in COVID-19 was also highlighted in a separate study investigating the SARS-CoV-2 human protein interactome. Herein, Gordon et al. (10) expressed 26 of the 29 putative SARS-CoV-2 viral proteins in HEK293 cells and screened for potential interaction partners through affinity purification followed by MS quantification. For the SARS-CoV-2 protease Mpro, screening was conducted using wild-type and a catalytically inactive mutant version of Mpro (Nsp5 C145A) to circumvent promiscuous covalent binding to the catalytic cysteine sulfhydryl group. Three interacting proteins of high confidence were identified, histone deacetylase 2 (HDAC2), tRNA (guanine-26-(N-2))-dimethyltransferase (TRMT1), and GPX1. Taken together, the parallel findings of Mpro inhibition by the GPX1-mimetic ebselen and a physical interaction between Mpro and GPX1 further allude to an important protective role for GPX1 against COVID-19.
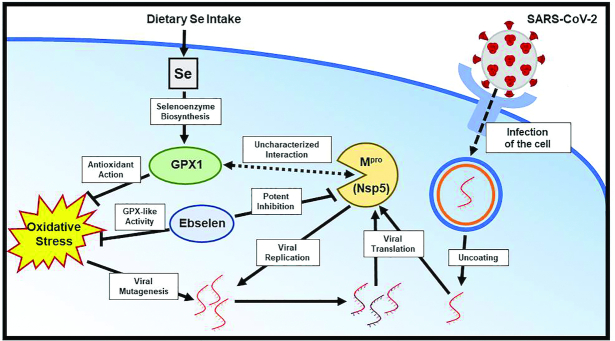
Here, we suggest that the interaction between the GPX1 detoxifying system and the main protease (Mpro) of SARS-CoV-2 represents a novel molecular target for COVID-19 (Figure 1). If proven, this interaction points to a key role for host selenium status in combating SARS-CoV-2 virulence, with selenium deficiency worsening clinical outcomes. This notion is further supported by the report of Zhang et al., demonstrating a positive association between selenium status and COVID-19 cure rates in China. Given that patient GPX1 activity may greatly impact prognosis and disease severity, probing for selenium concentrations among both symptomatic and asymptomatic SARS-CoV-2–infected individuals warrants prioritization. This may provide novel insights into mechanisms leading to the severity of COVID-19 and gauge the demographic most likely to benefit from selenium supplementation and/or ebselen treatment in the fight against COVID-19.
FIGURE 1.
The relation between selenium, GPX1, ebselen, and SARS-CoV-2 Mpro (Nsp5). Selenium (Se) availability regulates expression of GPX1, which, in turn, mitigates oxidative stress. If left unchecked, oxidative stress promotes viral mutagenesis. The selenium-containing compound ebselen mimics GPX activity and is a potent inhibitor of Mpro (Nsp5). GPX1 also interacts with Mpro in a yet-to-be-characterized fashion. Mpro is essential for viral replication. GPX1, glutathione peroxidase 1; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Acknowledgments
We thank Richard Yanagihara, Jeffrey Friedman, Margaret McFall-Ngai, and Edward Ruby for critical feedback on this commentary.
Notes
All authors conceptualized, drafted, and revised the text.
The authors are supported by NIH grants U54 MD007601, P20 GM103466, and R01DK47320.
The authors report no conflicts of interest.
Contributor Information
Lucia A Seale, Department of Cell and Molecular Biology, University of Hawaii, Honolulu, HI, USA.
Daniel J Torres, Department of Cell and Molecular Biology, University of Hawaii, Honolulu, HI, USA.
Marla J Berry, Department of Cell and Molecular Biology, University of Hawaii, Honolulu, HI, USA.
Matthew W Pitts, Department of Cell and Molecular Biology, University of Hawaii, Honolulu, HI, USA.
References
- 1. Guillin OM, Vindry C, Ohlmann T, Chavatte L. Selenium, selenoproteins and viral infection. Nutrients. 2019;11(9):2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang J, Taylor EW, Bennett K, Saad R, Rayman MP. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am J Clin Nutr. 2020;111(6):1297–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rayman MP. Selenium and human health. Lancet. 2012;379(9822):1256–68. [DOI] [PubMed] [Google Scholar]
- 4. Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stoffaneller R, Morse NL. A review of dietary selenium intake and selenium status in Europe and the Middle East. Nutrients. 2015;7(3):1494–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beck MA, Levander OA, Handy J. Selenium deficiency and viral infection. J Nutr. 2003;133(5 Suppl 1):1463S–7S. [DOI] [PubMed] [Google Scholar]
- 7. Yatmaz S, Seow HJ, Gualano RC, Wong ZX, Stambas J, Selemidis S, Crack PJ, Bozinovski S, Anderson GP, Vlahos R. Glutathione peroxidase-1 reduces influenza A virus-induced lung inflammation. Am J Respir Cell Mol Biol. 2013;48(1):17–26. [DOI] [PubMed] [Google Scholar]
- 8. Jin Z, Du X, Xu Y, Deng Y, Liu M, Zhao Y, Zhang B, Li X, Zhang L, Peng C et al. Structure of M(pro) from COVID-19 virus and discovery of its inhibitors. Nature. 2020;582:289–93. [DOI] [PubMed] [Google Scholar]
- 9. Sies H. Ebselen, a selenoorganic compound as glutathione peroxidase mimic. Free Radic Biol Med. 1993;14(3):313–23. [DOI] [PubMed] [Google Scholar]
- 10. Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, O'Meara MJ, Rezeij VV, Guo JZ, Swaney DL et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020. doi: 0.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]