Abstract
Background
Preliminary data from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia patients indicate that a cytokine storm may increase morbidity and mortality. Tocilizumab (anti-IL-6R) is approved by the Food and Drug Administration for treatment of cytokine storm associated with chimeric antigen receptor T-cell therapy. Here we examined compassionate use of tocilizumab in patients with SARS-CoV-2 pneumonia.
Methods
We report on a single-center study of tocilizumab in hospitalized patients with SARS-CoV-2 pneumonia. All patients had confirmed SARS-CoV-2 pneumonia and oxygen saturations <90% on oxygen support with most intubated. We examined clinical and laboratory parameters including oxygen and vasopressor requirements, cytokine profiles, and C-reactive protein (CRP) levels pre- and post-tocilizumab treatment.
Results
Twenty-seven SARS-CoV-2 pneumonia patients received one 400 mg dose of tocilizumab. Interleukin (IL)-6 was the predominant cytokine detected at tocilizumab treatment. Significant reductions in temperature and CRP were seen post-tocilizumab. However, 4 patients did not show rapid CRP declines, of whom 3 had poorer outcomes. Oxygen and vasopressor requirements diminished over the first week post-tocilizumab. Twenty-two patients required mechanical ventilation; at last follow-up, 16 were extubated. Adverse events and serious adverse events were minimal, but 2 deaths (7.4%) occurred that were felt unrelated to tocilizumab.
Conclusions
Compared to published reports on the morbidity and mortality associated with SARS-CoV-2, tocilizumab appears to offer benefits in reducing inflammation, oxygen requirements, vasopressor support, and mortality. The rationale for tocilizumab treatment is supported by detection of IL-6 in pathogenic levels in all patients. Additional doses of tocilizumab may be needed for those showing slow declines in CRP. Proof of efficacy awaits randomized, placebo-controlled clinical trials.
Keywords: COVID-19, SARS-CoV2, acute respiratory distress syndrome, interleukin 6
We found that a single dose of tocilizumab 400 mg given intravenously for compassionate use in 27 patients with severe SARS-CoV-2 pneumonia appeared to offer benefits in reducing inflammation, oxygen requirements, vasopressor support, and mortality.
The coronavirus disease 2019 (COVID-19) epidemic is rapidly consuming the world. As of 15 April 2020, there are more than 2 million cases worldwide with 133 277 deaths [1]. In the United States, the epidemic is rapidly spreading with increasing numbers of confirmed cases and deaths. Initial data from China suggest the primary mortality risk of COVID-19 is attributable to severe pneumonia from the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that leads to acute respiratory distress syndrome (ARDS), which is reported to have a mortality of 60.5% [2–4]. The COVID-19 epidemic has shut down most of the world and is decimating the world’s economies. Vaccines are being developed, but are unlikely to be deployed in the near future. There is a clear and prescient need for new drug development to help save lives and our economies. Although promising agents are being tested in clinical trials, most studies to date are anecdotal and of unclear benefit.
ARDS is a syndrome that causes acute hypoxemic respiratory failure often resulting in mechanical ventilation [5]. The etiology of ARDS is diverse and can include pulmonary (eg, bacterial and viral pneumonia) and extrapulmonary (eg, sepsis, blood transfusions) causes. Those with severe disease can have a mortality rate as high as 45% [6]. The lung injury of ARDS induces a number of inflammatory cytokines that can cause both local and distal organ damage, and this dysregulated inflammation is a primary driver of the disease [7–9].
Interleukin (IL)-6 is a cytokine critical to the function of innate and adaptive immunity. IL-6 has diverse immunologic and physiologic activities including the direction of immune cell differentiation and initial responses to invading pathogens and ischemic injury. IL-6 transcriptional dysregulation is often seen in patients with autoimmune and inflammatory disorders, including capillary leak syndrome and macrophage activating syndrome [10]. IL-6 has also been found to be elevated in ARDS and is predictive of poorer outcomes [11, 12]. Emerging information also suggests IL-6 transcriptional dysregulation is present in patients with COVID-19 infection, and the extremes of IL-6 production are associated with and highly predictive of increased severity of disease and progression to ARDS from SARS-CoV-2 pneumonia [13–15].
Tocilizumab (Actemra®, Roche/Genentech, CA, USA) is the first in class humanized monoclonal antibody aimed at the IL-6 receptor (IL-6R), and functions by binding to both soluble and membrane-bound forms of the IL-6R and is approved by the Food and Drug Administration (FDA) for the treatment of rheumatoid arthritis (RA), and juvenile idiopathic arthritis and cytokine release syndrome (CRS) [16]. In patients with CRS after chimeric antigen receptor (CAR) T-cell therapy, elevations of IL-6 and C-reactive protein (CRP) can be seen that predict the severity of disease. Tocilizumab has an important and significant benefit in treating patients with CRS. The question is whether this benefit would translate to other diseases where extreme elevations of IL-6 and CRP are seen, such as COVID-19. Here, we report our experience using tocilizumab in patients with severe SARS-CoV-2 pneumonia.
METHODS
Patients
Patients reported in this study presented to Cedars-Sinai Medical Center with confirmed SARS-CoV-2 pneumonia by nasopharyngeal real-time polymerase chain reaction (PCR) and chest imaging. Routine laboratory tests, including a COVID-19 panel (IL-6, CRP, ferritin, D-dimer, lactate dehydrogenase (LDH), and troponin I), were checked prior to study entry and intermittently afterward. Patients were not considered for compassionate use of tocilizumab unless the following criteria were met: signs of respiratory compromise consisting of tachypnea, dyspnea, or peripheral capillary oxygen saturation (SpO2) <90% on 4L or increasing oxygen requirements over 24 hours, plus 2 or more of the following predictors for severe disease:
IL-6 > 10 pg/mL
CRP > 35 mg/L
Ferritin > 500–600 ng/mL
D-dimer > 1 mcg/L
Neutrophil-Lymphocyte Ratio > 4
LDH > 200 U/L
Increased troponin in a patient without known cardiac disease
After informed consent was obtained, patients were administered a single dose of tocilizumab (anti-IL-6Rα monoclonal antibody) 400 mg by intravenous injection.
Study Oversight
This observational study was supported by Cedars-Sinai Medical Center and was approved by the Institutional Review Board of Cedars-Sinai Medical Center. The study was conducted in accordance with the Declaration of Helsinki with the ethics guidelines based on federal regulations and the Common Rule. Cedars-Sinai Medical Center also has a federal-wide assurance. This study was designed, conducted, and evaluated solely by the investigators after approval. After informed consent was obtained, the data were gathered and analyzed, and the manuscript was prepared by the investigators, all of whom validated the completeness and accuracy of the results and the fidelity of the study to the protocol.
Clinical Assessment
Clinical data were collected until death, hospital discharge, or the date of last follow-up on 13 April 2020. We analyzed vital signs, vasopressor usage, oxygen requirements, and markers of acute lung injury prior to tocilizumab and up to 27 days post-tocilizumab administration. We also measured cytokines (IL-6, IL-10, IL-17A, IL-1β, tumor necrosis factor α [TNF-α], IL-2, and γ interferon [γ -IFN]) using Luminex platform assays. Pre- and post-tocilizumab administration values were compared using the paired t-test. All adverse events (AEs) and serious adverse events (SAEs) deemed possibly related to tocilizumab and unexpected as common complications of SARS-CoV-2 pneumonia were graded and reported to the Cedars-Sinai Medical Center Institutional Review Board.
RESULTS
Baseline Characteristics
Between 13 March 2020 and 1 April 2020, a total of 27 consecutive patients received a single dose of tocilizumab. Of these, 21 were on mechanical ventilation on the day of administration, and 6 were receiving oxygen supplementation through nasal cannula. Baseline characteristics of the overall population and both subgroups are shown in Table 1. Nearly all patients with the exception of 4 were male (85%). Although most were older (median: 63 years; interquartile range [IQR]: 51–75 years), there were 6 patients younger than 50 years old in the study population, of whom 4 were started on mechanical ventilation before tocilizumab administration. No patients were current smokers, and most had no prior tobacco history (24/27; 89%). Seventeen patients (63%) had a comorbid condition, the most common of which was hypertension (12/17; 71%). The median number of days in the hospital before tocilizumab administration was 2 (IQR: 1–3 days). A total of 22 patients (81%) overall required mechanical ventilation; of these, 21 were on mechanical ventilation at the time of tocilizumab administration. Seventeen patients (63%), all in the invasive oxygen support group, required norepinephrine for vasopressor support.
Table 1.
Baseline Characteristics
| Overall Population | Invasive Oxygen Support | Noninvasive Oxygen Support | |
|---|---|---|---|
| N = 27 | N = 21 | N = 6 | |
| Age, median (years) | 63 | 63 | 67 |
| Range | 37–89 | 37–89 | 41–81 |
| 25th–75th percentile | 51–75 | 55–74 | 45–79 |
| Male (%) | 23 (85) | 17 (81) | 6 (100) |
| Race/Ethnicity (%) | |||
| White | 18 (67) | 13 (62) | 5 (83) |
| Black | 4 (15) | 4 (19) | 0 (0) |
| Hispanic | 3 (11) | 2 (10) | 1 (17) |
| Asian | 2 (7) | 2 (10) | 0 (0) |
| Smoker (%) | |||
| Never | 24 (89) | 18 (86) | 6 (100) |
| Former | 3 (11) | 3 (14) | 0 (0) |
| Current | 0 (0) | 0 (0) | 0 (0) |
| Comorbid conditions (%) | |||
| Hypertension | 12 (44) | 7 (33) | 5 (83) |
| Diabetes | 3 (14) | 3 (14) | 0 (0) |
| Cardiovascular disease | 7 (26) | 6 (29) | 1 (17) |
| Pulmonary disease | 9 (33) | 8 (38) | 1 (17) |
| Malignancy | 2 (7) | 1 (5) | 1 (17) |
| Vasopressor use (%) | 17 (63) | 17 (81) | 0 (0) |
| Days in hospital before intubation, median (25th–75th percentile) | 2 (0–3) | 2 (0–3) | 5 |
| Days in hospital before tocilizumab administration, median (25th–75th percentile) | 2 (1–3) | 3 (2–3) | 2 (1–2) |
All patients received azithromycin and 26/27 received hydroxychloroquine. Seven patients (4 of whom required mechanical ventilation at the time of tocilizumab administration) were also enrolled in a placebo-controlled trial investigating the use of remdesivir; their treatment assignment was blinded and not known.
Clinical Outcomes
Blood gas data were available on 20/21 patients requiring mechanical ventilation on the day of tocilizumab administration. Among these, the median PaO2 was 86 mm Hg (IQR: 71–172 mm Hg), median FiO2 was 60% (IQR: 50–80%), and median PaO2/FiO2 was 161 (IQR: 113–253). Two of the 21 (10%) had a PaO2/FiO2 > 300 (ratio 358 and 359), 6/21 (29%) had a PaO2/FiO2 201–300, 9/21 (43%) had a PaO2/FiO2 101–200, and 3/21 (14%) had a PaO2/FiO2 < 100 on the day of tocilizumab administration.
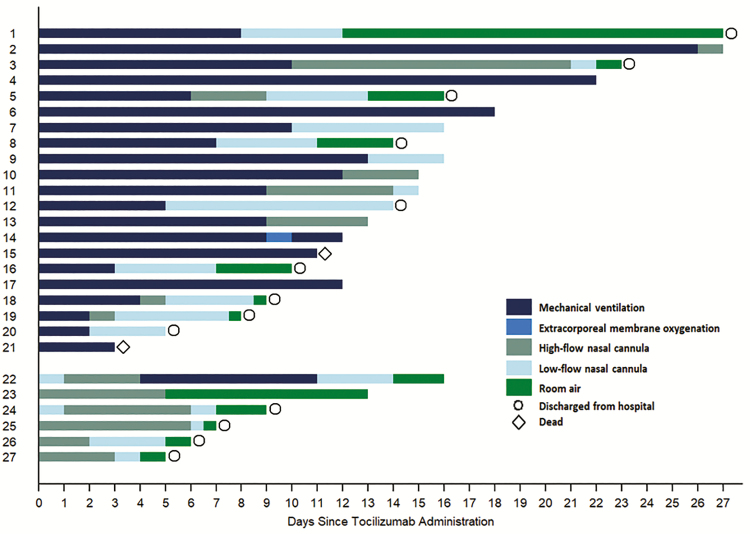
Figure 1 shows the progression of oxygen support requirement over the study period for each individual patient. Of the 21 patients requiring mechanical ventilation at the time of tocilizumab administration, 15 were extubated at a median 8 days after tocilizumab (IQR: 4–10 days). Nine patients were discharged from the hospital at a median 14 days after tocilizumab (IQR: 9–16; Table 2). Two patients died at day 3 and day 11 after tocilizumab. Four patients remained intubated at last follow-up (median 15 days after tocilizumab; IQR: 12–20).
Figure 1.
Oxygen support requirement following tocilizumab administration. Each bar represents an individual patient from the study population. Changes in color represent changes in oxygen support modality administered over time. Four patients remain intubated at last follow-up. Indicators of hospital discharge (n = 13; circle) and death (n = 2; diamond) are represented.
Table 2.
Outcomes Following Tocilizumab Administration
| Invasive Oxygen Support | Noninvasive Oxygen Support | |
|---|---|---|
| N = 21 | N = 6 | |
| Extubated (%) | 15 (71) | 1a (100) |
| Days to extubation, median (25th–75th percentile) | 8 (5–10) | 7a |
| Hospital discharge (%) | 9 (43) | 4 (67) |
| Days to hospital discharge, median (25th–75th percentile) | 14 (9–16) | 7 (6–8) |
| Dead (%) | 2 (10) | 0 (0) |
aOne patient in the noninvasive oxygen support group progressed to mechanical ventilation and was extubated 7 days later.
Among 6 patients administered tocilizumab while on noninvasive oxygen support, only 1 had deterioration in respiratory status requiring mechanical ventilation 4 days after tocilizumab. This patient was extubated 7 days later and was weaned to room air by the end of follow-up. Each of the remaining 5 patients was free of supplemental oxygen at the end of follow-up, and 4 have been discharged to home (Figure 1 and Table 2).
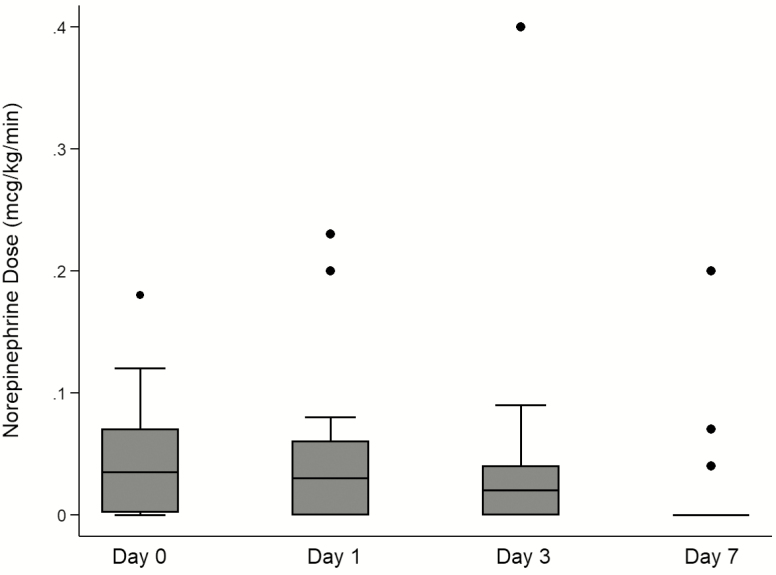
Figure 2 indicates that there was a reduction in the requirement for vasopressors observed within 1 week of tocilizumab administration. Seventeen of 27 patients (63%) required norepinephrine for blood pressure support on the day of tocilizumab administration. Within 3 days, 5 patients were weaned off norepinephrine, and only 3 patients remained on norepinephrine by day 7.
Figure 2.
Vasopressor support over time among patients requiring mechanical ventilation on the day of tocilizumab administration (n = 21). In sum, 17 patients required norepinephrine at the time of tocilizumab administration (day 0), 16 patients on day 1, 12 patients on day 3, and 3 patients on day 7.
Markers of Inflammation
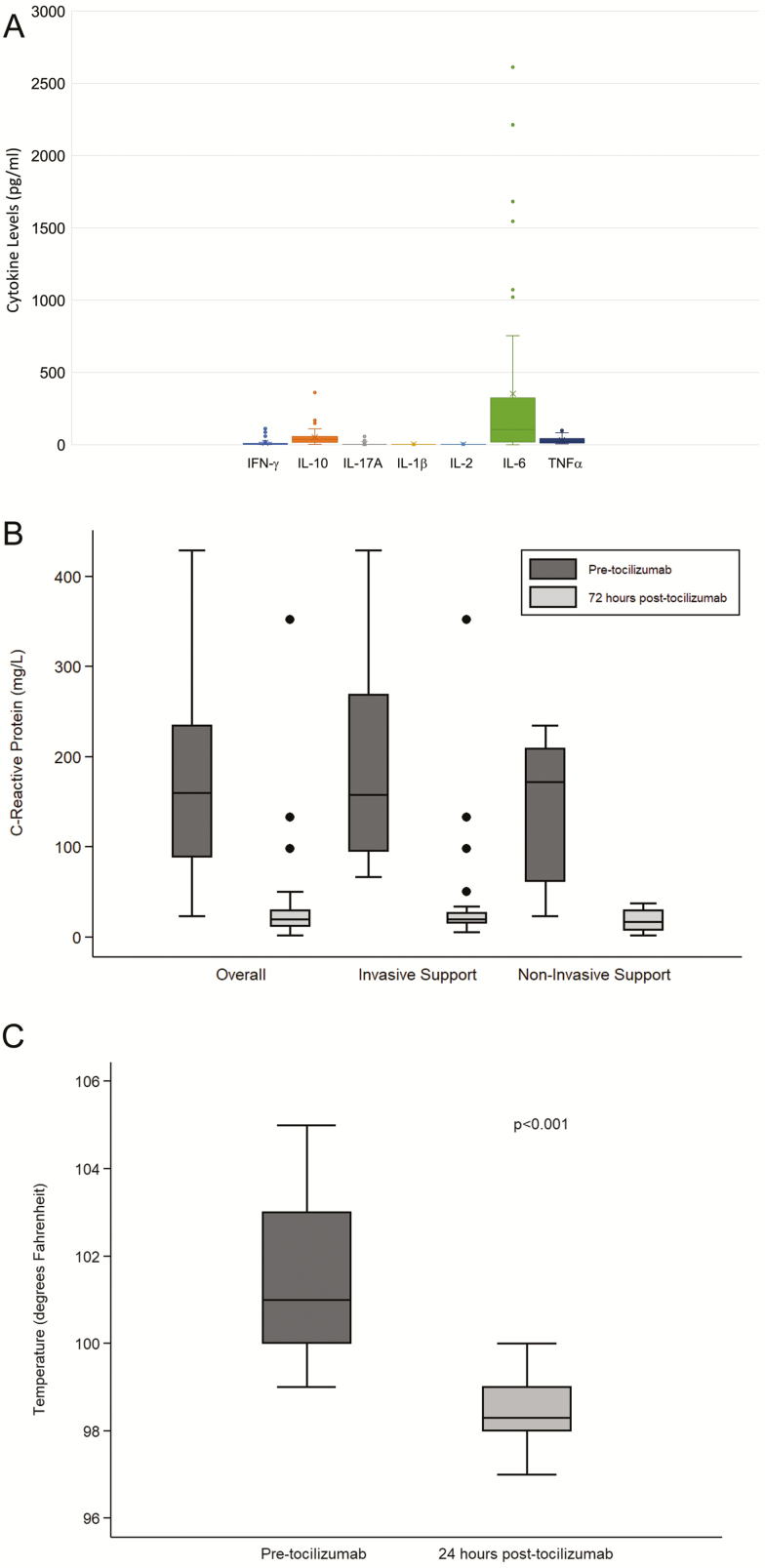
The cytokine profile for all patients at time of tocilizumab administration is shown in Figure 3A. The predominant cytokine seen in all patients was IL-6 with significantly higher levels compared to all other cytokines, (mean 356.07 ± 616 pg/mL, normal 0–5 pg/mL, P < .001). Modest elevations were seen in γ-IFN and IL-10, but not to the degree seen with IL-6. Post-tocilizumab cytokine levels were not routinely obtained in these patients due to the known ability of tocilizumab to increase circulating IL-6 levels due to blocking IL-6’s ability to bind to cellular IL-6 receptor α (IL-6Rα) and stabilizing IL-6/IL-6R complexes in the circulation [17]. Here the best way to assess the efficacy of inhibition of IL-6/IL-6R blockade by tocilizumab is to measure CRP. These data are shown in Figure 3B. We observed significant reductions in CRP levels within 72 hours post-tocilizumab administration (pre-tocilizumab: median 160 mg/L; IQR: 89–235 compared to post-tocilizumab: median 20 mg/L; IQR: 12–30; P < .001). CRP levels did not differ at baseline or post-tocilizumab infusion between patients requiring mechanical ventilation and those on noninvasive oxygen support at the time of tocilizumab administration. Four patients, all of whom were on mechanical ventilation at tocilizumab administration, did not show a rapid decline in CRP. Of these, 2 patients died and 1 required prolonged mechanical ventilation for 26 days, but was ultimately extubated. The fourth required mechanical ventilation for only 2 days. It is possible that a single 400-mg dose of tocilizumab does not uniformly block all cellular IL-6Rα, especially if there is a large amount of circulating IL6Rα/IL-6 complexes that negate efficacy of tocilizumab. However, we can conclude that the 400-mg dose was sufficient to yield CRP reductions in most patients.
Figure 3.
A, Cytokine profile at time of toclizumab administration. Cytokines were measured using Luminex platform assays. Normal values for IL-6 are 0–5 pg/mL. Although other cytokines showed minimal elevations at time of tocilizumab administration (IL-10, TNF-α, and IFN- γ), IL-6 was the predominant cytokine expressed in SARS-CoV-2 patients. B, Change in CRP after tocilizumab administration. Figure shows the CRP values obtained at initiation and 72 hours post-tocilizumab administration. Tocilizumab administration resulted in a significant reduction in CRP values at 72 hours. C, Body temperature after tocilizumab administration. This figure shows the temperatures of SARS-CoV-2 patients pre- and 24 hours post-tocilizumab. Tocilizumab was associated with a significant reduction in body temperature within 24 hours of administration. Abbreviations: CRP, C-reactive protein; IFN, interferon; IL, interleukin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TNF, tumor necrosis factor.
We also examined the impact of tocilizumab on temperature. This is shown in Figure 3C. Here, tocilizumab administration resulted in a significant reduction in body temperature within 24 hours (pre-tocilizumab: median: 101o F; IQR: 100–103o F to post-tocilizumab: median: 98.3o F; IQR: 98–99 o F; P < .001).
Safety
There were no cases of neutropenia observed following tocilizumab administration. One patient developed thrombocytopenia 9 days after tocilizumab administration. There were multiple other factors that were felt more likely to be the cause of the thrombocytopenia, although we cannot exclude the possibility that tocilizumab may have played a contributory role.
Two SAEs were noted; both were patient deaths. The first patient was admitted to the intensive care unit in severe septic shock with SARS-CoV-2 pneumonia. He was also noted to have kidney failure and developed a pneumothorax due to high pressure ventilation. The patient subsequently developed hypotension and could not be ventilated and expired. The second patient was admitted to the intensive care unit with severe SARS-CoV-2 pneumonia and septic shock. After an initial good response, he developed severe bilateral pneumothoraces due to high pressure ventilation, hypotension, and atrial fibrillation and expired. Neither of the deaths were felt to be related to tocilizumab administration. No other AEs or SAEs were reported that were thought to be related directly to tocilizumab therapy by attending physicians.
DISCUSSION
The fundamental pathophysiology of ARDS is dysregulated inflammation that leads to the histological finding of diffuse alveolar damage [8]. A number of studies have reported evidence of a cytokine storm associated with SARS-CoV-2 infection [13–15]. This information has led to a growing interest in agents that might alter or ameliorate the cytokine induced injury [18]. Persistent lung injury can perpetuate the cytokine storm that results in damage to distal organs, such as the kidneys, and this biotrauma can lead to increasing morbidity and mortality [7, 9]. IL-6 in particular has emerged as the primary mediator of the cytokine storm initiated by SARS-CoV-2 infection. In this regard, tocilizumab (anti-IL-6R) has received interest as a potential therapy in the battle against the severe and often fatal pneumonia seen in SARS-CoV-2 patients due to its known efficacy in treating cytokine storm associated with CAR T-cell therapy [19]. In addition, several case reports have shown encouraging results [20–22].
Based on these observations and our own experience with tocilizumab for other indications, we undertook a compassionate use study to determine if patients with SARS-CoV-2 pneumonia would benefit from early tocilizumab administration. Data presented here indicate that IL-6 was the predominant cytokine elevated at the time of SARS-CoV-2 diagnosis, thus confirming the rationale for the use of tocilizumab to mitigate the effect of cytokine-associated injury. Tocilizumab treatment resulted in early significant reductions in inflammatory markers including CRP and temperature.
More importantly, tocilizumab treatment was associated with improvements in oxygenation and a reduction in vasopressor support. Blood pressure response occurred early, and most patients were weaned off of vasopressors within a week of tocilizumab administration. Among patients who required mechanical ventilation, most (73%) have been extubated at the time of this writing, and only 2 have died. In comparison, a series from China reported that 31 of 32 patients (97%) who required invasive mechanical ventilation because of COVID-19 died [4]. Similarly, an early series from the United States reported a 50% mortality rate among patients admitted to the intensive care unit for hypoxemic respiratory failure [2]. Data from a Spanish study indicated a mortality rate of 33.6% [23].
The primary limitation of this study is the absence of a control group. Additionally, due to drug shortages, we used a single 400-mg (1 vial) dose of tocilizumab, which is lower than the recommended dose of 8 mg/kg for CRS. Our center was quick to implement tocilizumab in a protocolized fashion for patients with severe respiratory compromise based on our prior experience with tocilizumab for cytokine storm and early reports suggesting its effectiveness in COVID-19. Despite clinical equipoise regarding the efficacy of tocilizumab in COVID-19, we were reluctant to not offer this treatment to patients given the high mortality associated with hypoxemic respiratory failure related to COVID-19 from earlier reports. Second, the follow-up time in this study is limited, and 4 patients remain ventilator-dependent at the time of this writing. It is possible that the reported death rate in this study could increase with longer follow-up, although the percentage of deaths in this preliminary series would still be lower than that observed in other reports. Finally, all patients were treated with at least 1 other therapy that has been under investigation for treatment of COVID-19, including hydroxychloroquine, azithromycin, and/or remdesivir. The impact of these treatments on the outcomes observed in this study is not known, particularly because prior reports have not uniformly found these treatments to be beneficial [24–26].
In summary, our early experience with tocilizumab suggests that targeted treatment against cytokine storm related to SARS-CoV2 infection may lead to clinical improvement, recovery from respiratory failure, and prevention of death. Despite these encouraging results, we are mindful that proof of efficacy awaits results from a placebo controlled trial (Roche-Genentech) that is now underway. However, we continue to offer tocilizumab to patients admitted with SARS-CoV-2 pneumonia based on these preliminary results.
Notes
Acknowledgments. The authors want to express their gratitude to the nurses, physicians, respiratory therapists, social workers, laboratorians, and support staff whose dedication and devotion to their patients is exemplary. They also want to acknowledge and thank the patients who participated in this study. Finally, they remember all those who have perished from COVID-19; their thoughts and prayers are with them and their families.
Potential conflicts of interest. S. C. J. and E. H. have received research grant funding from Vitaeris. S. C. J. has patents on anti-IL-6 for treatment of allograft rejection and desensitization, consulting contracts with Vitaeris for development of anti-IL-6 in kidney transplant rejection, and grants evaluating anti-IL-6 (clazakizumab) for treatment of COVID 19 pneumonia, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. https://www.worldometers.info/coronavirus/. [Google Scholar]
- 2. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. . COVID-19 in critically ill patients in the Seattle region: case series. N Engl J Med 2020; 382:2012–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang X, Yu Y, Xu J, et al. . Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou F, Yu T, Du R, et al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med 2017; 377:562–72. [DOI] [PubMed] [Google Scholar]
- 6. Ranieri VM, Rubenfeld GD, Thompson BT, et al. ; ARDS Definition Task Force Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307:2526–33. [DOI] [PubMed] [Google Scholar]
- 7. Imai Y, Parodo J, Kajikawa O, et al. . Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA 2003; 289:2104–12. [DOI] [PubMed] [Google Scholar]
- 8. Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest 2012; 122:2731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ranieri VM, Suter PM, Tortorella C, et al. . Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 1999; 282:54–61. [DOI] [PubMed] [Google Scholar]
- 10. Jordan SC, Choi J, Kim I, et al. . Interleukin-6, a cytokine critical to mediation of inflammation, autoimmunity and allograft rejection: therapeutic implications of IL-6 receptor blockade. Transplantation 2017; 101:32–44. [DOI] [PubMed] [Google Scholar]
- 11. Parsons PE, Eisner MD, Thompson BT, et al. . Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med 2005; 33:1–6; discussion 230–232. [DOI] [PubMed] [Google Scholar]
- 12. Ware LB, Koyama T, Billheimer DD, et al. ; NHLBI ARDS Clinical Trials Network Prognostic and pathogenetic value of combining clinical and biochemical indices in patients with acute lung injury. Chest 2010; 137:288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McGonagle D, Sharif K, O’Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev 2020; 19:102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ulhaq ZS, Soraya GV. Interleukin-6 as a potential biomarker of COVID-19 progression [published online ahead of print, 2020 Apr 4]. Med Mal Infect. 2020; 50:382–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125276s114lbl.pdf. [Google Scholar]
- 17. Chen F, Teachey DT, Pequignot E, et al. . Measuring IL-6 and sIL-6R in serum from patients treated with tocilizumab and/or siltuximab following CAR T cell therapy. J Immunol Methods 2016; 434:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fu B, Xu X, Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med 2020; 18:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Le RQ, Li L, Yuan W, et al. . FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist 2018; 23:943–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Luna G, Habibi A, Deux JF, et al. . Rapid and severe Covid-19 pneumonia with severe acute chest syndrome in a sickle cell patient successfully treated with tocilizumab. Am J Hematol 2020; 95:876–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol 2020; 92:814–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Michot JM, Albiges L, Chaput N, et al. . Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: a case report. Ann Oncol 2020; 31:961–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barrasa H, Rello J, Tejada S, et al. . SARS-CoV-2 in Spanish intensive care: early experience with 15-day survival in Vitoria. Anaesth Crit Care Pain Med. 2020; 9:S2352-5568(20)30064–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beigel JH, Tomashek KM, Dodd LE, et al. . Remdesivir for the treatment of COVID-19: preliminary report. N Engl J Med. 22 May 2020;NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- 25. Hernandez AV, Roman YM, Pasupuleti V, Barboza JJ, White CM. Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review. Ann Intern Med. 27 May 2020. doi: 10.7326/M20-2496 [DOI] [PubMed] [Google Scholar]
- 26. Wang Y, Zhang D, Du G, et al. . Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020; 395:1569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]