Abstract
Background
The coronavirus disease 19 (COVID-19) pandemic has spread globally, causing extensive illness and mortality. In advance of effective antiviral therapies, countries have applied different public health strategies to control spread and manage healthcare need. Sweden has taken a unique approach of not implementing strict closures, instead urging personal responsibility. We analyze the results of this and other potential strategies for pandemic control in Sweden.
Methods
We implemented individual-based modeling of COVID-19 spread in Sweden using population, employment, and household data. Epidemiological parameters for COVID-19 were validated on a limited date range; where substantial uncertainties remained, multiple parameters were tested. The effects of different public health strategies were tested over a 160-day period, analyzed for their effects on intensive care unit (ICU) demand and death rate, and compared with Swedish data for April 2020.
Results
Swedish mortality rates are intermediate between rates for European countries that quickly imposed stringent public health controls and those for countries that acted later. Models most closely reproducing reported mortality data suggest that large portions of the population voluntarily self-isolate. Swedish ICU use rates remained lower than predicted, but a large fraction of deaths occurred in non-ICU patients. This suggests that patient prognosis was considered in ICU admission, reducing healthcare load at a cost of decreased survival in patients not admitted.
Conclusions
The Swedish COVID-19 strategy has thus far yielded a striking result: mild mandates overlaid with voluntary measures can achieve results highly similar to late-onset stringent mandates. However, this policy causes more healthcare demand and more deaths than early stringent control and depends on continued public will.
Keywords: COVID-19, individual-based modeling, public health mandates, individual behavior, healthcare capacity
Swedish public health responses to coronavirus disease 2019 (COVID-19) were analyzed using individual-based modeling. Partial voluntary self-isolation explains the reported mortality rate. Intensive care utilization is lower than anticipated and age-skewed, suggesting that additional care could benefit older adults.
Since its emergence, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread globally, owing to a lack of prior immunity combined with relatively high infectiousness [1–3]. This has caused substantial illness, deaths, and strain on the healthcare systems of affected countries. Reported hospitalization rates are in the range of 12% [4] to 17% [5] of diagnosed cases, increasing with age. In severely affected regions, the availability of medically necessary care has become limiting.
In advance of effective vaccines and therapies for coronavirus disease 19 (COVID-19), countries have adopted different public health measures to reduce transmission. These have been classified as suppressive approaches, which aim to arrest transmission, and mitigation approaches, which aim to slow spread and shield vulnerable populations without truncating transmission [6, 7]. Sweden is perhaps the most prominent example of mitigation—limiting the extent of socially and economically disruptive interventions while still aiming to slow spread and allow for an effective medical response [7, 8]. Studying the effects of this strategy, which elements are key to reducing mortality rates and healthcare needs, and how it might compare with other approaches is thus of critical importance to the global understanding of pandemic responses.
In addition to direct mortality, the potential of COVID-19 to saturate national healthcare systems is a key concern, because this affects both survival of patients with COVID-19 and the ability to provide care for others in need. One critically limiting resource is the capacity of intensive care unit (ICU) beds with mechanical ventilation, given that many COVID-19 patients die from causes related to respiratory failure [9, 10]. In global settings where ICU resources were not limiting, infection fatality rates have been estimated in the range 0.36%–1.2% [11–13]. Reported Swedish case-fatality rates were relatively high at 15% for April 2020 [14], although infection fatality rates were certainly lower and much more difficult to determine reliably.
Numerical models of pandemic spread provide the ability to prospectively predict the effects of different public health decisions [15] and help guide national policy [6, 7, 16–20]. In addition, they provide the critical ability to analyze, prospectively or retrospectively, which factors are key determinants of public health outcome. The absolute predictions—numbers of deaths, precise hospital use—from such models include many uncertainties, but relative predictions have been highly informative. We classify predictive models into aggregate population models that yield basic principles of epidemic spread and discrete models that take into account geographic and demographic structure, permitting more specific assessment of different interventions. Discrete models offer a particular advantage here because the nonuniform structure of individual interactions alters infection spread, and differences in demography and social network structure are potential confounders when comparing pandemic behavior across countries.
In particular, individual-based models provide a flexible platform to analyze the propagation of emerging infectious diseases and the effect of social distancing and similar behavioral changes [21]. Because household, workplace, and community structure are explicitly represented in individual-based models, they provide a natural means to examine the effects of public health strategies such as school and workplace closure or home-quarantine measures.
Because of Sweden’s unique strategy of not imposing stringent public health mandates, we used an individual-based model parameterized on Swedish demographics to assess the impact of measures deployed against COVID-19. Sweden’s approach has advertised particular reliance on social distancing and voluntary individual behaviors supplementing public health mandates, so we examined which of these measures is most likely to explain Swedish COVID-19 spread to date, what the future implications are for Sweden, and what lessons the global public health community can glean.
METHODS
Individual-based Model for Pandemic Spread
We used an individual agent-based model based on the framework published by Ferguson and coworkers [6, 22, 23] that we have reimplemented [24]. Details of the transmission model are given elsewhere [24] and briefly summarized here.
Geographic and Demographic Placement
Individuals were assigned location, age, and household randomly sampled from Swedish demographic data [25], capturing the small household size (2.2 per household; 39.8% single-occupancy [25]) that has been postulated as a reason for COVID-19 transmission different from the rest of Europe. Households were placed on a 30-arcsec lattice using LandScan population data [26] and initially assigned 1 adult aged >20 years old. The remainder of the population for each lattice site was randomly distributed among households. In Sweden, only 5% of adults >70 years old are in congregate living facilities [25]; these were not treated explicitly.
School and Workplace Assignment
Each individual was placed in a school or workplace. Individuals <1 or >75 years old did not attend school or work. Schools were classified into 3 age levels, and workplaces into 2 types. Following Swedish school attendance data [27], 78% of children aged 1–3, 95% of children aged 3–5, and all children aged 6–15 years were placed in schools in their city. Students 15–22 years old were assigned to secondary and tertiary schools in their county, using the mean Swedish school size of 220 [27].
Individuals were placed in workplaces of 15 people each following Swedish employment rates: 77.3% for age 22–65 and 17.2% for age 65–75 years [25]. Approximately 95% of Swedish workplaces have 1–9 employees [25]; the 15-person size was chosen to incorporate interactions between workplaces and the skewing effect of larger workplaces. Hospitals were treated as separate workplaces, with 120 employees on average and ≥1 per county, consisting of 4.3% of the total workforce [25]. Hospital workers were considered occupationally exposed to each other as well as patients, but at 25% the exposure risk of other workplaces to account for infection-control precautions (see Supplementary Material for discussion of Swedish precautions).
Initial Infections
Initial infections were randomly seeded to match the per-county case distribution reported as of 25 March 2020 (date of Sweden’s 100th death) [14]. To correct for undertesting bias, the number of initial infections was estimated from COVID-19 ICU admissions from 17 to 31 March, using prior estimates that 2.95% of infections overall result in hospitalization, and 30% of those in ICU admission [6].
Transmission Dynamics
Disease transmission was modeled via discrete-time stochastic simulation on an individual basis; further details are given in the Supplementary Material and reported elsewhere [24]. Briefly, transmission can occur in workplaces or schools, households, and communities. An individual’s probability of becoming infected is the sum of these exposures.
Transmissibility Factors
Individual-based models have been used for a number of emerging diseases; one key disease-specific parameter is the transmissibility within the studied population (analogous to the beta parameter in compartmental SEIR models [28]). Twenty values of this parameter were tested and selected for best agreement with either aggregate growth in cases across Europe [29] or growth in reported Swedish deaths [14] from 21 March to 6 April. The resulting transmissibility factors correspond to doubling rates of 3 days (shown in the Supplementary Material) and 5 days (main text results), respectively.
Statistical Sampling
Ten independent runs were performed per parameter set; the 90% confidence intervals (CIs) for measured outcomes across these runs were relatively tight, indicating that uncertainties in biological parameters rather than statistical sampling are most limiting on overall error. For death estimates, 95% CIs for age-adjusted infection fatality rates in China [11] were calculated and propagated through the model (Supplementary Figure 1).
Public Health Interventions
Several public health interventions were simulated, including both universal mandates and voluntary individual behaviors. The mandates considered were chosen to approximate public health options considered or adopted elsewhere. Transmission scaling factors in each of the 9 mandate-based interventions, listed below, are based on previous work [6] and also follow our initial implementation [24].
Swedish public health mandates: This intervention models Swedish government mandates (not including voluntary behavior) through April 2020. Students aged 15–22 years old did not attend school, removing school transmission and increasing community transmission by 25% and household transmission by 50%. Persons aged >70 years practiced moderate self-isolation, reducing workplace and community transmission by 75%. Symptomatic individuals self-isolated after 1 day with a 90% compliance rate, abrogating workplace transmission and reducing community transmission by 75%. Additional interventions considered below were implemented as additions to these mandates.
Case isolation of entire households: Everyone sharing a household with a symptomatic person was advised to self-quarantine. For these individuals, community transmission was reduced by 75%, workplace transmission was removed, and household transmission increased by 50%. Compliance was estimated at 70% for asymptomatic and 90% for symptomatic individuals.
School closure: All schools were closed in this intervention. Students had no school transmission, but household transmission increased by 50% and community transmission by 25%.
Simple closure of schools and nonessential businesses: Schools and nonessential businesses were closed, but social distancing was not advised. School transmission was removed, workplace transmission was reduced by 75%, household transmission increased by 75%, and community transmission increased by 50%.
Closure of schools and nonessential businesses with social distancing: This was as per intervention 4, but with social distancing. School transmission was removed, workplace transmission was reduced by 75%, household transmission increased by 50%, and community transmission decreased by 75%. This was practiced with 90% compliance.
Voluntary work from home: A specified fraction of individuals worked from home; their community transmission was decreased by 25%, and household transmission increased by 50%.
Voluntary self-isolation: A specified fraction of individuals self-isolated; their workplace transmission was removed, community transmission was decreased by 75%, and household transmission increased by 100%.
Voluntary work from home overlaid on mild social distancing: As per intervention 6, but all other individuals reduced community transmission by 25%.
Voluntary self-isolation overlaid on mild social distancing: As per intervention 7, but all other individuals reduced community transmission by 25%.
Healthcare Capacity
Swedish prepandemic healthcare capacity was assessed based on the most recent pan-European reports available: 5.8 ICU beds per 100 000 inhabitants [30].
Implementation
Code implementing the model and interventions tested is freely available online (https://github.com/kassonlab/covid19-epi). Data files are available on Zenodo (doi:10.5281/zenodo.3836195).
RESULTS
Models of COVID-19 spread were initialized with data for the period until 21 March, validated against reported death rates for the period from 21 March to 6 April, and evaluated against death data from 6 April onward. Because reverse-transcription polymerase chain reaction testing was not performed on a widespread basis in Sweden, we estimated initial infections in the period until 21 March via back-calculation from ICU admissions.
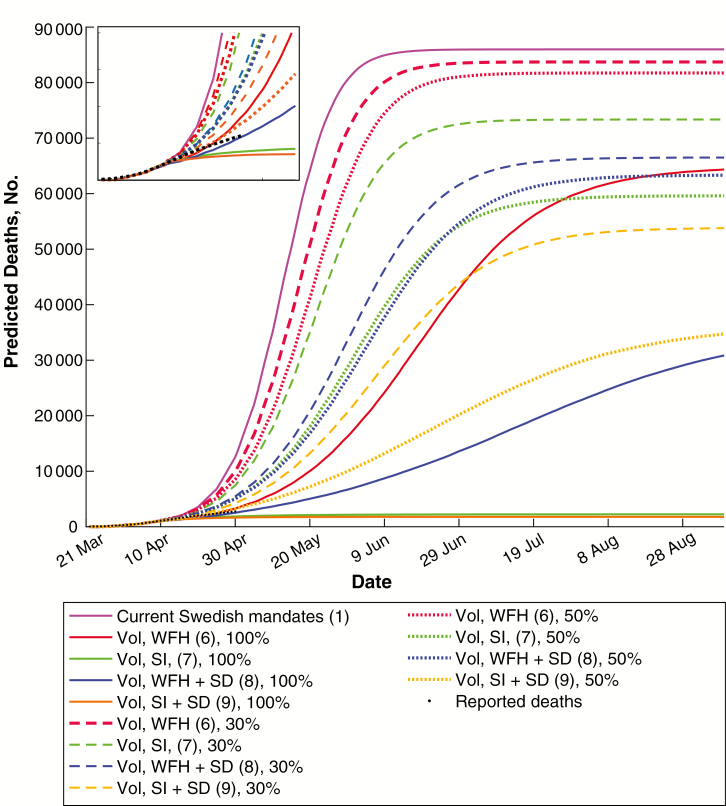
Numbers of infected individuals, hospitalization need, ICU admission need, and deaths were estimated using either a mandate-only strategy or a voluntary isolation strategy across a range of epidemiological parameters. These were evaluated against reported COVID-19 deaths in Sweden over the period from 6 to 30 April. The resulting mortality estimates (Figure 1 and infected individuals in Supplementary Figure 2) show that voluntary isolation can have a substantial effect on COVID mortality. Models of the Swedish public health mandates alone predict substantially more deaths in April than were reported, whereas self-isolation by a moderate fraction of the population can well reproduce the reported death toll. Although redundancies in the model parameter space and uncertainty regarding delayed death registrations preclude assignment of a unique set of “best fit” parameters, the agreement with reported death tolls demonstrates that the model is able to capture Swedish mortality data with epidemiological parameters within the range of international best estimates.
Figure 1.
Predicted coronavirus disease 2019 (COVID-19) deaths in Sweden with different voluntary adherence strategies. Plotted are median numbers of COVID-19 deaths predicted by modeling current Swedish public health mandates and individual voluntary behaviors. These are compared against reported COVID-19 deaths in Sweden. A moderate level of individual self-isolation (SI) is sufficient to well reproduce the reported death tolls. Data are shown for 3-day doubling times (see Supplementary Figures 4 and 5 for alternates). Numbers in parentheses represent interventions listed in Methods. April predictions are enlarged in the inset. Abbreviation: SD, social distancing; Vol, Voluntary; WFH, work from home.
Perhaps most importantly, this analysis suggests that approximately 30% of Swedish residents have self-isolated in some form. Mobile-phone location data can provide rough estimates of this quantity. Although detailed interpretation of these data is a complex undertaking, reports from Google and others [31, 32] suggest a decrease in workplace presence of between 18% and 33% during the month of April. Our analysis is consistent with these numbers. As with any voluntary measures, adoption varied both over the course of April and in different regions of the country; those variations are not treated explicitly here. Furthermore, because diagnosed cases and deaths are lagging indicators of infection, individual modulation of voluntary measures is unlikely to result in optimal choice of control measures. For instance, premature relaxation of voluntary measures may contribute to further spread.
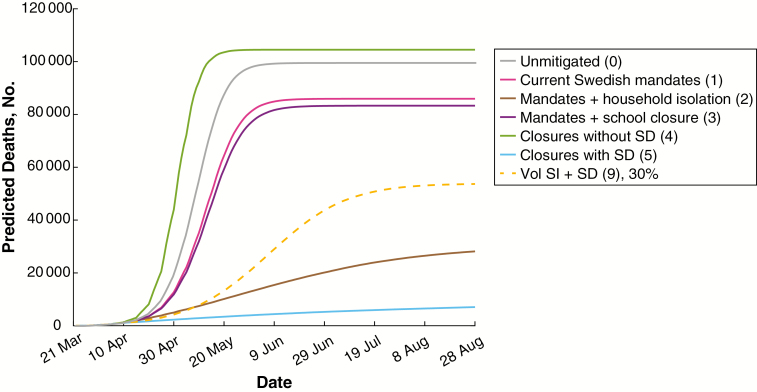
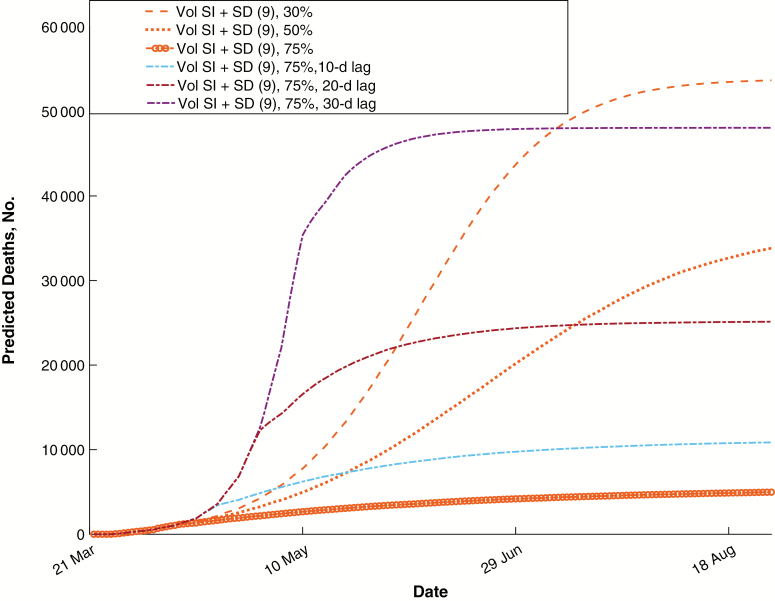
We also evaluated alternative public health mandates. Each of these was considered from 21 March onward, and we estimated the relative impact on mortality rate, ICU demand, and individuals infected (Figure 2 and Supplementary Figure 3). In agreement with prior predictions [33, 34], these results suggest that strong public health mandates greatly reduce the mortality rate and healthcare needs. Surprisingly, voluntary self-isolation overlaid on the existing public health mandates achieved results within 9-fold of strong mandates at a voluntary adherence rate of 30%, within 7-fold if mild social distancing were overlaid, and within 5-fold at an adherence rate of 50% with mild social distancing. This suggests that voluntary control efforts, if widely implemented, can have a substantial effect on transmission. Working from home reduced the mortality rate and ICU demand substantially, but the decreased community transmission resulting from self-isolation had a substantial additional effect at all adherence rates. For all public health interventions considered, prompt implementation is critical to success. The predicted effects of variable delays in voluntary adherence are plotted in Figure 3, showing a substantial loss of effectiveness with increasing delay.
Figure 2.
Predicted coronavirus disease 2019 (COVID-19) deaths in Sweden with different public health mandates. Plotted are median numbers of COVID-19 deaths predicted by modeling current Swedish public health mandates and alternate strategies. A single voluntary behavior is plotted as a comparator. Numbers in parentheses represent interventions listed in Methods. Abbreviations: SD, social distancing; SI, self-isolation; Vol, Voluntary.
Figure 3.
Effects of delayed implementation on mortality rate. Predicted numbers of deaths are plotted for the strongest set of interventions, considered at varying voluntary ratios and time lags before implementation. Infection could still be suppressed effectively with a 10-day delay, but a 30-day delay greatly increased the predicted death toll. Number 9 in parentheses represents intervention 9 in Methods. Abbreviations: SD, social distancing; SI, self-isolation; Vol, Voluntary.
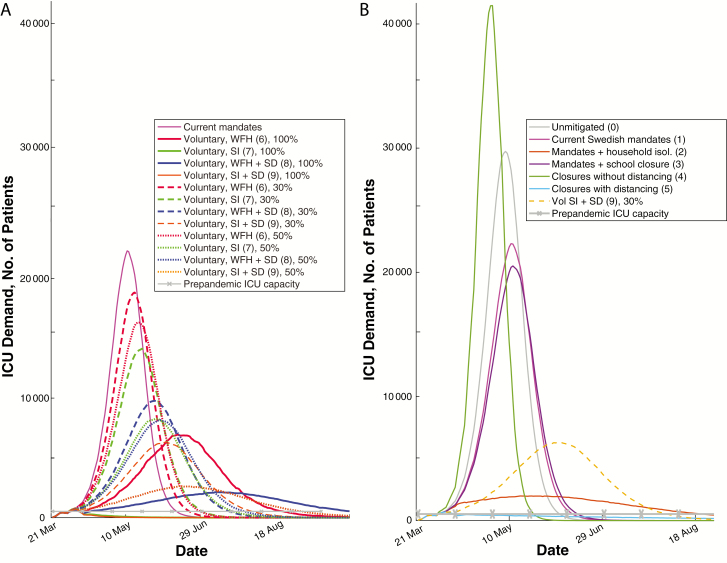
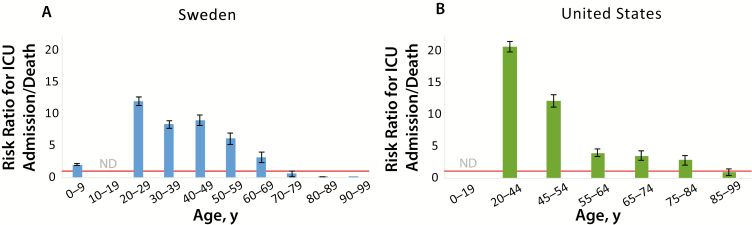
Model predictions suggest that the Swedish public health mandates alone would have resulted in approximately 40-fold more patients (median, 42-fol; 90% CI, 42–43-fold; statistical sampling not limiting on error) who could benefit from ICU care than ICU beds available before the pandemic (Figure 4). Voluntary self-isolation of 50% of the population reduced this to 5-fold (90% CI, 4.8–5.1-fold), and strong suppressive mandates would have reduced it to 1.5-fold (1.4–1.50-fold). As part of its public health response, Sweden approximately doubled its number of ICU beds during spring 2020. However, not all ICU beds were occupied—the number of unique patients receiving COVID ICU care was approximately 53% of the total COVID-diagnosed deaths at the start of May 2020 [14, 35] To analyze this, we examined the demographic characteristics of patients with COVID-19 diagnosed, patients admitted to the ICU, and patients who died with a confirmed COVID-19 diagnosis [25]. Analyzed by categorical age group, older Swedish patients with confirmed COVID-19 were more likely to die than to be admitted to the ICU (Figure 5), suggesting that predicted prognosis may have been a factor in ICU admission. This likely reduced ICU load at the cost of more high-risk patients dying outside the ICU.
Figure 4.
Predicted intensive care unit (ICU) demand with different mandate and voluntary strategies. A, ICU demand predicted by modeling selected public health mandates and voluntary behaviors, with Swedish prepandemic capacities shown as dotted lines. B, Median number of individuals predicted to require intensive care at the indicated date for each of the indicated public health mandates, again compared with the reported Swedish prepandemic ICU capacity. Numbers in parentheses represent interventions listed in Methods. Abbreviations: SD, social distancing; SI, self-isolation; Vol, Voluntary; WFH, work from home.
Figure 5.
Risk of intensive care unit (ICU) admission versus death due to coronavirus disease 2019 (COVID-19). The risks of ICU admission due to COVID-19 versus death due to COVID-19 are plotted for reported cases for categorical age ranges in Sweden (A) and the United States (B). Age categorization is plotted according to original data sources. Swedish data show a substantially higher likelihood of death than of ICU admission for patients aged >70 years (ratio <1.0). Red lines represent a risk ratio of 1; error bars, standard errors. Abbreviation: ND, no reported deaths.
DISCUSSION
Sweden has attracted much international attention for its different approach to the COVID-19 pandemic. By applying only very light mandates—closure of high schools and universities only and advising isolation by symptomatic individuals and those aged >70 years—it appears to be a substantial outlier in its public health strategy. Our analysis suggests that individual actions in Sweden have created a more graduated scenario: voluntary self-isolation in Sweden has provided a combined population response intermediate between the public health mandates alone and the stronger mandate regimen implemented by other countries and regions. Sweden has had a similarly intermediate number of reported COVID-19 deaths—fewer per capita than Italy, Spain, and the United Kingdom but more than its Scandinavian neighbors that implemented strong measures promptly and more than most other European countries (Supplementary Table 1; 35 of 100 000 in Sweden versus 9.3 in Denmark, 5.2 in Finland, and 4.7 in Norway, as of 15 May 2020).
Benefits of the individual-response approach include increased flexibility; drawbacks include decreased coordination in the maintenance and strategic relaxation of controls. Predicted deaths and ICU demand are also greater with voluntary adherence than with stringent mandates until adherence rates exceed 75%. Whether mandated or voluntary, self-isolation of a substantial fraction of the population profoundly reduces ICU need and mortality rates if applied early and with substantial adherence rates. It therefore also follows that greater self-isolation in Sweden would have commensurately reduced deaths. Most national strategies consider both public health and economic effects of infection-control mandates; based on preliminary European data, the economic impacts on Sweden appear similar to those of its neighbors [36].
Our analysis yields results qualitatively similar to those obtained using other model formalisms [8, 33]; one advantage of an individual-based model is explicit representation of demographic data, so differences between countries can be analyzed based purely on data rather than parameterized. In addition, individual-based models facilitate examination of nonuniform behaviors across a population: self-isolation by 50% of the population all the time has markedly different effects than self-isolation by all of the population 50% of the time. Different models have yielded convergent predictions that individual action can increase the efficacy of public health measures, but high adoption is necessary for disease suppression, and both rapid and sustained action are required. This suggests that the underlying findings are indeed robust.
Our analyses demonstrate how individual-based modeling can account for both the unmitigated spread of the COVID-19 pandemic and its control via either suppressive measures or substantial voluntary self-isolation. We demonstrate that Sweden is likely not exceptional in its demography or other parameters controlling the epidemic spread of COVID-19; instead, the course of the pandemic in Sweden likely results from the overlay of public health mandates and individual control measures. We further note that control of ICU load during the pandemic may reflect ICU admission criteria applied. Substantial uncertainty remains regarding the key biological variables controlling COVID-19 spread as well as human behavioral changes, and models of this nature are not designed to predict the future precisely. Our analyses nonetheless suggest that voluntary control strategies are highly dependent on continued individual adherence, which may prove difficult over time.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Åke Sandgren and Lars Viklund for code optimization and Milad Sharifpour, Nele Brusselaers, and Wouter van der Wijngaart for helpful discussions.
Financial support. This work was supported by the Knut and Alice Wallenberg Foundation (grants 2015.0198 to P. M. K. and 2018.0140 and 2019.0431 to S. C. L. K.) and the Swedish National Infrastructure for Computing (grant SNIC 2020/5–176) at the High Performance Computing Center North, Uppsala Multidisciplinary Center for Advanced Computational Science, National Supercomputer Center Sweden, and the PDC Center for High Performance Computing.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020; 382:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-19. Nature 2020; 581:465–9. [DOI] [PubMed] [Google Scholar]
- 3. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579:270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19). Morb Mortal Wkly Rep 2020; 69:343–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Täglicher lagebericht des RKI zur coronavirus-krankheit-2019 (COVID-19). Berlin: Robert Koch Institute, 2020. Available at: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/2020-05-12-de.pdf?__blob=publicationFile. Accessed 12 May 2020. [Google Scholar]
- 6. Ferguson NM, Laydon D, Nedjati-Gilani G, et al. Impact of non-pharmaceutical interventions (NPIs) to reduce COVID-19 mortality and healthcare demand. London: Imperial College, UK Govt / SAGE, 2020. doi: 10.25561/77482. [cited 2020 Mar 16]. Available at: https://www.gov.uk/government/publications/impact-of-non-pharmaceutical-interventions-npis-to-reduce-covid-19-mortality-and-healthcare-demand-16-march-2020. [DOI]
- 7. Walker PGT, Whittaker C, Watson O, et al. The impact of COVID-19 and strategies for mitigation and suppression in low- and middle-income countries. Science 2020. in press. [DOI] [PMC free article] [PubMed]
- 8. Flaxman S, Mishra S, Gandy A, et al. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature doi: 10.1038/s41586-020-2405-7. [DOI] [PubMed] [Google Scholar]
- 9. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of COVID-19 disease: a model-based analysis. Lancet Infect Dis 2020; 20:669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Russell TW, Hellewell J, Jarvis CI, et al. Estimating the infection and case fatality ratio for coronavirus disease (COVID-19) using age-adjusted data from the outbreak on the Diamond Princess cruise ship, February 2020. Euro Surveill 2020; 25:2000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Streeck H, Schulte B, Kuemmerer B, et al. Infection fatality rate of SARS-CoV-2 infection in a German community with a super-spreading event. medRxiv [Preprint]. 2 June 2020. Available from: 10.1101/2020.05.04.20090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Public Health Agency of Sweden (Folkhälsomyndigheten) Available at: https://www.folkhalsomyndigheten.se/. Accessed 12 May 2020.
- 15. Adam D. Special report: the simulations driving the world’s response to COVID-19. Nature 2020; 580:316–8. [DOI] [PubMed] [Google Scholar]
- 16. Fan C, Liu L, Guo W, et al. Prediction of epidemic spread of the 2019 novel coronavirus driven by spring festival transportation in China: a population-based study. Int J Environ Res Public Health 2020; 17:1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang S, Diao M, Yu W, Pei L, Lin Z, Chen D. Estimation of the reproductive number of novel coronavirus (COVID-19) and the probable outbreak size on the Diamond Princess cruise ship: a data-driven analysis. Int J Infect Dis 2020; 93:201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Petropoulos F, Makridakis S. Forecasting the novel coronavirus COVID-19. PLoS One 2020; 15:e0231236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang H, Wang Z, Dong Y, et al. Phase-adjusted estimation of the number of Coronavirus disease 2019 cases in Wuhan, China. Cell Discov 2020; 6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science 2020; 368:860–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Willem L, Verelst F, Bilcke J, Hens N, Beutels P. Lessons from a decade of individual-based models for infectious disease transmission: a systematic review (2006–2015). BMC Infect Dis 2017; 17:612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ferguson NM, Cummings DA, Cauchemez S, et al. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature 2005; 437:209–14. [DOI] [PubMed] [Google Scholar]
- 23. Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature 2006; 442:448–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gardner JM, Willem L, van der Wijngaart W, Kamerlin SCL, Brusselaers N, Kasson P. Intervention strategies against COVID-19 and their estimated impact on Swedish healthcare capacity. medRxiv [Preprint]. 25 April 2020. Available from: 10.1101/2020.04.11.20062133. [DOI] [Google Scholar]
- 25. Statistics Sweden (SCB). Population statistics Available at: http://www.statistikdatabasen.scb.se/pxweb/sv/ssd/. Accessed 12 May 2020.
- 26. Oak Ridge National Laboratory. LandscanTM Global: geographic information science & technology. Available at: https://landscan.ornl.gov/. Accessed 27 March 2020. [Google Scholar]
- 27. The Swedish National Agency for Education (Skolverket). Available at: https://www.skolverket.se/. Accessed 11 April 2020. [Google Scholar]
- 28. Xu R, Ma Z. Global stability of a SIR epidemic model with nonlinear incidence rate and time delay. Nonlinear Anal-Real 2009; 10:3175–89. [Google Scholar]
- 29. Pellis L, Scarabel F, Stage HB, et al. Challenges in control of Covid-19: short doubling time and long delay to effect of interventions. medRxiv [Preprint]. 11 June 2020. Available from: 10.1101/2020.04.12.20059972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rhodes A, Ferdinande P, Flaatten H, Guidet B, Metnitz PG, Moreno RP. The variability of critical care bed numbers in Europe. Intensive Care Med 2012; 38:1647–53. [DOI] [PubMed] [Google Scholar]
- 31. Apple. In: Mobility trends reports. 2020. Available at https://www.apple.com/covid19/mobility. Accessed 30 April 2020. [Google Scholar]
- 32. Google. COVID-19 community mobility reports. 2020. Available at: https://www.google.com/covid19/mobility/. Accessed 30 April 2020.
- 33. Rocklov J. COVID-19 health care demand and mortality in Sweden in response to nonpharmaceutical (NPIs) mitigation and suppression scenarios. medRxiv [Preprint]. 10 May 2020. Available from: 10.1101/2020.03.20.20039594. [DOI] [Google Scholar]
- 34. Wu JT, Leung K, Bushman M, et al. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat Med 2020; 26:506–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. The Swedish Intensive Care Registry Available at: https://www.icuregswe.org/data--resultat/covid-19-i-svensk-intensivvard/. Accessed 12 May 2020.
- 36. European economic forecast spring 2020. Luxembourg City, Luxembourg: European Commission, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.