Abstract
We are learning that the host response to severe acute respiratory syndrome coronavirus 2 ( SARS-CoV-2) infection is complex and highly dynamic. Effective initial host defense in the lung is associated with mild symptoms and disease resolution. Viral evasion of the immune response can lead to refractory alveolar damage, ineffective lung repair mechanisms, and systemic inflammation with associated organ dysfunction. The immune response in these patients is highly variable and can include moderate to severe systemic inflammation and/or marked systemic immune suppression. There is unlikely to be a “one size fits all” approach to immunomodulation in patients with coronavirus disease 2019 (COVID-19). We believe that a personalized, immunophenotype-driven approach to immunomodulation that may include anticytokine therapy in carefully selected patients and immunostimulatory therapies in others is the shortest path to success in the study and treatment of patients with critical illness due to COVID-19.
Keywords: COVID-19, immunoparalysis, cytokine storm, immune modulation, inflammation
There is unlikely to be a “one size fits all” approach to immunomodulation in patients with COVID-19. Clinical trials of immunomodulators in this setting should include prospective immunophenotyping and should adapt to the dynamic nature of the host immune response.
(See the Editorial Commentary by Fang and Schooley on pages 149–51; Brief Reports by Garcia-Vidal et al on pages 127–32 and Larson et al on pages 133–5.)
A vital component of our understanding of the pathogenesis of coronavirus disease 2019 (COVID-19) involves deconvoluting the complex spectrum of immune responses in patients with COVID-19. Despite the still emerging nature of the data, many early reports have enabled some characterization of the clinical course of this disease and associated immunological response against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Based on these reports, there seem to be 3 basic phases during the course of COVID-19 infection: the first being a largely asymptomatic incubation period, followed by symptom onset in the second phase that is nonspecific and nonsevere, with a subset of patients progressing to a third phase with severe lung disease, often with accompanying extrapulmonary organ dysfunction [1–4]. While most patients promptly recover, many have poor clinical outcomes including prolonged acute respiratory distress syndrome (ARDS) and/or death. Concurrent with the pathophysiology of COVID-19 infection, the immune responses in these patients may be categorized into phases: an early local innate immune response that is critical for the host in providing antiviral defense in the lung, and a later phase that results in severe local and systemic immune responses that contribute to morbidity and mortality [5].
A global race is on in search for a novel or repurposed antiviral agent to treat the infected and, ideally, decrease viral shedding and transmission. Concurrently, much interest exists in controlling the hyperinflammatory state elicited by the infection. The inflammatory response in COVID-19 has been likened to conditions including classical ARDS, macrophage activation syndrome (MAS), or hemophagocytic lymphohistiocytosis, or simply, “cytokine storm” [6]. It is likely that none of these syndromes precisely fit all patients with COVID-19, lending urgency to the development of personalized-medicine approaches to the diagnosis and management of the inflammatory effects of this complex and novel disease above and beyond supportive care. Defining the biological processes in the various stages of COVID-19 at a granular level is critical to identifying targets for drug development and, in the absence of proven effective therapies, informing empiric treatment decisions. It is also essential to look back over the history of treating deadly conditions like ARDS and sepsis in an effort to avoid pitfalls that our predecessors discovered decades ago.
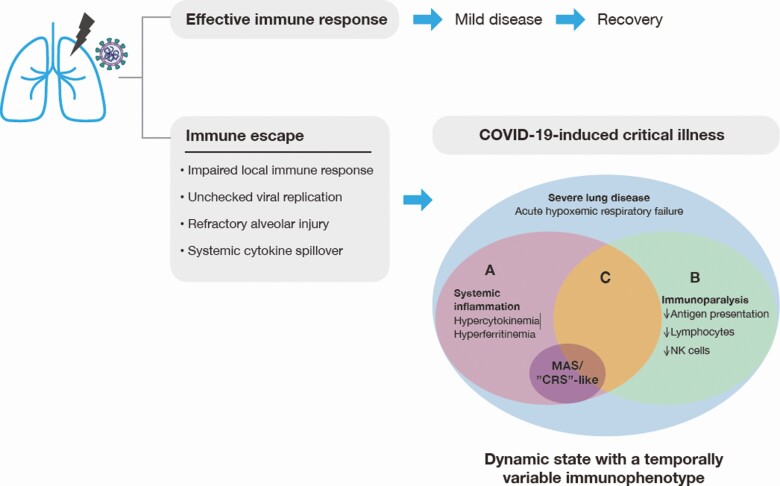
Here, we summarize what is known and unknown about the immunobiology of SARV-CoV-2 infection (Figure 1) and highlight the importance of following the science, past and present, in an effort to provide a balanced viewpoint on the development of therapeutic strategies for those affected with severe COVID-19.
Figure 1. .
Dynamic, heterogenous inflammatory processes subsequent to immune escape contribute to disease severity and tissue damage. Early, local inflammatory processes in the lung often result in an effective host response leading to pathogen clearance. In the event of escape of the virus from the immune system as the result of a suppressed or delayed immune response, there is unchecked viral replication. Increased viral load and direct tissue injury precipitate inflammatory processes, which lead to highly dynamic and variable local and systemic immune responses. Within the context of COVID-19–induced critical illness, including acute hypoxemic respiratory failure, systemic inflammation (A) and immunoparalysis (B) may occur independently or simultaneously (C). Systemic inflammation, including hypercytokinemia and hyperferritinemia, is common, but is not universally severe. Only a subset of patients have inflammatory biomarkers that are high enough to be consistent with MAS- or CRS-like presentations. Severe immune suppression (ie, immunoparalysis) may be characterized by decreased antigen-presenting capacity and decreased numbers of lymphocytes and NK cells. In sum, COVID-19–induced critical illness is a highly dynamic state with a temporally variable immunophenotype. Heterogeneity of the subsequent inflammatory responses in individual patients is common, and the use of immunomodulatory interventions likely needs to be based on the phase of the response and individual patient immunophenotype. Abbreviations: COVID-19, coronavirus disease 2019; CRS, cytokine release syndrome; MAS, macrophage activation syndrome; NK, natural killer.
EARLY INFLAMMATORY PROCESS
The angiotensin-converting enzyme 2 (ACE2)–expressing cells act as target cells for SARS-CoV-2 infection [7]. In the lung, virus can infect and impair the respiratory tract mucosal epithelium, alveolar epithelium, bronchial mucosal epithelium, and endothelial cells [8]. Importantly, the high expression of ACE2 in alveolar type II cells in the lung likely makes this cell type more susceptible to SARS-CoV-2 infection. Infection of alveolar type II cells may be an underpinning of COVID-19 pathogenesis as these cells are known to perform many critical functions that include production of pulmonary surfactant, airway epithelial barrier stabilization, and airway regeneration in response to injury. They also play a role in immune defense via secretion of cytokines in response to alveolar damage and pathogens in order to signal the recruitment and activation of macrophages and other immune cells in defense of the alveolus [9]. Given the high prevalance of mildly symptomatic patients, this early, local immune response can often successfully contain the initial viral infection and re-establish the homeostatic environment in the respiratory tract. Successful control of viral infections often depends on the initiation of types I–III interferon (IFN) responses and their downstream signaling events. These signaling events eventually also translate into an effective adaptive immune response [10]. Also critical during this phase may be the collaboration of transforming growth factor β (TGF- β) with interleukin (IL)-6 to induce the differentiation of naive CD4 into Th17 cells [11]. IL-6 is known to further synergize with IL-7 and IL-15 to induce the differentiation and cytolytic capacity of the CD8 T cells [12].
IMMUNE ESCAPE PROCESS
The IFN response can often be suppressed or delayed in infections caused by viruses such as severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV), as well as influenza virus, with impairment of the IFN response correlating with greater disease severity [13, 14]. Although speculative, members of the Betacoronavirus family such as SARS-CoV, MERS-CoV, and SARS-CoV-2 may have similarity in their mechanism of innate immune evasion. Recent studies have shown that the replication of SARS-CoV-2 in alveolar cells can result in a suppressed magnitude of antiviral response, with inhibition of IFN-I and IFN-III responses in both a human lung alveolar carcinoma cell line and in vivo in ferrets [15]. In addition, inoculation of SARS-CoV-2 onto ex vivo human lung tissue explants resulted in productive infection while inducing lower expression of IFNs and proinflammatory cytokines/chemokines, suggesting that SARS-CoV-2 is able to evade innate immune detection or suppress the downstream response [16]. Suppression of the innate immune response in the early phase would allow SARS-CoV-2 to replicate unchecked in the respiratory tract, achieving high viral load and eventually contributing to its efficient person-to-person transmission before onset of severe clinical symptoms [17]. Indeed, this notion is supported by the high prevalence of radiological changes in computed tomography (CT) scans of the lungs of patients with COVID-19, even in the early stages of illness. Evidence of adaptive immune dysfunction comes from autopsy reports of patients with COVID-19 demonstrating low numbers of CD8-positive T lymphocytes infiltrating lung tissue [18]. There have also been clinical reports of decreased immune cell populations including CD4, CD8, and natural killer (NK) cells in peripheral blood [19]. Additionally, the NK cells and cytotoxic lymphocytes appeared to be exhausted with a reduced ability to produce CD107a, IFN-γ, IL-2, granzyme B, and tumor necrosis factor α (TNF-α) [20]. The T cells from patients with COVID-19 also have significantly higher expression of the inhibitory molecule programmed death (PD)-1 compared with healthy controls. These increases in PD-1 and T-cell immunoglobulin and mucin domain 3 (Tim-3) expression on T cells were reported as patients progressed from mildly symptomatic to the severe stage, further indicative of T-cell exhaustion.
Coronaviruses can also impair host defense through their effects on the Th1 cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF). In addition to promoting the production of myeloid-lineage leukocytes in the bone marrow, GM-CSF is essential for the development and functional maturation of alveolar macrophages [21–23]. Alveolar macrophages are the sentinels of the innate immune system against respiratory pathogens. They act via secretion of oxygen metabolites, antimicrobial proteases, and by recruiting activated neutrophils into alveolar spaces. They can also aid in resolving inflammation after the infectious challenge is resolved by restoring surfactant homeostasis and orchestrating epithelial proliferation and barrier repair [24]. Lung epithelial cells transfected with the SARS-CoV protease 3CLPro were shown to have decreased GM-CSF mRNA and protein expression, suggesting that SARS-CoV may promote alveolar injury through the suppression of GM-CSF [25]. Whether this represents a mechanism of SARS-CoV-2–mediated immune suppression remains to be investigated.
SUBSEQUENT INFLAMMATORY PROCESS
Severe suppression of the host immune response is paradoxically associated with increased levels of cytokines in the systemic circulation in patients with COVID-19 pneumonia [26, 27]. Severe lung damage due to unchecked viral replication can cause breakdown of epithelial barrier function, leading to diffuse alveolar damage with increased microvascular permeability. There is leakage of proinflammatory cytokines such as IL-6, IL-8, and IL-1β from parenchymal lung tissue into the systemic circulation and a concurrent recruitment of circulating immune cells into the lung. A counterregulatory response is activated simultaneously, resulting in increased local and systemic levels of suppressive cytokines such as IL-10 and TGF-β [28]. This concurrent systemic elevation of both pro- and anti-inflammatory cytokine levels is frequently referred to as a “cytokine storm,” the proinflammatory arm of which can result in malperfusion and worsening pulmonary and extrapulmonary organ function while its anti-inflammatory arm can result in severe suppression of circulating leukocyte function, or “immunoparalysis” [29]. The local proinflammatory milieu in the lung can overwhelm homeostatic tissue repair functions, leading to irreversible tissue damage and depletion of alveolar macrophages [30]. During this process, activated fibroblasts can deposit excess collagen, which further impairs pulmonary gas exchange. Epithelial cell death may also expose the basement membrane to secondary microbial pathogens, offering them access to the systemic circulation [31]. Loss of alveolar macrophages can therefore represent a potential chief contributor to refractory respiratory failure in patients with COVID-19, with almost complete depletion of these cells reported in severely infected patients [32].
A CAUTIONARY TALE OF IMMUNOMODULATION
Most forms of acute critical illness have, at their roots, an initial proinflammatory insult, with sepsis and ARDS being the classic examples of disorders whose predominant pathophysiologic effects are due to an exaggerated host response. The use of anti-inflammatory drugs including prostaglandin E1 and ketoconazole (a thromboxane inhibitor) failed to improve ARDS outcomes in adults [33]. The use of corticosteroids in ARDS remains highly controversial, with some studies showing improvement in outcomes following prolonged treatment with low-dose glucocorticoids [34]. The evidence was not, however, strong enough to merit inclusion in current pediatric or adult ARDS treatment guidelines [35–37]. The Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial group recently published a preliminary report indicating that low-dose dexamethasone may improve outcomes in adults with respiratory failure due to COVID-19 [38]. Conversely, the use of GM-CSF in adults with ARDS, while safe, has not been associated with improved clinical outcomes [39]. None of these trials used a priori immune phenotyping to identify subjects who would be most likely to benefit from these interventions.
The 1980s and 1990s saw a profusion of clinical trials in septic adults that targeted reduction in the proinflammatory response through removal or blockade of specific inflammatory mediators. Treatments that were evaluated included those aimed at reducing or removing endotoxin, TNF-α, IL-β, bradykinin, and others [40]. These therapies, along with high-dose glucocorticoids, were nearly all failures in phase III clinical trials, with some demonstrating increased mortality. Over the next 2 decades, the field of immunomodulation in critical illness pivoted to focus more on the immunosuppressive phase of the host response, as we learned that critical illness–induced innate and adaptive immune suppression often occurs simultaneously with a cytokine storm. Immunoparalysis, with reduced antigen presentation capacity, impaired cytokine production capacity, and lymphopenia, has been strongly associated with adverse outcomes, from critical illness including nosocomial infection, prolonged organ failure, and death [41]. There are now clinical trials being performed that target stimulation of immune function in selected critically ill patients with therapies such as recombinant GM-CSF (NCT03769844, NCT00252915), IFN-γ (NCT03332225), and antibodies against PD-1 pathway members (NCT03332225). To be sure, there are several examples where specific anticytokine therapies have been shown to be beneficial, including anakinra for the treatment of MAS and tocilizumab for the treatment of chimeric antigen receptor (CAR)-T cell therapy-induced cytokine release syndrome (CRS). These therapies, however, have specific biologic plausibility (eg, inflammasome dysregulation in MAS) or particularly severe elevations in systemic cytokine levels [42]. For example, the serum levels of IL-6 seen in severe CAR-T cell therapy–induced CRS are in the many thousands of picograms per milliliter, one or more orders of magnitude higher than those seen in most reports of severe COVID-19 disease to date [43]. The conflict inherent in the field’s current approach to immunomodulation in adults hospitalized with COVID-19 is perhaps best exemplified by the fact there is at least 1 ongoing clinical trial that uses recombinant GM-CSF therapy (NCT04326920) while another active trial promotes GM-CSF blockade (NCT04341116) in a similar population.
There is unlikely to be a “one size fits all” approach that can be taken with regard to immunomodulation in patients with COVID-19. A recent immune-monitoring study in COVID-19-positive adults showed a high degree of heterogeneity of immune phenotypes within the cohort [19]. Profound depletion of CD4+ lymphocytes and NK cells was common, as was marked reduction in monocyte antigen-presenting capacity as measured by human leukocyte antigen (HLA)-DR expression. MAS, with marked hyperferritinemia, occurred only in a minority of subjects, as did severe elevations in systemic IL-6 levels. Immunomodulatory therapies that are, in our view, likely to be successful in patients with COVID-19 are those that are tailored to the patient’s immunophenotype in real time. Further suppression of the immune response has the potential to enhance the risk of bacterial and viral infections, promote the development of opportunistic infections, and potentiate the reactivation of latent viruses. It is also important to acknowledge that cytokine levels in peripheral blood do not necessarily reflect systemic leukocyte function or cytokine profiles at the actual site of infection in lung, and may peak after the nadir of respiratory function [44].
While nonpharmaceutical interventions such as physical distancing have played a key role in the management of the COVID-19 pandemic across the globe, additional approaches are needed to combat this virus in the longer term. These include accelerated vaccine development, effective antiviral medications, and targeted immunomodulatory approaches in the right patient at the right time. A subset of patients with the most severe elevations in systemic proinflammatory cytokines (eg, IL-6 levels >1000 pg/mL) may benefit from drugs like tocilizumab or anakinra. Still others may benefit from immune stimulation and enhancement of alveolar macrophage function with drugs like GM-CSF, which has been shown to boost host immunity against pathogens in critically ill patients without worsening the cytokine storm [45, 46]. The design of clinical trials of immune modulators for the treatment of COVID-19 should therefore include prospective immunophenotyping and/or subject stratification based on cell counts, immune function assays, cytokine levels, or other markers of inflammation. Further, we believe that these trials should employ short-acting agents so that protocols can adapt to the dynamic nature of the immune response, as subjects’ immunomodulatory needs may change over time and with the trajectory of their illness. Until data from these clinical trials are available, it will be essential to have equipoise around the use of therapies that target reduction in the immune response, leaving the door open for the use of immunostimulatory therapies in selected patients. Using such an approach we can hope to restore lung homeostasis in patients with COVID-19 through promotion of (1) alveolar repair to ameliorate acute lung injury, (2) restoration of host defense, and (3) balance between pro- and anti-inflammatory responses. A carefully calibrated and personalized treatment approach in COVID-19 has the potential to arrest disease progression and improve outcomes from this devastating illness.
Note
Potential conflicts of interest. M. W. H. is a consultant for La Jolla Pharmaceuticals for whom he provides services that are unrelated to the content of this article. He is an unpaid advisory committee member for Partner Therapeutics, Inc; reports grants from the National Institutes of Health and licensing income from Kiadis, outside the submitted work. I. J. and L. L. are employees of Partner Therapeutics, Inc. E. E. O. is co-founder of Tychan Private Limited, which has a direct-acting candidate therapeutic antibody against SARS-CoV-2. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020; 382:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization; China Joint Mission on Coronavirus Disease 2019. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). Available at: www.who.int.
- 5. Shi Y, Wang Y, Shao C, et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ 2020; 27:1451–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vardhana SA, Wolchok JD. The many faces of the anti-COVID immune response. J Exp Med 2020; 217:e20200678. doi: 10.1084/jem.20200678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003; 426:450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 2020; 63:457–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Whitsett JA, Alenghat T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat Immunol 2015; 16:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 2017; 39:529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guglani L, Khader SA. Th17 cytokines in mucosal immunity and inflammation. Curr Opin HIV AIDS 2010; 5:120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cox MA, Kahan SM, Zajac AJ. Anti-viral CD8 T cells and the cytokines that they love. Virology 2013; 435:157–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol 2020; 38:1–9. [DOI] [PubMed] [Google Scholar]
- 14. Novak T, Hall MW, McDonald DR, et al. ; PALISI Pediatric Intensive Care Influenza (PICFLU) Network Investigators . RIG-I and TLR4 responses and adverse outcomes in pediatric influenza-related critical illness. J Allergy Clin Immunol 2020; 145:1673–80.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blanco-Melo C, Nilsson-Payant BE, Liu W, et al. SARS-CoV-2 launches a unique transcriptiona l signature from in vitro, ex vivo, and in vivo systems. bioRxiv [Preprint] 2020. 2020.03.24.004655. 10.1101/2020.03.24.004655 [DOI] [Google Scholar]
- 16. Chu H, Chan JF, Wang Y, et al. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin Infect Dis 2021; 71:1400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wei WE, Li Z, Chiew CJ, Yong SE, Toh MP, Lee VJ. Presymptomatic transmission of SARS-CoV-2—Singapore, January 23-March 16, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:411–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yao XH, Li TY, He ZC, et al. [A pathological report of three COVID-19 cases by minimally invasive autopsies.] Zhonghua Bing Li Xue Za Zhi 2020; 49:E009. [DOI] [PubMed] [Google Scholar]
- 19. Giamarellos-Bourboulis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe 2020; 27:992–1000.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol 2020; 17:533–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guilliams M, De Kleer I, Henri S, et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med 2013; 210:1977–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Naessens T, Schepens B, Smet M, et al. GM-CSF treatment prevents respiratory syncytial virus-induced pulmonary exacerbation responses in postallergic mice by stimulating alveolar macrophage maturation. J Allergy Clin Immunol 2016; 137:700–9.e9. [DOI] [PubMed] [Google Scholar]
- 23. Rösler B, Herold S. Lung epithelial GM-CSF improves host defense function and epithelial repair in influenza virus pneumonia—a new therapeutic strategy? Mol Cell Pediatr 2016; 3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Subramaniam R, Barnes PF, Fletcher K, et al. Protecting against post-influenza bacterial pneumonia by increasing phagocyte recruitment and ROS production. J Infect Dis 2014; 209:1827–36. [DOI] [PubMed] [Google Scholar]
- 25. Liao HH, Wang YC, Chen MC, et al. Down-regulation of granulocyte-macrophage colony-stimulating factor by 3C-like proteinase in transfected A549 human lung carcinoma cells. BMC Immunol 2011; 12:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intens Care Med 2020; 46:846–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev 2012; 76:16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Volk HD, Reinke P, Döcke WD. Clinical aspects: from systemic inflammation to “immunoparalysis”. Chem Immunol 2000; 74:162–77. [DOI] [PubMed] [Google Scholar]
- 30. Wong JJM, Leong JY, Lee JH, Albani S, Yeo JG. Insights into the immuno-pathogenesis of acute respiratory distress syndrome. Ann Transl Med 2019; 7:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Plotkowski MC, Puchelle E, Beck G, Jacquot J, Hannoun C. Adherence of type I Streptococcus pneumoniae to tracheal epithelium of mice infected with influenza A/PR8 virus. Am Rev Respir Dis 1986; 134:1040–4. [DOI] [PubMed] [Google Scholar]
- 32. Liao M, Liu Y, Yuan J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med 2020; 26:842–4. [DOI] [PubMed] [Google Scholar]
- 33. McIntyre RC Jr, Pulido EJ, Bensard DD, Shames BD, Abraham E. Thirty years of clinical trials in acute respiratory distress syndrome. Crit Care Med 2000; 28:3314–31. [DOI] [PubMed] [Google Scholar]
- 34. Meduri GU, Siemieniuk RAC, Ness RA, Seyler SJ. Prolonged low-dose methylprednisolone treatment is highly effective in reducing duration of mechanical ventilation and mortality in patients with ARDS. J Intens Care 2018; 6:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Del Sorbo L, Goligher EC, McAuley DF, et al. Mechanical ventilation in adults with acute respiratory distress syndrome. summary of the experimental evidence for the clinical practice guideline. Ann Am Thorac Soc 2017; 14:261–70. [DOI] [PubMed] [Google Scholar]
- 36. Griffiths MJD, McAuley DF, Perkins GD, et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Respir Res 2019; 6:e000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. The Pediatric Acute Lung Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015; 16:428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Group RC, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - reliminary Report. New Engl J Med 2020. Jul 23. doi: 10.1056/NEJMe2025927 [DOI] [Google Scholar]
- 39. Paine R 3rd, Standiford TJ, Dechert RE, et al. A randomized trial of recombinant human granulocyte-macrophage colony stimulating factor for patients with acute lung injury. Crit Care Med 2012; 40:90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hall MA. The inflammatory response. In: Lucking SE, Maffei FA, Tamburro FA, Thomas NJ, eds/ Pediatric critical care: text and study guide. 2nd ed. New York, NY: Springer, 2020. [Google Scholar]
- 41. Greathouse KC, Hall MW. Critical illness-induced immune suppression: current state of the science. Am J Crit Care 2016; 25:85–92. [DOI] [PubMed] [Google Scholar]
- 42. Panacek EA, Marshall JC, Albertson TE, et al. ; Monoclonal Anti-TNF: a Randomized Controlled Sepsis Study Investigators. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab’)2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit Care Med 2004; 32:2173–82. [DOI] [PubMed] [Google Scholar]
- 43. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014; 371:1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ong EZ, Chan YFZ, Leong WY, et al. A dynamic immune response shapes COVID-19 progression. Cell Host Microbe 2020; 27:879–82, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hall MW, Knatz NL, Vetterly C, et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intens Care Med 2011; 37:525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Meisel C, Schefold JC, Pschowski R, et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med 2009; 180:640–8. [DOI] [PubMed] [Google Scholar]