Abstract
Many countries consider the lifting of restrictions of social contacts (RSC). We quantify the effects of RSC for Germany. We initially employ a purely statistical approach to predicting prevalence of Covid-19 if RSC had been upheld after 20 April. We employ these findings and feed them into our theoretical model. We find that the peak of the number of sick individuals would have been reached already end of April. The number of sick individuals would have fallen below 1000 at the beginning of July. If restrictions had been lifted completely on April 20, the number of sick should have risen quickly again from around 27 April. A balance between economic and individual costs of RSC and public health objectives consists in lifting RSC for activities that have high economic benefits but low health costs. In the absence of large-scale representative testing of CoV-2 infections, these activities can most easily be identified if federal states of Germany adopted exit strategies that differ across states.
Keywords: Covid-19, SARS-CoV-2, forecast Germany, epidemic, pandemic
Keywords: I18, E17, C63
1. Introduction
Authorities in most countries have imposed restrictions on social contacts (RSC in what follows) in various forms. They include contact restrictions outside the household, shut down of schools, the closing of businesses, quarantines, and in some cases curfews. Many countries are facing the question of how long RSC should last.
We study the situation in Germany (and briefly provide a comparison to other countries further below). Apart from some exceptions [like e.g. cancelling the travel and tourism fair Internationale Tourismus Börse Berlin (ITB) on 28 February 2020], no systematic public health measures were implemented before 14 March. After a meeting between the government and the heads of federal states on Friday, 13 March 2020, various coordinated measures to mitigate the spread of Covid-19 were implemented. Measures included the closing of schools and shops, suspending of sports events, the duty to wear masks, contact restrictions, or even contact bans (see Kleyer et al. 2020, for a detailed overview). As of 20 April, some of these measures were relaxed showing relatively heterogeneous exit strategies across federal states.1 We quantify both the effects of the RSC in place before 20 April and their effects in the long run in case they had been maintained. We also quantify the effect of a complete lift of RSC20.2
We find that neither permanent RSC nor a complete lift is desirable. Permanent RSC20 would yield an epidemic in Germany that would lead to around 167,000 sick individuals only. The epidemic would not be over, however, as most individuals would still likely be susceptible to an infection. Permanent RSC would also not be economically sustainable. A complete lift is likely to yield a fast increase of the number of sick that would overstrain the public health system. This points towards the need to think about exit options which promise to keep infection rates stable. Exit strategies should be reversible and tested for, say, four weeks and differ across regions. This would allow authorities to understand their health and economic effects. Learning about policy measures appears essential in this global pandemic.
We believe our analysis is useful both for as long as the Covid-19 epidemic is ongoing and for the time thereafter. Modelling, calibrating, and forecasting the dynamics of epidemics is crucial for understanding a possible second or third wave and the effects of public health measures. Understanding basic relationships today informs future policy decisions. Once the epidemic is over, the general interest will probably fall. Nevertheless there will be a long wave of analyses on how the epidemic can be understood in detail and how the income-health trade-off has been addressed. Our analysis is a starting point which is already being used for more elaborate analyses.
There is an exploding literature on Covid-19 characterized by a reproduction factor much larger than one. As even a minimal discussion would take up too much space in this introduction, we summarize the literature relevant for our work in an extra section further below.
The structure of the article is as follows. We first take a purely statistical perspective and describe the dynamics of the number of reported infected individuals over time. We employ both data from the Robert Koch Institute (RKI) (2020) and from Johns Hopkins University (JHU) (2020). We also provide a forecast of the number of reported sick individuals purely based on RKI observations and under the assumption that RSC rules in place until 19 April had not changed. Section 2 also briefly surveys the related literature. This allows us to work out the contribution of our article in more detail and provides a reference for future work. Section 3 presents the essentials of the model, the Appendix provides a complete overview. Our calibration is in Section 4, and Section 5 quantifies the effects of the RSC in place before 20 April and studies the effects of a complete exit. Section 6 concludes.
2. Data and Literature
2.1. A first look at data for Germany
2.1.1. Descriptive statistics
There are two datasets for Germany that are used to describe prevalence of Covid-19. The first is data from the Robert Koch Institute (RKI) (2020), the second data source is from Johns Hopkins University (JHU) (2020). In this section, we employ both to see their relative strengths and merits.
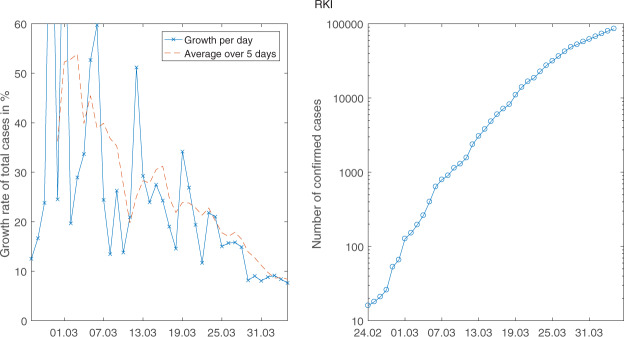
When we look at Figure 1, one might believe to identify a permanent break in growth rates end of March. Looking at the right picture gives the impression that the curve becomes flatter over time but there is a kink on 30 March: when looking at growth rates (crosses in left part of the figure), there is a permanent drop on this same 30 March.
Figure 1.
The daily growth rates (left) and the level of the number of sick (right) for RKI data (logarithmic scale).
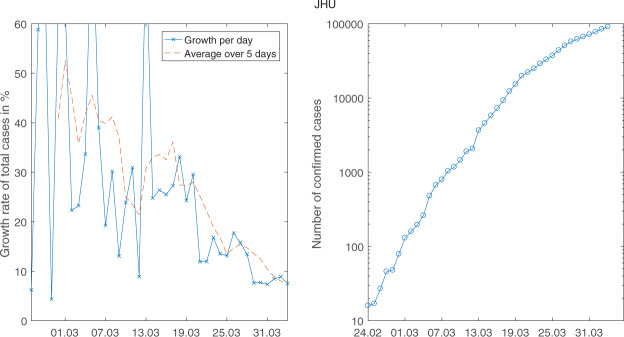
When we look at Johns Hopkins data in Figure 2, we can identify two break points. The first is on 20 March. It can clearly be seen in the left part of the figure with the drop in daily growth rates and in the right part on 20 march. This is the drop that was also identified econometrically by Hartl et al. (2020). It is also clear from these two figures that there is another break on 27 March. Looking at the sequence of public health measures in Germany (see Table 1) and the usual delay between infection and symptoms and reporting (see Linton et al. 2020 and Lauer et al. 2020 for medical evidence on incubation time with median 5.2 days for Covid-19 with Chinese data), one could try to identify the events behind these breaks.
Figure 2.
The daily growth rates (left) and the level of the number of sick (right) for JHU data (logarithmic scale).
Table 1.
Timeline of contact ban measures introduced in major European economies (University of Oxford, 2020)
| Major public events suspended | Schools closed | Domestic movements banned | Mortality rate (date of first measure and per 10 million)a | |
|---|---|---|---|---|
| Germany | March 20 | March 16 | March 22 | 2 |
| France | February 29 | March 16 | March 17 | 0.3 |
| Italy | March 5 | March 5 | March 10 | 24.5 |
| Spain | March 10 | March 16 | March 16 | 7.5 |
| United Kingdom | March 17 | March 23 | March 24 | 8.3 |
Data taken from Johns Hopkins University (2020).
In our analysis of the effect of public health measures below, we will focus on RKI data.3 Hence, we assume that the break took place on 30 March.4
2.1.2. Government responses to Covid-19
While we focus on Germany in our analysis, other countries also imposed social distancing rules. The following table provides a brief cross-country overview of government responses to Covid-19.5 The underlying data come from the University of Oxford (2020), similar overviews are available at www.acaps.org.6
When we look at the dates when governments became active, the timing of events differs. There are also strong differences with respect to the number of deaths before the governments became active for the first time. Yet, the table shows that countries qualitatively follow similar strategies. Our findings are therefore informative also beyond Germany.
2.1.3. Gompertz curves
The best, almost entirely observation-based, forecast for the evolution of Covid-19 in Germany, under the assumption that RSC20do not change, can be obtained from fitting a Gompertz-curve model to the data. The Gompertz curve is a reduced form, non-linear trend model which is characterized by an upper saturation point which is estimated endogenously. The model displays a double exponential form with three parameters and a time index t,
The parameter b is a horizontal shift parameter and c is the growth parameter. It can be thought of as the infection rate in this context. The parameter a denotes the saturation point: letting time t become larger and larger (we look further and further into the future) shows that yt approaches a as with c > 0 approaches zero. It is well-known that models of this type capture the s-shape of infection numbers quite well.
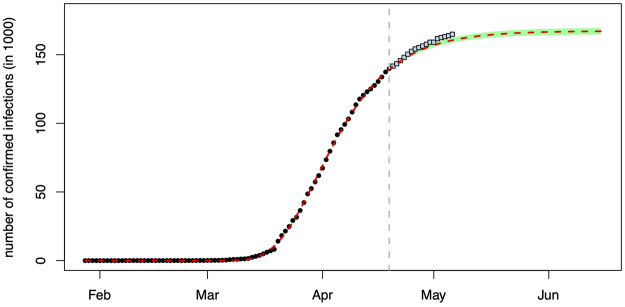
We employ data (dots) up to 19 April for estimation and data (squares) until 6 May for a first test of the effects of relaxing RSC as of 20 April. Figure 3 summarizes the estimated model (employing ordinary least squares and an additive error term). The dark dots are RKI observations and the red dashed curve is the prediction of the model. The green shaded area delineates the 95% confidence region for the forecast. With new data, the green area becomes smaller and approaches the dashed red curve.
Figure 3.
Predicting the number of reported infections under the regime in place before 20 April.
As this figure impressively shows, RSC as valid until 20 April made Germany head towards a stable number of reported Covid-19 infections. This number lies at around 167,000 individuals and would be reached around early June if RSC20 had not been changed. As the observations not employed for estimation show, relaxing RSC20 as of 20 April had a negative (though weak) health effect: Observations after 19 April tend to lie above the dashed red prediction and outside the confidence interval.
2.2. Covid-19 research
Given our interest on Covid-19 dynamics in Germany, we first briefly summarize general Covid-19 research applied to Germany. We then relate our work to projections of Covid-19 in Germany.
2.2.1. General questions
Despite Covid-19 having only emerged a few months ago, there are already several hundred research projects studying its effects in economics. A (non-exhaustive) repository is maintained by the European Economic Association.7 In this short time, many papers have addressed the implications of Covid-19 for the EU, and for Germany in particular. Chen et al. (2020) study the effects of the virus on economic activity proxied by electricity usage and mobility data from Google in Europe and the USA. They find that the drop in economic activity is negatively correlated with Covid-19-related deaths per capita, with Germany experiencing a year-on-year decline of over 10% in electricity usage and a drop of over 30% in visits to public places between pre- and post-Covid-19 periods. They posit that the reaction is driven by mitigation policies, which are proportional in their severity to the number of Covid-19-related fatalities. McKibbin and Fernando (2020) also study the economic impact of Covid-19, focusing on macroeconomic variables. They find that, under various scenarios ranging from less severe to most severe, aggregate labour supply and consumption drop substantially globally, while equity premiums and costs of production both rise in response to the health shock. They find that for Germany, this implies up to 350,000 deaths and a 8.7% drop in Gross domestic product (GDP) in 2020 under the worst case scenario. Finally, we also note the contribution of Chen and Qiu (2020), who assess the effectiveness of non-pharmaceutical interventions (NPIs) on the spread of the disease in a sample of countries. They forecast that Germany would experience a peak in active cases in the first half of April before seeing a sharp decline through to August. In particular, they find that choosing between stricter and more relaxed NPI combinations has little effect, so long as the core policies of mask wearing, school closing, and centralized quarantining remain in place.
2.2.2. Projections
A first survey is in Donsimoni et al. (2020a), a broader overview is in Gros et al. (2020). We build our analysis on the model and projection presented in Donsimoni et al. (2020a).8 In contrast to this article, we (i) provide a more precise calibration of the effect of no public health measures. The precision results from the availability of more observations. This is essential for quantifying the effects of lifting RSC. We (ii) can also quantify the effects of RSC in the present paper as sufficient data have become available since our earlier work. Our most recent observation employed for estimation now is from 19 April. Most importantly, due to the availability of enough observations, we can (iii) employ purely statistical methods to make a forecast for the RSC in place until April 19. This allows us to work without assumptions about long-run infection and sickness rates. For judging the effect of a lift of RSC20, we do need to return to long-run assumptions, however, as we need to work with the theoretical model developed in Donsimoni et al. (2020a) again.
Adamik et al. (2020) also quantitatively analyse the situation in Germany. They employ a microsimulation model which allows to better understand the effect of heterogeneity across households. They argue that reaching herd immunity without violating the capacity limit of the health care system is likely to fail. They do not explicitly analyse the effects of RSC and do not discuss the fit of their model to observed data. Dehning et al. (2020) estimate parameters of their model in a statistically very convincing way. They focus on constant transition rates for different RSC-regimes (but do allow for time-dependency to smooth between regimes). They make forecasts for a period of two to three weeks and use data up to 31 March.9
The letter by Rothe et al. (2020) points to the importance of asymptomatic carriers when assessing the diffusion of CoV-2. They specifically isolate several cases where patients did not present symptoms for an extended period of time allowing them to further contaminate others. The relevance of asymptomatic carriers is echoed by Fuhrmann and Barbarossa (2020), who argue that ignoring them could have dire consequences in terms of lives lost.
Stübinger and Schneider (2020) analyse the progression of the virus exploiting a ‘lead–lag structure’ among various countries around the world. They employ a purely empirical approach which makes a comparison of their findings more difficult. Khrapov and Loginova (2020) in their susceptible-infected-removed (SIR)-based analysis support the idea that the situation in Germany is unlikely to become unstable, as they forecast a peak number of active cases of 80,000 and a peak number of fatalities below 10,000. In contrast to our work, they do not explicitly take changes in public health measures into account. Hidden or asymptomatic infections are also ignored.
There are many further, so far unpublished analyses that also address various dimensions of projections and virus spread. Barbarossa et al. (2020a, 2020b) analyse the impact of control measures and their effectiveness in slowing down the spread of Covid-19 in Germany. German et al. (2020) study exit strategies from lockdown, paying particular attention to the role of antibody testing. Khailaie et al. (2020a) quantity the reproduction rate Rt and also evaluate the implications for policy.10 These analyses are very interesting as they are based on considerably generalized SIR models taking, for example, hidden infections and hospitalization into account. One distinction of our approach to all SIR-type models we are aware of is the modelling of the infection rate. This rate is usually a linear function of the infectious in the model. In our setup, we generalize the infection rate in (2) below and make it a function of the number of healthy, infected and recovered. This reflects the idea behind matching models in economics and makes calibration of parameters much more flexible and therefore leads to a better data fit. Zhang et al. (2020) consider the effects of the size of the population that can be infected on the evolution of the pandemic.
3. The Model
The model is described fully in the Appendix. In the main text, we present only those parts that are important for understanding our calibration below and our forecasts.
3.1. The basic structure
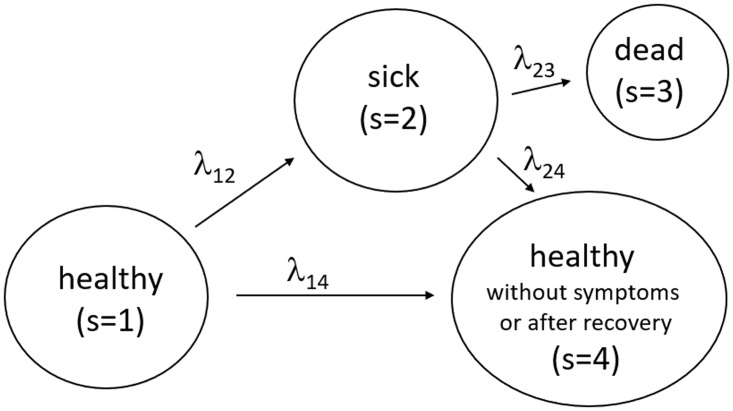
The basic structure of the model is illustrated in Figure 4. The most well-known background in economics are search and matching models in the tradition of Diamond (1982), Mortensen (1982), and Pissarides (1985). The background in mathematics is continuous time Markov chains. We employ this structure and assume four states.
Figure 4.
Transitions between the state of health (initial state), sickness, death and health despite infection or after recovery.
We employ this figure to offer precise definitions about which individuals we consider to be in which state. State 1 is the state of being healthy in the sense of never having been infected by CoV-2. State 2 captures all individuals that have been reported to be infected with CoV-2. As these reports are based in Germany up to now on tests of individuals that have some (e.g. respiratory) symptoms, we call this the group of sick individuals. The sum of all individuals that are ever reported to be sick is the data collected and published by RKI and JHU that we will employ below. The term sick is also useful as it stresses the differences to individuals that are infected but do not display symptoms. This process is captured in the model by the flows from state 1 to state 4. The size of these flows is a big unknown empirically speaking and several tests are currently being undertaken to measure the number of infected but not sick individuals.11 State 3 counts the number of deceased individuals. All individuals that have recovered from being sick or that were never reported or never displayed symptoms after infection are in state 4.
We will employ the terms prevalence and incidence distinctly throughout the article. Incidence is the number of individuals that are reported for the first time to be sick on a given day. This is the inflow into state 2. Prevalence is identical to which denotes the (expected) number of sick individuals at a point in time t in state 2. Prevalence at t is the sum over all incidences from the beginning of the epidemic up to t minus the deceased and the recovered individuals.
Data reported by RKI or JHU have traditionally consisted of the number of individuals that were ever reported to be sick, that is, the sum (integral in terms of the model) of all the inflows into state 2. This quantity at t amounts to prevalence plus the deceased plus the recovered.12 The incidence is the daily difference between data reported by RKI or JHU on 1 day minus the value reported on the day before. This corresponds to incidence above, that is, in (12).
The population in our model is characterized by an infection rate which is simply the ratio of the number of infected individuals (sick and in state 2 or without symptoms in state 4) to individuals that are alive. Letting denote the number of individuals in state s at t, the infection rate is simply
| (1) |
The infection rate is zero initially at On 24 February 2020 and for Germany, a number of sick individuals is introduced into the system and infections and sickness start occurring.
The central transition rate in our model is the individual sickness rate that captures flows from state 1 to state 2. We specify it as
| (2) |
where allows for some non-linearity in the process. The contact rate with which individuals meet other individuals is The first term captures the idea that more healthy individuals reduce the individual sickness rate. The second term increases the sickness rate when there are more infectious individuals. The parameter η describes the fact that individuals that are infected but do not display symptoms (and are therefore in state 4 of our model) nevertheless can infect other individuals. The third term in squared brackets makes sure that the arrival rate is zero when a share of society is sick (state 2) or healthy after infection (state 4).
The sickness rate satisfies ‘no sickness without infected individuals’, and ‘end of spread at sufficiently high level’, In between these start- and endpoints, the infection rate will first rise and then fall. This specification makes sure that in the long run a share of around will not have left state 1, that is, will never have been infected.13 We refer to as the long-run share of infected individuals once the epidemic is over.
In our calibration procedure below we consider the contact rate a and elasticities γ to be a function of public health measures. We would especially expect that RSC lead to a drop in the contact rate a.
3.2 The model as an ordinary differential equation system
After some steps (see Donsimoni et al. 2020a), our model can be summarized by an ordinary differential equation system. The (expected) number of individuals in state s is described by system (3). Parameters not described above are r, , and N. The probability to become sick after an infection with CoV-2 is denoted by r. The death rate for the transition of sick individuals from state 2 to state 3 visible in Figure 4 is denoted by We assume that it takes (on average) days to recover from being sick, that is, to move from state 2 to state 4. Finally, the population size (before the epidemic) is given by N.
| (3a) |
| (3b) |
| (3c) |
| (3d) |
We employ this system for calibration and for prediction. Initial conditions for our solution are for 24 February 2020 (Robert Koch Institute 2020), and , where N = 83.1 million is the population size in Germany before the epidemic. Initial conditions for our calibration of the RSC20 regime are numbers where tr = 30 March 2020 is the day when the RSC20 regime starts. Initial conditions for predicting the effect of a potential lift of RSC20 correspond to model predictions for tl = 27 April 2020.14
4. Calibration and Model Fit
4.1. Calibration
The parameters in our model are either chosen exogenously or are the outcome of our data fitting procedure. Exogenous parameters are displayed in Table 2.
Table 2.
Exogenously chosen parameters
| Average recovery in days | Share of reported infections r | Share of infectious recovered individuals η |
|---|---|---|
| 14 | 0.1 | 0.4 |
As in our earlier work, we assume that recovery takes an average of 14 days. This implies a recovery rate of which captures heterogeneity in the course of the disease (Guan et al. 2020) to some extent. The share r of individuals that becomes sick (and is reported) after an infection is 10%. The share η of infected individuals without symptoms that can infect other individuals is 40%.15
The model makes a clear prediction about the long-run number of individuals that were ever reported to be sick. This number is given by
| (4) |
We would like to emphasize that this property of our model is crucial for our long-run predictions and the short-run findings. The long-run number of individuals that, once the epidemic is over, were ever reported to be sick is the probability to get sick after an infection, times the long-run share of infected individuals, times population size, N = 83.1 million, that is, the long-run number of sick individuals equals 5 million. This is the number of sick individuals in the ‘normal’ scenario of Donsimoni et al. (2020a,b). In their ‘optimistic Hubei scenario’, they assume that the population share of ever infected individuals once the epidemic is over amounts to only. In this scenario, the long-run number of sick individuals is million =498.6 thousand individuals, that is, roughly 0.5 million individuals. Once this quantity is fixed, any public health measure in our model only shifts the number of sick individuals over the duration of the epidemic. RSC reduces the sickness rate λ12 from (2) in the short-run but only delays the infection of the rest of from (4). We admit that this is a strong implication of our model but we only ‘translate’ assumptions made in more general not model-based discussions.16
Given that this is a strong assumption and given our Gompertz-curve estimation of the situation in Germany until April 19 illustrated in Figure 3, we are now in the lucky situation that we can do without a strong assumption for for RSC before April 20. For this regime (but not for the end of the entire Covid-19 epidemic), Figure 3 tells us that we are converging in May or June to a value of roughly 167,000 sick individuals. To make clear that this value is valid only for the RSC in place before 20 April, we denote it by This estimate implies a parameter restriction on our long-run value.17 Put differently, we can compute
| (5) |
This is the share of sick individuals in the population when the epidemic is over and if the RSC20 were preserved forever. The value for the long-run share of infected individuals is therefore computed such that (5) is satisfied.18
We finally fix various parameters such that we match data reported by RKI. To do so, we minimize the Euclidean distance between the reported data and the predicted values of the model. We undertake two separate calibrations, one for each sub-period described above after the discussion of Figure 1. We target a weighted sum of the squared difference between and observation and the newly sick and observation. More precisely, parameters a, , and γ are obtained from
| (6) |
We impose constraints for α, β, γ to lie between zero and one and for a to be positive. None of the constraints are binding. Table 3 presents these and all other parameter values both for and
Table 3.
Calibrated parameters for RKI data before and after the break
| Death rate | Contact rate | Infection elasticities | Long-run infection rate | |||
|---|---|---|---|---|---|---|
| λ 23 | a | α | β | γ | ||
| 24 February to 29 March | 1/500 | 0.5751 | 0.8662 | 0.6459 | 0.06 | |
| 30 March to 19 April | 1/500 | 0.2782 | 0.8983 | 0.7764 | 0.0207 |
We want to match the number of reported deaths from Covid-19 for our two sub-periods. Hence, the constant death rate for the period from t1 to t2 can be computed from
| (7) |
where is the number of dead individuals at T. Employing this equation yields the values in Table 3.
4.2 Parameters and model fit
The calibration in Donsimoni et al. (2020a) employed RKI data from 24 February 2020 to T = 21 March 2020. Given the impression from Figure 1, there is a break in the growth rate of the number of sick only on 30 March (and not on 20 March). We therefore identify two regimes in the RKI data, one from 24 February to 29 March and one starting 30 March.
The calibration results for both regimes are in Table 3. The figure also displays for the pre-RSC20 regime up to 29 March. We set it equal to 6% and therefore choose the ‘optimistic Hubei scenario’. The value for for the RSC20 regime as of 30 March is the value from (5) divided by r from Table 2. The death rate λ23 is such that the model matches the number of deceased individuals according to (7).
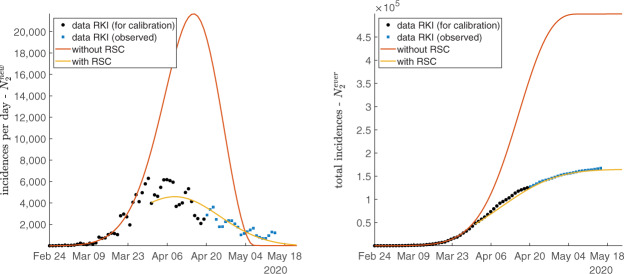
The fit of the calibration can be judged by looking at Figure 5.
Figure 5.
Fit for RKI data, incidences on left and total incidences on right.
Our minimization procedure in (6) takes both incidences and total incidences into account without weighting observations explicitly. As a consequence, the fit is unlikely to be equally good. The red curve in the left part of Figure 5 shows that incidences up to 29 March are well explained by our model. By contrast, as visible when looking at the yellow curve, daily incidences in the RSC20 regime are harder to capture. We clearly see, however, that the calibrated model employing data up to 19 April already captures the turning point in the number of incidences. This is also what the purely statistical Gompertz approach shown in Figure 3 has identified.
The fit for total incidences on the right is very good. The red curve fits data up to 29 March very well and shows where the number reported by RKI would have gone if no RSC had been imposed. The yellow curve is calibrated with data up to 19 April. From the prediction of the model we are around the turning point now in Germany.
The figure also tests our model prediction by plotting observations as of 20 April which were not taken into account in the calibration procedure. We see that the model predicts the effects after 19 April almost as well as before. This seems to contradict the finding from the Gompertz-curve estimation in Figure 3. The analysis there argues that relaxing RSC20 led to a (small) increase in incidence. The purely statistical approach assumes one data-generating process up to 19 April. Our calibration of the theoretical model allows for a break in the spread of Covid-19 on 30 March. Hence, the fit is adjusted to the period of RSC and therefore should match the observations as of 20 April better. We see this as a confirmation that the easing of RSC on 20 April did not have a significant effect on infections.
5. The Effects of RSC and of Relaxing Them
5.1. The effects of RSC
We now quantify the health effects of restrictions of social contacts (RSC). Our central variable of interest is again the prevalence of Covid-19, the number of individuals that are simultaneously sick— in terms of our model. This section also shows what the effects of keeping social distancing forever and relaxing it as of 20 April are.
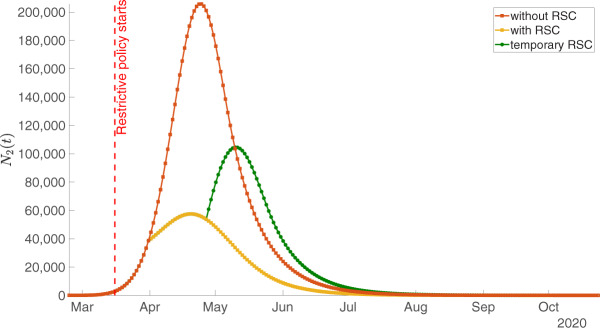
The red curve shows the evolution of the epidemic in the absence of any public intervention. This curve employs parameters as calibrated above and as reported in Table 2 and the first row of Table 3. As the yellow curve in Figure 6 shows, social distancing measures and the shutdown were useful and considerably ‘flattened the curve’. This curve is plotted using parameter values again from Table 2 and from the second row of Table 3.
Figure 6.
The epidemic without restrictions of social contacts (RSC, red curve), the effect of permanent RSC (yellow) and the effect of a temporary RSC (green) as measured by prevalence .
From a pure health perspective, this is of course very desirable. As an example, we can again look at the corresponding probabilities to become sick on a given day or over the period of one week. As the red curve in the left part of Figure 5 illustrates, in a situation without RSC, the number of incidences would have continued to increase and so would have the risk to get infected. The yellow curve shows that incidences are now falling and so does the risk to get infected.
While this was expected and predicted by many, our quantitative model can make predictions about the long-run effects of these distancing measures. If measures were upheld permanently, the peak of Covid-19-prevalence would be reached end of April already. We can define the end of an epidemic such that prevalence falls below 1000 or the daily incidences are below 100. Prevalence would be lower than 1000 beginning of July and incidences would be below 100 beginning of May. We stress again that these are expected dates that should hold if RSC20 are upheld permanently. We also stress that this would not mean a complete end of the epidemic in the sense of herd immunity. There would still be many individuals in state 1 that are not immune and that can be infected and become sick.
5.2. A complete exit from RSC
Let us return to Figure 6 and inquire about the effects of lifting social distancing rules as of 20 April. Due to the delay between infection and reporting also discussed in the context of Figures 1 and 2, we assume that the effects of a lift are visible as of 27 April. We therefore plot a green curve in Figure 6 that starts on 27 April.
Plotting this curve requires again parameters for our ordinary differential equation (ODE) system in (3). We assume that Covid-19 would continue to spread according to the sickness rate λ12 from (2). The question is which parameter values we should choose. We do employ parameters in Table 2 as always. As it is a projection under a different regime, we cannot employ parameter values from the days before. Hence, concerning parameters from Table 3, we assume that the sickness rate is characterized by the same parameter values as before RSC20. This leads us to employing the parameter values which we obtained for our calibration of the period from 24 February to 29 March in the first row of Table 3.
We should stress that this does not imply that the spread is with the same speed as of 24 February. The number of individuals in states 1, 2, and 4, which are the arguments in the sickness rate (2), differ on 27 April as compared to those before any RSC. As a consequence, the speed of the spread will differ.
Plotting the projection for 27 April onwards also requires a value for This share of the population that will have been infected once the epidemic is over is the most difficult parameter to pin down. If we keep the value of RSC would just imply a shifting of the number of sick over the length of the epidemic. It would, however, not reduce the overall number of sick. It seems natural to assume, however, that RSC not only affect current infection rates but also the long-run share of individuals that are ever infected. We therefore assume a lower value for the long-run infection rate of 19 As is clear from this discussion, a complete lifting of social distancing rules under RSC20 should lead to an increase in the number of sick individuals again.
Figure 6 therefore summarizes the trade-off decision makers faced. Preserving RSC20 would be good from a public health perspective but would imply further very high economic costs. A complete lift on 20 April would have run the risk of returning to fast growth of the number of sick individuals. The conclusion discusses options that could strike a balance between both scenarios.
6 Conclusion
Neither perpetuating the situation with restrictions on social contacts in place before 20 April (RSC20) nor a complete lift of RSC20 is desirable. Preserving RSC20 would imply social and economic costs that cannot be sustained for long. Lifting RSC20 completely would yield high health risks with a quick increase in the number of sick individuals.
A way out must consist in measures that reduce economic costs without increasing infection risks substantially (see Abele-Brehm et al. 2020, for suggestions). At the same time one should not follow a one-rule-fits-all policy for all regions in Germany. If different regions (or even smaller communities) run different policies and data are well-recorded for smaller areas as well, decision makers could quickly learn about which measures are most effective in terms of reducing infection rates as well as reducing economic and social costs. As an example, some regions could allow for schools to open again as of grade 9, others only as of grade 5. Other regions could allow restaurants (preserving a distance of 2 m between tables) to open, while others do not. A trial period of four weeks with partially relaxed rules in some parts of Germany should be enough to identify the effects. One should then be prepared to adjust the measures (both upwards or downwards depending on the outcomes) in around four weeks after relaxing the measures.20
These measures would not be required if truly large-scale testing of the population and isolation of infected and sick was possible.21 In the absence of medical testing, one can only learn by coordination of heterogeneous regional responses to Covid-19. This would be a good example of how a federal system can be used to learn from each other. If this option is ignored, it will be just as difficult in one month’s time to judge which measures help economically and are not too costly from a health perspective.
Acknowledgements
The first version of this article became available on 8 April 2020 as Donsimoni et al. (2020). This is an updated version using data up to 6 May for reasons explained in the text. We are grateful to Claudius Gros, Albrecht Ritschl, Hilmar Schneider, Hans-Werner Sinn, to many members of the ‘Makrorunde’ and to seminar participants of the ‘Forecasting COVID19’ workshop at the Johannes Gutenberg University for comments and discussions.
Appendix
This Appendix briefly summarizes the model of Donsimoni et al. (2020a). The model builds on a continuous time Markov chain with four states as illustrated in Figure 4.22 In addition to the infection rate from (1) and the sickness rate from (2), we allow for a flow of individuals that are infected but do not display any symptoms. This captures hidden infections. The corresponding individual rate is denoted by As data on λ14 are basically non-existent, we assume that a constant share r of infected individuals display symptoms. Hence, the transition rate λ14 is related to λ12 via : The outflow of newly infected in squared brackets times the share r of individuals that show symptoms after infection gives the flow into sickness on the right-hand side. We therefore obtain the transition rate from state 1 to state 4 as
| (8) |
Individual mortality and recovery rates are constants. When we assume average recovery of days, we obtain
| (9) |
The number of deaths from Corona by t is determined by the constant death rate λ23 applied to those that are currently sick,
| (10) |
We employ this equation to quantify
The number of individuals that have been reported sick since the onset of the epidemic, which is also the time series that is typically reported by authorities is denoted by in our model. It follows from
| (11) |
When data provide incidences on a given day t, we need to compare this with the inflow between yesterday t − 1 and t into state 2. Hence, our theoretical counterpart to incidences is
| (12) |
Employing these equations allows to formulate our differential equation system in (3).
Footnotes
While definite answers need to await future research, the general perception goes that exit strategies were much more heterogeneous than strategies for restricting social contacts.
Restrictions of social contacts in some general sense are abbreviated by RSC. When we refer to restrictions of social contacts in effect before 20 April 2020, we abbreviate them by RSC20.
Attention is restricted to the period up to 3 April. Our choice of the dataset would not change if we had used a longer time period. Our Gompertz-curve analysis employs data up to 6 May.
We have undertaken analyses with JHU data as well where we assumed that the effects of public health measures are visible as of 20 March. While there are obviously (small) quantitative differences, the broad picture remains the same.
We are grateful to the editor for this suggestion.
For a much more detailed overview of public health measures in Germany, see Kleyer et al. (2020).
The European Economic Association (EEA) only lists existing projects studying the effects of Covid-19, which does not constitute a publication but merely a database. The list can be found at https://www.eeassoc.org/index.php? site=JEEA&page=298&trsz=299.
See Donsimoni et al. (2020b) for a summary in German.
Khailaie et al. (2020b) maintain a model of infection forecasting that they update regularly. This report offers an up-to-date view of Rt for Germany and for each federal state. The data can be found at https://gitlab.com/simm/covid19/secir/-/wikis/Report.
See (8) in the Appendix on how we quantify this flow and the corresponding transition rate. The crucial assumption concerns the share of infected individuals that do not display symptoms or are not reported. We assume this share is around 80–90%. In terms of model parameters, this means we assume (see below).
We denote this by in Donsimoni et al. (2020).
We employ ‘around’ as some individuals will have ended up in state 3 whose number does not enter the expression in (1).
As discussed below, we assume that a lift on 20 April would imply observable effects only around one week later.
Robustness analyses were undertaken in Donsimoni et al. (2020a).
In ongoing work, we study the historical evidence about from other epidemics and pandemics. No systematic evidence seems to be available at this point. We are grateful to dozens of epidemiologists, virologists, economists, and decision makers for discussions of this point.
We are grateful to Hilmar Schneider for having raised this point.
We emphasize again that preserving the RSC would be unlikely to set an end to the epidemic as some infected individuals will remain within the population also by June. A lift of RSC only in June would then lead to a next rise of infections.
We emphasize that this is the outcome of many comments and discussions about the effects of shutdowns on long-run infection rates. It is generally argued that more social separation does not only reduce the infection rate instantaneously but also in the long run.
A detailed description of a potential application of this diff-in-diff approach to Germany is in Barbaro et al. (2020).
Romer (2020) proposes a national testing strategy for the USA. He suggests testing everyone once every two weeks and isolating all those who test positive.
These three papers were presented at the ‘Forecasting COVID19’ workshop at the Johannes Gutenberg University on 6 April 2020.
For a very accessible introduction to the underlying Poisson processes and their arrival rates see e.g. Wälde (2012, part IV).
References
- Abele-Brehm A., Dreier H., Fuest C., Grimm V., Kräusslich H.-G., Krause G., Leonhard M., Lohse A., Lohse M., Mansky T., Peichl A., Schmid R. M., Wess G., Woopen C. (2020), Die Bekämpfung der Coronavirus-Pandemie tragfähig gestalten, https://www.ifo.de/publikationen/2020/monographie-autorenschaft/die-bekaempfung-der-coronavirus-pandemie-tragfaehig (last accessed 20 April 2020).
- Adamik B., Bawiec M., Bezborodov V., Bock W., Bodych M., Burgard J., Götz T., Krueger T., Migalska A., Pabjan B., Ozanski T., Rafajlowicz E., Rafajlowicz W., Skubalska-Rafajlowiczc E., Ryfczynska S., Szczureki E., Szymanski P. (2020), Mitigation and herd immunity strategy for COVID-19 is likely to fail, medRxiv preprint, 10.1101/2020.03.25.20043109. [DOI]
- Barbaro S., Frölich M., Jung P., Kosfeld R., Merkl C., Schank T., Timmer J., van Ewijk R., Gaudecker H-M v, Wälde K. (2020), Lockerung der COVID-19 Kontaktregeln nach Landkreisen randomisieren, Wissenschaftlicher Aufruf in www.oekonomenstimme.org (last accessed 20 April 2020).
- Barbarossa M., Fuhrmann J., Heidecke J., Varma H. V., Castelletti N., Meinke J., Krieg S., Lippert T. (2020), A first study on the impact of current and future control measures on the spread of COVID-19 in Germany, medRxiv preprint, doi: 10.1101/2020.04.08.20056630.
- Barbarossa M., Fuhrmann J., Meinke J., Krieg S., Varma H. V., Castelletti N., Lippert T. (2020), The impact of current and future control measures on the spread of COVID-19 in Germany, medRxiv preprint, doi: 10.1101/2020.04.18.20069955. [DOI] [PMC free article] [PubMed]
- Chen S., Igan D., Pierri N., Presbitero A. (2020), Tracking the economic impact of COVID-19 and mitigation policies in Europe and the United States, International Monetary Fund Research, Special Series on COVID-19.
- Chen X., Qiu Z. (2020), Scenario analysis of non-pharmaceutical interventions on global Covid-19 transmissions, CEPR Press, Covid Economics, Vetted and Real-Time Papers, 7.
- Dehning J., Zierenberg J., Spitzner F. P., Wibral M., Neto J. P., Wilczek M., Priesemann V. (2020), Inferring COVID-19 Spreading Rates and Potential Change Points for Case Number Forecasts, Mimeo Max Planck Institute for Dynamics and Self-Organization, Göttingen. [Google Scholar]
- Diamond P. A. (1982), “ Aggregate Demand Management in Search Equilibrium”, Journal of Political Economy 90, 881–94. [Google Scholar]
- Donsimoni J. R., Glawion R., Plachter B., Wälde K. (2020. a), “ Projecting the Spread of COVID19 for Germany”, German Economic Review 21, 181–216. [Google Scholar]
- Donsimoni J. R., Glawion R., Plachter B., Wälde K. (2020. b), “ Projektion Der COVID-19-Epidemie in Deutschland”, Wirtschaftsdienst 100, 272–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donsimoni J. R., Glawion R., Plachter B., Wälde K., Weiser C. (2020), “Should Contact Bans Be Lifted in Germany? A Quantitative Prediction of Its Effects”, CESifo Working Papers 8242, IZA Discussion Papers 13151. [DOI] [PMC free article] [PubMed]
- Fuhrmann J., Barbarossa M. (2020), “The Significance of the Detection Ratio for Predictions on the Outcome of an Epidemic - A Message from Mathematical Modelers”, Preprints 2020, 2020050011 (doi: 10.20944/preprints202005.0011.v1). [DOI] [PMC free article] [PubMed]
- German R., Djanatliev A., Maile L., Bazan P. (2020), “Modeling Exit Strategies From COVID-19 Lockdown With A Focus On Antibody Tests”, medRxiv preprint, 10.1101/2020.04.14.20063750. [DOI]
- Gros C., Valenti R., Valenti K., Gros D. (2020), “Strategies for Controlling the Medical and Socio-economic Costs of the Corona Pandemic”, https://arxiv.org/abs/2004.00493v1 (last accessed 20 April 2020).
- Guan Z., Ni Z., Hu Y., Liang W., Ou C., He J., Liu L., Shan H., Lei C., Hui D. S. C., Du B., Li L., Zeng G., Yuen K.-Y., Chen R., Tang C., Wang T., Chen P., Xiang J., Li S., Wang J., Liang Z., Peng Y., Wei L., Liu Y., Hu Y., Peng P., Wang J., Liu J., Chen Z., Li G., Zheng Z., Qiu S., Luo J., Ye C., Zhu S., Zhong N. (2020), “ Clinical Characteristics of Coronavirus Disease 2019 in China”, The New England Journal of Medicine 382, 1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl T., Wälde K., Weber E. (2020), “Measuring the Impact of the German Public Shutdown on the Spread of COVID19”, Covid Economics, Vetted and Real-Time Papers, CEPR Press, 1, 25–32. [Google Scholar]
- Khailaie S., Mitra T., Bandyopadhyay A., Schips M., Mascheroni P., Vanella P., Lange B., Binder S., Meyer-Hermann M. (2020), Estimate of the development of the epidemic reproduction number Rt from Coronavirus SARS-CoV-2 case data and implications for political measures based on prognostics, medRxiv preprint, doi: 10.1101/2020.04.04.20053637. [DOI] [PMC free article] [PubMed]
- Khrapov P., Loginova A. (2020), “ Comparative Analysis of the Mathematical Models of the Dynamics of the Coronavirus COVID-19 Epidemic Development in the Different Countries”, International Journal of Open Information Technologies 8, 17–22. [Google Scholar]
- Kleyer C., Kosfeld R., Wälde K. (2020), Public Health Measures Addressing Covid-19 in Germany: A Systematic Overview, Mimeo Johannes Gutenberg-University Mainz and University Kassel.
- Lauer S., Grantz K., Bi Q., Jones F., Zheng Q., Meredith H., Azman A., Reich N., Lessler J. (2020), “ The Incubation Period of Coronavirus Disease 2019 (COVID-19) from Publicly Reported Confirmed Cases: Estimation and Application”, Annals of Internal Medicine 172, 577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton N., Kobayashi T., Yang Y., Hayashi K., Akhmetzhanov A. R., Jung S., Yuan B., Kinoshita R., Nishiura H. (2020), “ Incubation Period and Other Epidemiological Characteristics of 2019 Novel Coronavirus Infections with Right Truncation: A Statistical Analysis of Publicly Available Case Data”, Journal of Clinical Medicine. 9,538–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns Hopkins University (2020), COVID19 dataset, https://raw.githubusercontent.com/CSSEGISandData/COVID-19/master/csse_covid_19_data/csse_covid_19_time_series/time_series_covid19_confirmed_global.csv.
- University of Oxford (2020), Coronavirus Government Response Tracker, https://raw.githubusercontent.com/OxCGRT/covid-policy-tracker/master/data/OxCGRT_latest.csv.
- McKibbin W., Fernando R. (2020), The Global Macroeconomic Impacts of COVID-19: Seven Scenarios, CEPR Press, Covid Economics, Vetted and Real-Time Papers, 10.
- Mortensen D. T. (1982), “ Property Rights and Efficiency in Mating, Racing, and Related Games”, American Economic Review 72, 968–79. [Google Scholar]
- Pissarides C. A. (1985), “ Short-Run Equilibrium Dynamics of Unemployment Vacancies, and Real Wages”, American Economic Review 75, 676–90. [Google Scholar]
- Robert Koch Institut (RKI) (2020), COVID-19: Fallzahlen in Deutschland und weltweit, https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Fallzahlen.html. [Google Scholar]
- Romer P. (2020), Roadmap to responibly reopen America, https://roadmap.paulromer.net/paulromer-roadmap-report.pdf.
- Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C., Zimmer T., Thiel V., Janke C., Guggemos W., Seilmaier M., Drosten C., Vollmar P., Zwirglmaier K., Zange S., Wölfel R., Hoelscher M. (2020), “ Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany”, The New England Journal of Medicine 382, 970–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stübinger J., Schneider L. (2020), “Epidemiology of Coronavirus COVID-19: Forecasting the Future Incidence in Different Countries”, Healthcare 8, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wälde K. (2012), Applied Intertemporal Optimization. Know Thyself - Academic Publishers, www.waelde.com/KTAP.
- Zhang X., Liu H., Tang H., Zhang M., Yuan X., Shen X. (2020), “The Effect of Population Size for Pathogen Transmission on Prediction of COVID-19 Pandemic Spread”, https://arxiv.org/abs/2004.10527v1 [DOI] [PMC free article] [PubMed]