Abstract
Background
Various types of pulmonary diseases are associated with iron deficiency. However, information on iron status in coronavirus disease 2019 (COVID-19) is scarce.
Methods
This study included 50 hospitalized patients with confirmed COVID-19. The role of serum iron in predicting severity and mortality of COVID-19 was evaluated.
Results
The most common symptoms of COVID-19 patients in this study were cough (82%), fever (64%), and chest distress (42%). Of the 50 patients, 45 (90%) patients had abnormally low serum iron levels (<7.8 μmol/L). The severity of COVID-19 was negatively correlated with serum iron levels before and after treatment and was positively correlated with C-reactive protein, serum amyloid A, D-dimer, lactate dehydrogenase, urea nitrogen, and myoglobin levels. Decreased serum iron level could predict the transition of COVID-19 from mild to severe and critical illness. Seven (53.8%) patients with a lower serum iron level after treatment in the critical group had died. There was a significant difference in posttreatment serum iron levels between COVID-19 survivors and nonsurvivors.
Conclusions
Serum iron deficiency was detected in the patients with COVID-19. The severity and mortality of the disease was closely correlated with serum iron levels. Low serum iron concentration was an independent risk factor for death in COVID-19 patients.
Keywords: coronavirus disease 2019, mortality, prediction, serum iron, severity
Serum iron deficiency occurs in COVID-19. Critically ill patients with COVID-19 who died during hospitalization had significantly lower serum iron levels compared with those survivors. Decreased serum iron level can predict the transition of mild to critical COVID-19.
In December 2019, a novel coronavirus was identified among patients with pneumonia in Wuhan, Hubei Province, China, after which the virus rapidly spread worldwide [1]. As of June 7, 2020, there were 7 047 922 laboratory-confirmed cases worldwide and 402 827 deaths. A disease spectrum analysis of 44 415 patients diagnosed with coronavirus disease 2019 (COVID-19) showed the case-fatality rate (CFR) of critically ill patients to be as high as 49% [2]. In 2003, severe acute respiratory syndrome (SARS) caused 774 deaths in 29 countries and the CFR was approximately 10%. Thus, the mortality rate of COVID-19 critically ill patients was much higher than that of SARS [3]. Therefore, it is urgent to search for valuable and potential clinical laboratory parameters that can act as early warning indicators of the severity and mortality of COVID-19.
It has been shown that this emerging infection is caused by a new type of enveloped ribonucleic acid coronavirus B, and thus it was named SARS coronavirus 2 (SARS-CoV-2) [4]. Scientists have noted that SARS-CoV-2 enters the human body through angiotensin-converting enzyme 2 (ACE2) receptors on the membrane of cells [5]. The ACE2 receptors are found in type II alveolar epithelial cells of the human lungs, and thus the lungs became the main target of SARS-CoV-2 in COVID-19 [6]. However, the specific pathogenesis of impaired lung function is still unclear. The iron uptake system has been demonstrated to be implicated in hospital-acquired pneumonia and chronic lung infections [7]. Lower body iron and high tissue iron levels have been associated with lower lung function and severe pulmonary inflammation, respectively [8]. Although the role of iron in COVID-19 is unknown, we hypothesized that lower serum iron levels would be associated with a greater risk of inflammation and a high severity and mortality of COVID-19. To test this hypothesis, we evaluated the level of serum iron before and after treatment and investigated the correlation between serum iron levels and the severity and mortality of the disease.
METHODS
Study Population
This study was approved by the Institutional Ethics Board of Hubei No. 3 People’s Hospital of Jianghan University. Oral consent was obtained from the patients, and written informed patient consent was waived by the ethics board of the Hubei No. 3 People’s Hospital of Jianghan University for quarantine in Wuhan, China. All patients with COVID-19 enrolled in this research were diagnosed according to the guidance of World Health Organization (WHO) and the National Health Commission of China from February 1 to February 29, 2020.
Diagnostic and Classified Criteria
Nasopharyngeal swab samples were collected for detection, and reverse transcription-polymerase chain reaction was used to detect open reading frame 1aboratory (open reading frame1ab [ORF1ab]) and nucleocapsid protein (nucleocapsid protein N) of SARS-CoV-2. Reverse transcription-polymerase chain reaction was carried out as per the protocol issued by the WHO. The SARS-CoV-2-positive case confirmation needs to meet the requirement that both the target genes are positive at the same time or that ORF1ab is positive in 2 different samples of the same patient. According to the seventh edition of COVID-19’s diagnosis and treatment plan issued by the Chinese National Health Commission, patients with COVID-19 are divided into 3 main types according to the following clinical manifestations: (1) mild - with fever, respiratory or digestive symptoms, and physician-diagnosed pneumonia by chest computed tomography (CT); (2) severe - one of the following conditions: shortness of breath and respiratory rate ≥30 times/minute, oxygen saturation ≤93% at rest, or chest CT imaging showing lesion progression of more than 50% within 24–48 hours; (3) critical - meet any of the following rules: respiratory failure and need for mechanical ventilation, shock, and any other organ failure needing critical care and treatment.
Data Collection
Blood routine, serum iron before and after treatment, liver function, renal function, C-reactive protein (CRP), serum amyloid A (SAA), D-dimer, myocardial enzyme, brain natriuretic peptide, arterial blood gas analysis, creatine kinase, and lactate dehydrogenase (LDH) were obtained. Data were analyzed by the research team of the Third People’s Hospital of Hubei Province. Exposure history, course of disease, clinical symptoms, laboratory tests, chest CT images, and treatment data were obtained from medical records.
Statistical Analysis
Categorical variables were described as frequency rates and percentages, and continuous variables were described using median and interquartile range (IQR) values. Median for continuous variables were compared using nonparametric Wilcoxon rank-sum test. For categorical variables, group comparisons were carried out by the Fisher exact test. To evaluate the value of serum iron and other laboratory indexes in predicting the severity and mortality of the disease, a receiver-operating characteristics (ROC) curve and the area under the ROC curve (AUROC) were determined. Multivariate logistic regression analysis was used to determine the independent risk factors for death in COVID-19 patients. All statistical analyses were performed using SPSS 26 software. A P value less than .05 was considered statistically significant.
RESULTS
Demographic and Clinical Characteristics of Patients With Coronavirus Disease 2019
This study included 50 hospitalized patients with confirmed COVID-19. The median age of the patients was 55 years (IQR, 44–66) and 20 (40%) were women. All patients were residents of Wuhan and had been in contact with patients with COVID-19. Compared with patients in the mild group, those in the critical group were significantly older (median age, 66 years [IQR, 56–74] vs 49 years [IQR, 36–65]; P < .001) and were more likely to have clinical comorbidities, including hypertension (7 [41.2%] vs 4 [23.5%], diabetes (4 [30.8%] vs 0 [0%]), cardiovascular disease (3 [23.1%] vs 2 [10.5%]), and cerebrovascular disease (2 [15.4%] vs 0 [0%]) (Table 1).
Table 1.
Demographic and Clinical Characteristics of Patients Infected With COVID-19
| Characteristics | No. (%) | P Value | |||
|---|---|---|---|---|---|
| Total (n = 50) | Mild (n = 19) | Severe (n = 18) | Critical (n = 13) | ||
| Age, median (IQR), years | 55 (44–66) | 49 (36–65) | 55 (46–63) | 66 (56–74) | .052 |
| Sex | .035 | ||||
| Men | 30 (60) | 7 (36.8) | 14 (77.8) | 9 (69.2) | |
| Women | 20 (40) | 12 (63.2) | 4 (22.2) | 4 (30.8) | |
| Any Comorbidity | |||||
| Hypertension | 17 (34) | 4 (23.5) | 6 (35.3) | 7 (41.2) | .159 |
| Diabetes | 6 (12) | 0 (0) | 2 (11.1) | 4 (30.8) | .013 |
| Chronic pulmonary disease | 2 (4) | 0 (0) | 0 (0) | 2 (15.4) | .064 |
| Coronary heart disease | 6 (12) | 2 (10.5) | 1 (5.6) | 3 (23.1) | .417 |
| Cerebrovascular atherosclerosis | 2 (4) | 0 (0) | 0 (0) | 2 (15.4) | .064 |
| Malignancy | 1 (2) | 0 (0) | 1 (5.6) | 0 (0) | .620 |
| Chronic liver disease | 1 (2) | 1 (5.3) | 0 (0) | 0 (0) | 1.000 |
| Signs and Symptoms | |||||
| Fever | 32 (64) | 12 (63.2) | 10 (55.6) | 10 (76.9) | .461 |
| Cough | 41 (82) | 14 (73.7) | 17 (94.4) | 10 (76.9) | .255 |
| Chest distress | 21 (42) | 8 (42.1) | 7 (38.9) | 6 (42.2) | .937 |
| Myalgia or fatigue | 10 (20) | 3 (15.8) | 6 (33.3) | 1 (7.7) | .210 |
| Sputum production | 5 (10) | 1 (5.3) | 2 (11.1) | 2 (15.4) | .726 |
| Anorexia | 4 (8) | 1 (5.3) | 2 (11.1) | 1 (7.7) | .826 |
| Diarrhea | 2 (4) | 1 (5.3) | 1 (5.6) | 0 (0) | 1.000 |
| Dyspnea | 2 (4) | 0 (0) | 0 (0) | 2 (15.4) | .064 |
| Palpitation | 2 (4) | 1 (5.3) | 0 (0) | 1 (7.7) | .721 |
| Chest pain | 2 (4) | 1 (5.3) | 1 (5.6) | 0 (0) | 1.000 |
Significant values are shown in bold.
Abbreviations: COVID-19, coronavirus 2019; IQR, interquartile range.
Among the 50 patients included in our study, the most common symptoms at the onset of COVID-19 were cough (82%), fever (64% on admission), and chest distress (42%). The second most common symptoms were fatigue (20%), sputum production (10%), anorexia (8%), diarrhea (4%), dyspnea (4%), and cardiovascular symptoms such as palpitations (4%) (Table 1).
Laboratory Parameters in Patients With Coronavirus Disease 2019
Lymphopenia was the main feature of patients with COVID-19, and lower lymphocyte counts were found in the critical group than in the mild group (0.39 [0.30–0.53] vs 1.9 [1.4–2.3]) and the severe group (0.39 [0.30–0.53] vs 0.63 [0.56–0.72]). D-dimer, fibrinogen, CRP, and SAA levels were significantly increased in the critical group than in the mild and severe groups. There were no statistical differences in hepatic or renal function between the 3 groups of patients with mild COVID-19. Compared with patients with mild COVID-19, we found that 53.8% of critical patients had an elevated procalcitonin level (53.8% vs 0%). Serum iron levels were significantly decreased in all the groups. Moreover, serum iron levels in patients with severe COVID-19 were significantly lower than those in patients with mild COVID-19 (4.9 [4.0–8.1] vs 6.6 [5.4–10.9]). The levels of serum iron after treatment were significantly increased in all 3 groups (Table 2). There was no statistical difference in serum iron levels before treatment in COVID-19 survivors and nonsurvivors (6.2 [4.3–8.0] vs 4.1 [2.2–7.5]) (P = .118), and levels of serum iron in both COVID-19 survivors and nonsurvivors were all decreased compared with the normal range (7.8–32.3 µmol/L). However, there was a significant difference in serum iron levels of posttreatment between COVID-19 survivors and nonsurvivors (19.1 [13.2–25.6] vs 5.5 [3.5–11.1]) (P = .002), suggesting that lower serum iron levels after treatment indicate bad prognosis.
Table 2.
Laboratory Findings of Patients With COVID-19 During Hospitalization [Median (IQR)]
| Variable | Normal Range | Mild (n = 19) | Sever (n = 18) | Critical (n = 13) | P Value |
|---|---|---|---|---|---|
| Course of disease, day | / | 7 (4–11) | 7 (6–7) | 6 (3–8) | .567 |
| Lymphocyte count, ×109/L | 1.1–3.2 | 1.9 (1.4–2.3) | 0.63 (0.56–0.72)a | 0.39 (0.30–0.53)b,c | <.001 |
| Lymphocyte, % | 20–50 | 21 (17–26) | 7 (5–11)a | 5 (3–7) | <.001 |
| Red blood cell count, ×1012/L | 3.8–5.1 | 4.1 (3.6–4.6) | 4.2 (3.9–4.8) | 4.4 (4.0–4.7) | .270 |
| Hemoglobin, g/L | 115–150 | 127 (112–145) | 132 (117–146) | 134 (121–150) | .270 |
| D-dimer, µg/mL | 0–1 | 0.34 (0.27–0.67) | 1.07 (0.48–1.47)a | 9.05 (1.81–28.8)b,c | <.001 |
| Fibrinogen (FIB), g/L | 0–5 | 3.1 (2.0–4.0) | 4.8 (3.3–5.6)a | 16.3 (6.0–49.2)b,c | <.001 |
| CRP, mg/L | 0–5 | 3.6 (1.4–6.0) | 41.8 (13.1–70.7)a | 104.6 (51.0–165.1)b,c | <.001 |
| SAA, mg/L | 0–10 | 57 (27–121) | 501 (313–721)a | 1359 (499–1795)b,c | <.001 |
| PCT, ng/mL (PCT < 0.25) | 0.04–0.25 | 19 (100) | 15 (83.3) | 7 (53.8) | .003 |
| Serum iron, µmol/L | 7.8–32.3 | ||||
| Pretreatment | / | 6.6 (5.4–10.9) | 4.9 (4.0–8.1)a | 5.2 (2.6–7.3) | .320 |
| Posttreatmentt | / | 21.6 (16.3–26.6)d | 17.9 (11.6–22.8)d | 11.8 (7.8–21.7)c,d | .198 |
| BNP, ng/L | 0–125 | 68 (34–189) | 545 (290–890)a | 962 (223–1457)b,c | <.001 |
| Creatine kinase (CK), U/L | 25–200 | 68 (51–92) | 85 (38–175) | 78 (71–311) | .679 |
| Lactic dehydrogenase (LDH), U/L | 109–245 | 190 (164–244) | 297 (202–377)a | 398 (291–535)b,c | <.001 |
| PH (blood gas analysis) | 7.35–7.45 | 7.40 (7.38–7.45) | 7.44 (7.41–7.46) | 7.41 (7.39–7.47) | .641 |
| PaO2, mmHg | 80–100 | 104 (79–135) | 100 (74–132) | 69 (54–134) | .198 |
| Oxygen saturation | 95–97 | 98 (96–99) | 98 (96–99) | 95 (89–99) | .893 |
| Creatinine, µmol/L | 44–120 | 60 (49–66) | 66 (56–96) | 66 (45–78) | .320 |
| Urea nitrogen, mmol/L | 3.5–7.2 | 3.5 (2.6–4.6) | 4.6 (3.7–5.0)a | 6.2 (4.5–7.1)b,c | .016 |
| ALT, U/L | 5–40 | 26 (15–34) | 33 (19–72) | 34 ( (21–77) | .679 |
| AST, U/L | 8–40 | 34 (27–45) | 43 (35–52) | 50 (38–74)c | .094 |
| TNI, µg/L | 0.01–0.023 | .126 | |||
| TNI <0.01 | / | 18 (94.7) | 16 (88.9) | 9 (69.2) | |
| TNI >0.023 | / | 1 (5.3) | 2 (11.1) | 4 (30.8) | |
| Myohemoglobin, µg/L | 23–112 | 18 (10–25) | 34 (13–43)a | 52 (35–79)b,c | <.001 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BNP, B-type natriuretic peptide; COVID-19, coronavirus 2019; CRP, C-reactive protein; IQR, interquartile range; PaO2, partial pressure of oxygen; PCT, procalcitonin; PH, potential of hydrogen; SAA, serum amyloid A; TNI, troponin I.
NOTE: P < .05 was considered significant, significant values are shown in bold.
aP < .05, compared with mild group.
bP < .05, compared with severe group.
cP < .05, compared with mild group.
dP < .05 compared with pretreatment.
Correlation Between Type of Coronavirus Disease 2019 and Other Laboratory Parameters
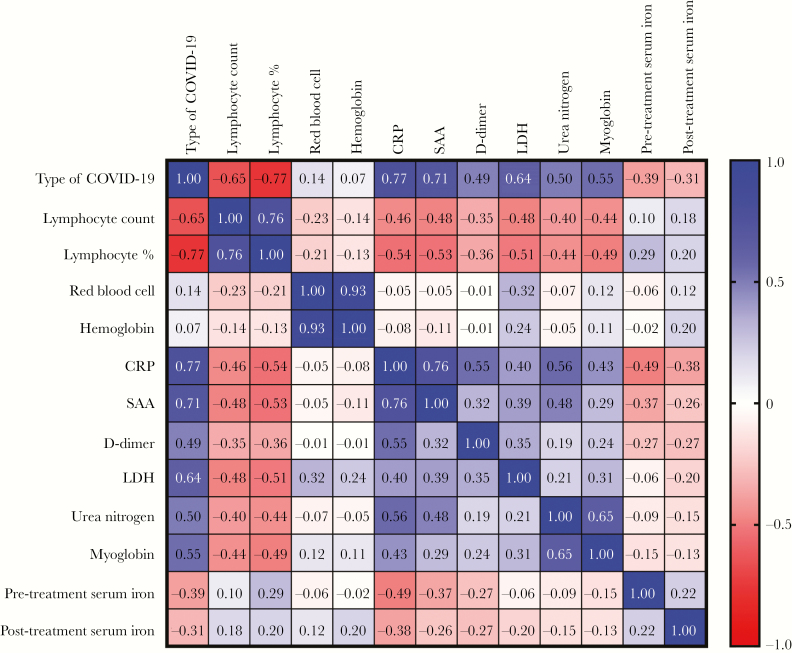
To investigate the possible role of serum iron in the pathogenesis of the disease, we analyzed the correlation between serum iron levels and other variables. We found that the severity of COVID-19 was negatively correlated with serum iron levels before and after treatment and was positively correlated with CRP, SAA, D-dimer, LDH, urea nitrogen, and myoglobin levels. A significant and negative correlation between severity of disease and serum iron levels before (r = −0.390, P < .001) and after treatment (r = −0.31, P = .034) was detected. There were also significant negative correlations between pretreatment serum iron and other laboratory parameters, including CRP (r = −0.49, P< .001) and SAA (r = −0.37, P = .010) (Figure 1).
Figure 1.
Correlation between the type of coronavirus 2019 (COVID-19) and other laboratory parameters. The severity of COVID-19 was negatively correlated with serum iron levels before and after treatment and was positively correlated with C-reactive protein (CRP), serum amyloid A (SAA), D-dimer, lactate dehydrogenase (LDH), urea nitrogen, and myoglobin levels. Pretreatment serum iron levels were negatively correlated with CRP and SAA levels.
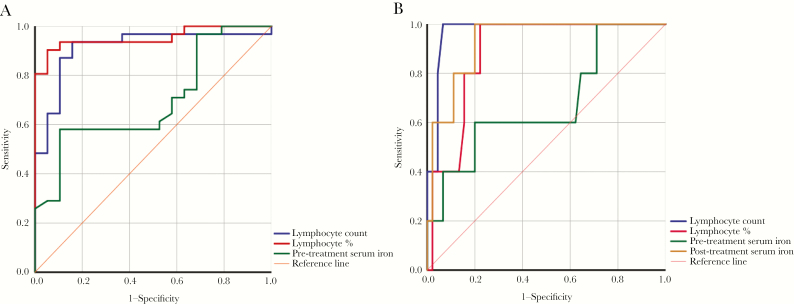
Analysis of Lymphocyte and Serum Iron in Predicting Severity and Mortality of Coronavirus Disease 2019
To evaluate the value of lymphocytes and serum iron in predicting the severity and mortality of COVID-19, a ROC curve and an AUROC were performed. A decrease in lymphocyte count, percentage of lymphocytes, and serum iron levels before treatment were of great value in predicting the transition of COVID-19 from mild to severe and critical illness (Figure 2A). The AUROC and 95% confidence interval (CI) of lymphocyte count, percentage of lymphocytes, and pretreatment serum iron were 0.913 (95% CI, 0.827–1.000), 0.952 (95%, CI 0.895–1.000), and 0.696 (95% CI, 0.548–0.844), respectively. The cutoff values of lymphocyte count and percentage were 0.83 × 109/L (sensitivity, 93.5%; specificity, 84.2%) and 13.15% (sensitivity, 90.3%; specificity, 94.7%). The cutoff value of serum iron before treatment was 5.26 μmol/L (sensitivity, 58.1%; specificity, 89.5%). Lymphocyte count, percentage of lymphocytes, and serum iron levels after treatment could be used to predict the mortality of COVID-19 (Figure 2B). The AUROC and 95% CI of lymphocyte count, percentage of lymphocytes, and posttreatment serum iron were 0.971 (95% CI, 0.927–1.000), 0.887 (95% CI, 0.785–0.988), and 0.929 (95% CI, 0.844–1.000), respectively. The cutoff values for lymphocyte count and percentage were 0.435 × 109/L (sensitivity, 100%; specificity, 91.1%) and 5.65% (sensitivity, 100%; specificity, 77.8%). The cutoff value for serum iron before treatment was 12.54 μmol/L (sensitivity, 100%; specificity, 77.8%). The ROC curve is shown in Figure 2B.
Figure 2.
Receiver-operating characteristics curve analysis for predicting the severity of coronavirus 2019 (COVID-19). (A) Lymphocyte count, percentage of lymphocytes, and pretreatment serum iron levels could be used to predict the severity of COVID-19. (B) Lymphocyte count, percentage of lymphocytes, and posttreatment serum iron levels could be used to predict the mortality of COVID-19.
Mutlivariate Analyses of Independent Risk Factors for Death in Coronavirus Disease 2019 Patients
Multivariate logistic regression analysis was used to determine whether the age, lymphocyte percentage, lymphocyte count, pretreatment serum iron level, and posttreatment serum iron level were the independent risk factors for death in patients with COVID-19. Death of the COVID-19 patients was set as the dependent variable, and mutlivariate analyses showed that old age and low posttreatment serum iron level were the independent risk factors for the death in COVID-19 patients (P = .013 vs P = .006) (Table 3).
Table 3.
Mutlivariate Analyses of Independent Risk Factors for Death in COVID-19 Patients
| B Value | 95% CI | P Value | |
|---|---|---|---|
| Age | 0.006 | 0.001–0.011 | .013 |
| Lymphocyte% | −0.002 | −0.014 to 0.011 | .812 |
| Lymphocyte count | −0.085 | −0.311 to 0.141 | .451 |
| Pretreatment serum iron | −0.001 | −0.022 to 0.021 | .961 |
| Posttreatment serum iron | −0.011 | −0.019 to −0.003 | .006 |
Abbreviations: CI, confidence interval; COVID-19, coronavirus 2019.
Treatments and Outcomes of Patients Infected With Coronavirus Disease 2019
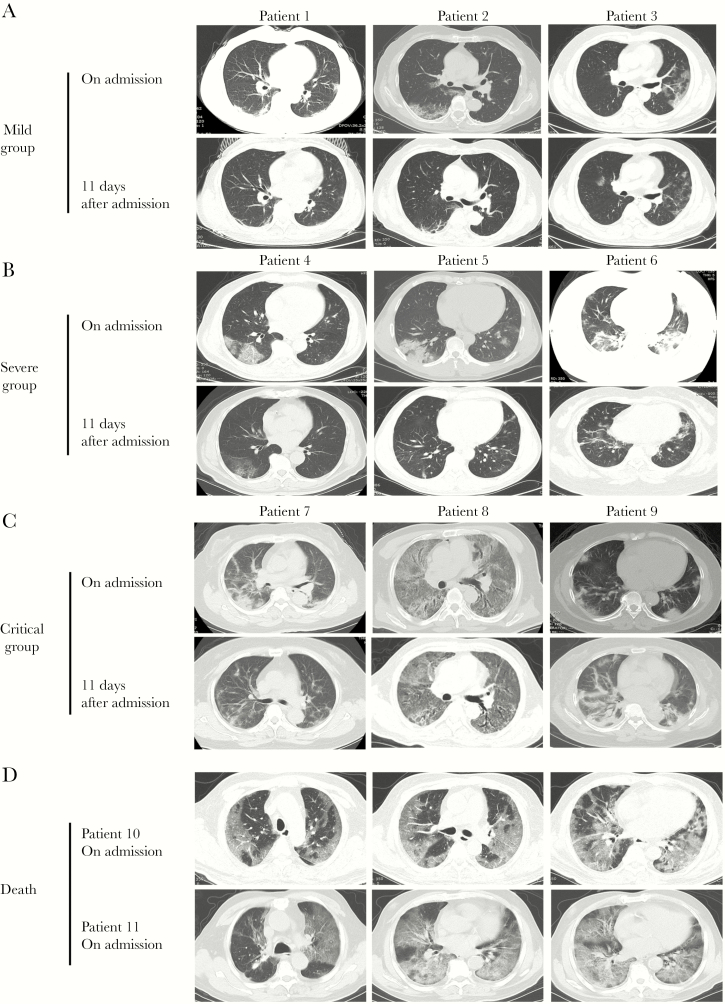
All patients in this study were administered with supportive treatments. All 50 (100%) patients received antiviral therapy (oseltamivir or arbidol), 36 (72%) received antibiotic therapy, 44 (88%) received immunomodulatory therapy (hydroxychloroquine or chloroquine phosphate), and 34 (68%) were given short-term (3–5 days) and low-dose systematic corticosteroids; a higher percentage of patients with severe COVID-19 received these therapies (Table 4). Patients in the severe and critical group had a higher percentage of antibiotic therapy and corticosteroid use than those in the mild group (P < .001). As of March 12, 2020, the final date of follow up, 42 of 50 patients (84%) had been discharged, 1 (2%) was under hospitalization, and 7 (14%) had died. Patients in the critical group had a higher percentage of hospitalization (7.7% vs 0%) and mortality (53.8% vs 0%) than those with mild disease (Table 4). Chest CT images showed multiple small patches and interstitial changes in the lungs after the onset of symptoms (Figure 3). Absorption of bilateral ground-glass lesions was found with increased level of posttreatment serum iron in all groups except for the critical group (Figure 3A–C). Patients in the critical group who died had more severe chest CT images, including multiple lobular and subsegmental areas of consolidation (Figure 3D).
Table 4.
Treatments and Outcomes of Patients With COVID-19
| No (%) | |||||
|---|---|---|---|---|---|
| Treatment | Total | Mild (n = 19) | Severe (n = 18) | Crtitical (n = 13) | P Value |
| Antiviral therapy | 50 (100) | 19 (100) | 18 (100) | 13 (100) | - |
| Antibiotic therapy | 36 (72) | 6 (31.6) | 17 (94.4) | 13 (100) | <.001 |
| Use of corticosteroid | 34 (68) | 6 (31.6) | 15 (83.3) | 13 (100) | <.001 |
| Use of gamma globulin | 34 (68) | 10 (52.6) | 12 (70.6) | 12 (92.3) | .060 |
| Immunomodulatory therapy | 44 (88) | 17 (89.5) | 16 (88.9) | 11 (84.6) | 1.000 |
| Cough and sputum treatment | 44 (88) | 16 (84.2) | 16 (88.9) | 12 (92.3) | .872 |
| Chinese patent medicine therapy | 20 (40) | 0 (0) | 12 (66.7) | 8 (61.5) | <.001 |
| Prognosis | |||||
| Hospitalization | 1 (2) | 0 (0) | 0 (0) | 1 (7.7) | .260 |
| Discharge | 42 (84) | 19 (100) | 18 (100) | 5 (38.5) | <.001 |
| Death | 7 (14) | 0 (0) | 0 (0) | 7 (53.8) | <.001 |
Significant values are shown in bold.
Abbreviations: COVID-19, coronavirus 2019.
Figure 3.
Chest computed tomography (CT) images of patients with coronavirus 2019 (COVID-19). (A) Transverse chest CT images showed bilateral ground-glass opacity from 3 mild patients 1 week after symptom onset and 11 days after admission. (B) Chest CT images showed subsegmental areas of consolidation from another 3 severe patients 1 week after symptom onset and 11 days after admission. (C) Extensive bilateral pulmonary changes of consolidation were found in the lungs of critical patients. (D) Patients who died in the critical group had more severe lesions in bilateral lungs than those in the mild and severe groups.
DISCUSSION
We first reported an iron deficiency in COVID-19 patients. We then reported a close correlation between low serum iron and high severity of the disease. A total of 45 of 50 (90%) patients had abnormally low serum iron levels (<7.8 μmol/L) in this study. Various types of pulmonary diseases have been associated with iron deficiency. A lack of iron resulted in allergic asthma, whereas iron supplementation could decrease chronic cough hyperresponsiveness and allergic inflammation in the lungs [8, 9]. Iron deficiency was highly prevalent in idiopathic pulmonary arterial hypertension, and the extent of iron deficiency was also related to hemodynamics [10]. The level of serum iron was thought to be an independent predictor of in-hospital mortality in critically ill patients [11]. We noted that patients with COVID-19 who died during hospitalization had markedly severe chest CT images and significantly lower serum iron levels compared with those who survived before and after treatment.
Combination of a variety of chronic diseases was one of the factors that contributed to the high mortality of critically ill patients with COVID-19. In our study, coexisting disorders in patients with severe COVID-19 included hypertension (34%), cardiovascular disease (12%), and diabetes (12%). A higher percentage of comorbidities (hypertension [41.2%] and diabetes [30.8%]) was found in critical patients than in mild and severe patients, and with a greater mortality in the severe group than in the other 2 groups (53.8% vs 0%). The most common symptoms at the onset of illness were cough (82%), fever (64% on admission), and chest distress (42%), which is consistent with a previous study. However, the percentage of patients with a cough was higher in our study (82% vs 67.8%) [12]. Serum iron was found to be closely correlated with chronic cough [9, 13, 14]. Previous research showed that nonsmoking women with chronic unexplained cough should be checked for iron deficiency and iron supplementation may resolve this symptom [9]. It was intriguing that oral intake of iron attenuated ACE inhibitor therapy related cough through its effect on nitric oxide generation [13]. These results suggest that iron deficiency might be one of reasons why cough symptoms accounted for a major percentage of the symptoms in COVID-19 patients. We also noted that 44 (88%) patients in our study received noniron anticough therapy, indicating that oral of iron supplements can be used as an alternative treatment for cough in COVID-19 patients.
C-reactive protein and SAA were acute phase reactants associated with an overactive inflammatory response. C-reactive protein was associated with 28-day mortality and had prognostic significance in patients hospitalized with community-acquired pneumonia [15]. In this study, D-dimer, fibrinogen, CRP, SAA, and LDH levels were significantly increased in critically ill patients than in mild and severe patients, which is consistent with a previous study [12]. Four (30.8%) patients in the critical group showed abnormally increased TNI levels. Patients in the critical group had higher B-type natriuretic peptide and myohemoglobin levels than those in the mild and severe groups, suggesting the cardiovascular system is implicated in the progression of COVID-19. The specific role of the cardiovascular system in the pathogenesis of this emerging infectious disease is still unclear. The high expression of ACE2 in the endothelial cells of coronary arteries and the immune damage caused by cytokines released by inflammatory storms in critically ill patients might explain why the cardiovascular system is affected [16, 17]. After analyzing the correlation between severity of disease and laboratory indexes, we found that the severity of COVID-19 was negatively correlated with serum iron levels before and after treatment. Further ROC analysis demonstrated that posttreatment serum iron level could predict the mortality of the illness. However, serum iron levels before treatment did not have a role in predicting the mortality of COVID-19.
As a retrospective study, this study has some notable limitations. First, it was only performed in a single center, and only 50 subjects were enrolled in this study. Thus, the results of our study need to be confirmed in a large population study. Second, because data generation was clinically driven and not systematic, we did not include other iron metabolism markers such as serum ferritin and transferrin. Third, most patients did not undergo sputum pathogenic bacteriologic or fungal detection during hospitalization due to overwhelmed medical resources.
CONCLUSIONS
In conclusion, we demonstrated that serum iron deficiency was one of the features in the patients with COVID-19. The severity and mortality of the disease was closely correlated with serum iron levels. Low serum iron concentration after treatment was an independent risk factor for death in COVID-19 patients.
Acknowledgments
Author contributions. K. Z. and S. N. conceptualized the paper. S. N. analyzed the data, with input from K. Z., J. H., and Y. F. S. N. and L. L. wrote the initial draft with all authors providing critical feedback and edits to subsequent revisions. All authors approved the final draft of the manuscript.
Financial support. This work was supported by Young medical talents Program in Hubei Province (Grant to L.L.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Zhu N, Zhang D, Wang W, et al. ; China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239–42. [DOI] [PubMed] [Google Scholar]
- 3. Mahase E. Coronavirus covid-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ 2020; 368:m641. [DOI] [PubMed] [Google Scholar]
- 4. Guan WJ, Ni ZY, Hu Y, et al. . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wan Y, Shang J, Graham R, et al. . Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol 2020; 94:e00127–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reyfman PA, Walter JM, Joshi N, et al. . Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am J Respir Crit Care Med 2019; 199:1517–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Minandri F, Imperi F, Frangipani E, et al. . Role of iron uptake systems in Pseudomonas aeruginosa virulence and airway infection. Infect Immun 2016; 84:2324–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brigham EP, McCormack MC, Takemoto CM, Matsui EC. Iron status is associated with asthma and lung function in US women. PLoS One 2015; 10:e0117545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bucca C, Culla B, Brussino L, et al. . Effect of iron supplementation in women with chronic cough and iron deficiency. Int J Clin Pract 2012; 66:1095–100. [DOI] [PubMed] [Google Scholar]
- 10. Ruiter G, Manders E, Happé CM, et al. . Intravenous iron therapy in patients with idiopathic pulmonary arterial hypertension and iron deficiency. Pulm Circ 2015; 5:466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xia JJ, Wang F, Jiang XN, et al. . Serum iron levels are an independent predictor of in-hospital mortality of critically ill patients: a retrospective, single-institution study. J Int Med Res 2019; 47:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu XW, Wu XX, Jiang XG, et al. . Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 2020; 368:m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bhalla P, Singh NP, Ravi K. Attenuation of angiotensin converting enzyme inhibitor induced cough by iron supplementation: role of nitric oxide. J Renin Angiotensin Aldosterone Syst 2011; 12:491–7. [DOI] [PubMed] [Google Scholar]
- 14. Lee SC, Park SW, Kim DK, et al. . Iron supplementation inhibits cough associated with ACE inhibitors. Hypertension 2001; 38:166–70. [DOI] [PubMed] [Google Scholar]
- 15. Lee JH, Kim J, Kim K, et al. . Albumin and C-reactive protein have prognostic significance in patients with community-acquired pneumonia. J Crit Care 2011; 26:287–94. [DOI] [PubMed] [Google Scholar]
- 16. Donoghue M, Hsieh F, Baronas E, et al. . A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res 2000; 87:E1–9. [DOI] [PubMed] [Google Scholar]
- 17. Chen C, Chen C, Yan JT, et al. . [Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19]. Zhonghua Xin Xue Guan Bing Za Zhi 2020; 48:E008. [DOI] [PubMed] [Google Scholar]