Abstract
Aim
To determine the incidence, patient characteristics, and related events associated with new-onset atrial fibrillation (AF) during a national COVID-19 lockdown.
Methods and results
Using nationwide Danish registries, we included all patients, aged 18–90 years, receiving a new-onset AF diagnosis during the first 3 months of 2019 and 2020. The main comparison was between patients diagnosed during lockdown (12 March 12–1 April 2020) and patients diagnosed in the corresponding period 1 year previously. We found a lower incidence of new-onset AF during the 3 weeks of lockdown compared with the corresponding weeks in 2019 [incidence rate ratios with 95% confidence intervals (CIs) for the 3 weeks: 0.66 (0.56–0.78), 0.53 (0.45–0.64), and 0.41 (0.34–0.50)]. There was a 47% drop in total numbers (562 vs. 1053). Patients diagnosed during lockdown were younger and with a lower CHA2DS2-VASc score, while history of cancer, heart failure, and vascular disease were more prevalent. During lockdown, 30 (5.3%) patients with new-onset AF suffered an ischaemic stroke and 15 (2.7%) died, compared with 45 (4.3%) and 14 (1.3%) patients during the corresponding 2019 period, respectively. The adjusted odds ratio of a related event (ischaemic stroke or all-cause death) during lock-down compared with the corresponding weeks was 1.41 (95% CI 0.93–2.12).
Conclusions
Following a national lockdown in Denmark, a 47% drop in registered new-onset AF cases was observed. In the event of prolonged or subsequent lockdowns, the risk of undiagnosed AF patients developing complications could potentially translate into poorer outcomes in patients with AF during the COVID-19 pandemic.
Keywords: COVID-19 pandemic, Atrial fibrillation, National lockdown, Collateral damage
Graphical Abstract
Graphical Abstract.
Introduction
During March 2020, leaders in many Western countries issued nationwide quarantines and limitations due to the coronavirus disease (COVID-19) pandemic. Most notably from a healthcare perspective, all non-critical medical appointments in hospitals were postponed, and general practitioners (GPs) and private specialists were asked to see only patients with the utmost important needs.1 The major focus of the healthcare system has been on morbidity and mortality of individuals with SARS-CoV-19 infection, but an increasing number of reports of reduced volumes of patients with non-infectious disease have gradually emerged.2,3 Atrial fibrillation (AF) is the most common cardiovascular condition resulting in a hospital contact,4,5 and correct and timely treatment is important when managing this condition.6 The consequences of a national lockdown have never been described and could pose accompanying collateral damage on top of the COVID-19 pandemic. Hence, we aim to estimate the incidence, patient characteristics, and related outcomes of new-onset AF during a period with a national lockdown.
Methods
Context
Universal access and coverage for healthcare need is provided for all Danish residents through a taxpayer-funded healthcare system.
The first step after the lockdown announcement in the evening of 11 March 2020 was the closure of schools and daycare institutions, postponement or cancellation of all non-critical medical appointments and elective surgery in hospitals, followed by the closure of the Danish borders on 14 March. On 18 March, gatherings of >10 people were not allowed. GPs and hospitals were still operating, but the public were advised to only seek medical attention with urgent matters. These restrictions persisted from 12 March until the end of data collection (1 April) and will be referred to as the lockdown.
Databases
Diagnoses, recent prescription claims, and vital status at the individual level were obtained from contemporary administrative Danish healthcare registries.7–9 We extracted information on diagnoses according to International Classification of Disease 10th Revision (ICD-10), date, and setting (data available until 1 April 2020) from the National Patient Registry. We identified filled prescription claims according to ATC codes (data available until 31 January 2020) from the National Prescription registry. We identified if the individual was alive or not, including the date of death, where applicable (data available until 1 April 2020), from the Civil Personal Registry. Highest achieved educational level was obtained from The Danish Education registry.10
Study population and study periods
We identified all patients with a first-time, new-onset AF diagnosis (ICD-10 code: DI48) between 18 and 90 years of age. The type of contact and the diagnostic coding were noted by a hospital physician. The setting of new-onset AF was divided into three groups: (i) virtual outpatient contact [patients would have had a physical visit to have an electrocardiograph (ECG) or be equipped with an ECG Holter monitor; patients deemed capable of receiving important information via phone or video would be contacted by a physician after this work-up for the final diagnosis]; (ii) physical contact exceeding 2 h was defined as a hospitalization; and (iii) physical contact lasting <2 h was defined as an outpatient visit.
We defined two study periods. (i) The first study period was the first 3 months of 2019 and 2020, i.e. from 1 January and 2 January through 1 April for 2019 and 2020, respectively, ensuring an equal amount of days due to 2020 being a leap year. (ii) The second study period was 3 weeks of lockdown (starting from 12 March 2020 through 1 April 2020) and three corresponding weeks the year before (12 March 2019 through 1 April 1 2019). Corresponding weeks in 2017 and 2018 were assessed as well to ensure that the 2019 comparison was generalizable. In all study periods, patients were categorized in weekly intervals. All patients with a new-onset diagnosis of AF prior to the start of either study period were excluded.
Patient characteristics
In the second study period (12 March–1 April 2020 compared with the same period in 2019), on the date of new-onset AF, all patients were characterized according to comorbidity from ICD-10 codes including prior history of heart failure, ischaemic stroke, vascular disease (including ischaemic heart disease and peripheral artery disease), cancer (excluding non-melanoma skin cancer), chronic kidney disease, chronic obstructive pulmonary disease, gastrointestinal bleeding, and diabetes mellitus. All diagnoses have previously been validated.7 To define hypertension, a combination of two antihypertensive drugs within the last 6 months was used (Supplementary material online, Table S1)11. The CHA2DS2‐VASc score for this study was calculated using the relevant characteristics described above.11–13
Related events
We defined related events as occurrence of ischaemic stroke within 1 week prior to or 1 week after the day of new-onset AF for each patient, the latter also including death from any cause. As a supplementary analysis, we investigated hospitalizations with heart failure or vascular disease in relation to new-onset AF (1 week prior to or 1 week after).
Statistical analyses
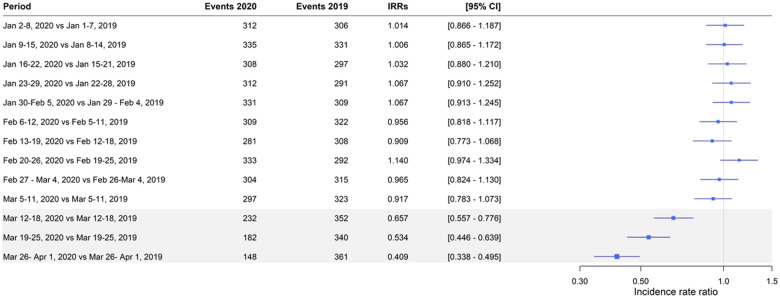
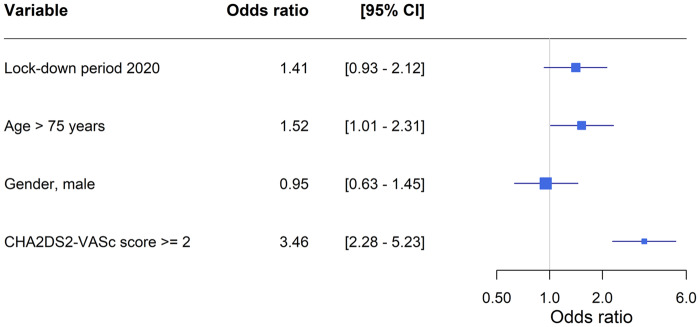
Numbers are presented as percentages, and continuous variables as the median with interquartile range. Baseline characteristics are shown for patients diagnosed with new-onset AF in the 3 weeks during lockdown with the corresponding 3 weeks in 2019. P-values were calculated, using χ2 test for categorical variables and Wilcoxon rank tests for continuous variables, comparing all 3 weeks of lockdown in 2020 with the corresponding weeks in 2019. Incidence rates (IRs) per 1000 person-years of new-onset AF, stratified by admission, as well as proportions of related events, were identified from 1 January and 2 January through 1 April for 2019 and 2020, respectively (Take home figure; Supplementary material online, Figure S1).Unadjusted incidence rate ratios (IRRs) with 95% confidence intervals (CIs) were computed using Poisson regression comparing each week (separately) in the 2020 study period with the corresponding week in 2019 (Figure 1). IRs (95% CI) per 1000 person-years of new-onset AF and IRRs (95% CI) were calculated comparing lockdown weeks with corresponding weeks in 2017 and 2018 as well (Supplementary material online, Table S2). A multivariable logistic regression model was used to associate odds of suffering a related event during the lockdown vs. the corresponding weeks in 2019. Other than the diagnosis period, we included age, gender, and CHA2DS2-VASc score (points for age and female gender removed in order to include these factors as independent variables) in the model. Reported were odds ratios (ORs) with 95% CIs (Figure 2). We used SAS software 9.4 (SAS Institute, Inc., Cary, NC, USA) and R (version 3.5.0 for Windows, R Foundation for Statistical Computing),14 including packages ‘Oddsratio, ‘Publish’, and ‘haven’.
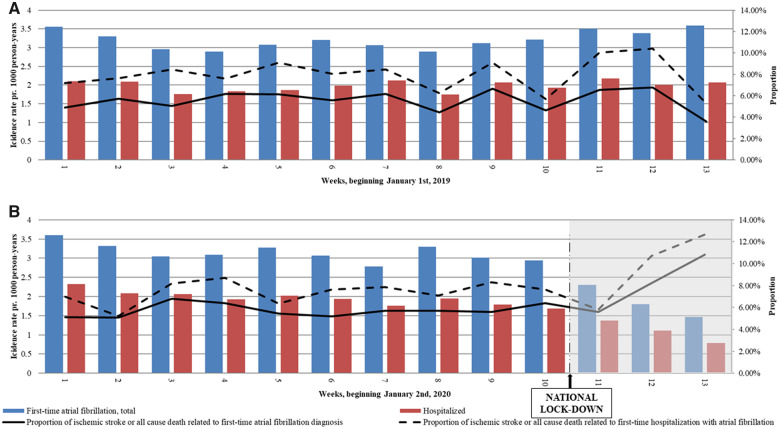
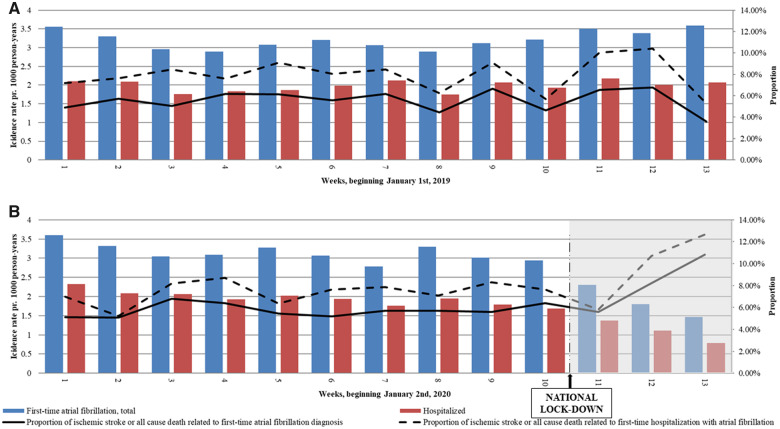
Take home figure.
Incidence of new-onset atrial fibrillation and related ischaemic stroke and death during the first 3 months of 2019 and 2020. (A and B) Incidence rates of new-onset atrial fibrillation (AF) within the first 3 months of 2019 and 2020 including hospitalizations. Incidence rates per 1000 person-years are read on the left y-axis. The black line shows the proportion of AF diagnoses with a related event (ischaemic stroke or all-cause death within 7 days of AF diagnosis); the dotted line shows the proportion among hospitalized patients with new-onset AF. Proportions in % are read on the right y-axis.
Figure 1.
Weekly incidence rate ratios comparing the first 3 months of 2020 and 2019. IRR, incidence rate ratio; CI, confidence interval. As of 1 January 2019, 5.26 million persons contributed to the analysis. As of 2 January 2020, 5.30 million persons contributed to the analysis. Period refers to the exact dates of 2020 and 2019 which are compared. The 1-day gap the first nine periods was due to 2020 being a leap year. The grey area indicates lockdown compared with corresponding weeks. Incidence rate ratios are unadjusted and were calculated using a Poisson regression.
Figure 2.
Odds ratios of related stroke or all cause death to atrial fibrillation diagnosis. CI = confidence intervals. A multivariable logistic regression, including the variables shown, was fitted to calculate odds of a related event comparing patients diagnosed with atrial fibrillation during lockdown with the patients diagnosed during the corresponding weeks in 2019. Scores for gender and age have been removed from the CHA2DS2-VASc score, since those factors are independent variables in the model.
Approval
Retrospective studies using administrative health databases do not need ethical approval in Denmark. The study was approved by the Data Protection agency (Journal no. FSEID-00004169-DST-project no. 706582, Approval no. P-2019-191).
Results
Incidence of new-onset AF
IRs of new-onset AF diagnoses were comparable and steady during the first 10 weeks of both 2019 and 2020 (Take home figure). Comparing the lockdown weeks in 2020 with the corresponding weeks in 2019 showed a significant decrease in registration of new-onset AF diagnoses (Figure 1). Extending the comparison period to the corresponding weeks in 2017 and 2018, the decline in new-onset AF diagnoses remained significant (Supplementary material online, Table S2).
Comparing the 3 weeks of lockdown with the corresponding 3 weeks in 2019, the registration of new-onset AF declined by 47%. Patients diagnosed with AF during the lockdown were younger, had a lower CHA2DS2-VASc score, and a higher prevalence of a history of cancer, heart failure, and vascular disease as compared with those diagnosed in 2019 (Table 1).
Table 1.
Characteristics of patients with new-onset atrial fibrillation from a 3-week national lockdown and three corresponding weeks in the previous year
| Characteristics | 12–18 March 2019 (n = 352) | 19–25 March 2019 (n = 340) | 26 March–1 April 2019 (n = 361) | 12–18 March 2020 (n = 232) | 19–25 March 2020 (n = 182) | 26 March–1 April 2020 (n = 148) | 12 March–1 April 2019 (n = 1053) | 12 March–1 April 2020 (n = 562) | P-value* |
|---|---|---|---|---|---|---|---|---|---|
| Age, median [IQR] | 76 [67, 82] | 74 [67, 82] | 74 [67, 80] | 73 [65, 80] | 73 [65, 80] | 74.5 [67, 80] | 75 [67, 81] | 74 [66, 80] | 0.014 |
| Male, n (%) | 202 (57.4) | 193 (56.8) | 212 (58.7) | 147 (63.4) | 115 (63.2) | 83 (56.1) | 607 (57.6) | 345 (61.4) | 0.16 |
| Type of AF diagnosis, n (%) | 0.06 | ||||||||
| Primary | 239 (67.9) | 220 (64.7) | 255 (70.6) | 149 (64.2) | 109 (59.9) | 96 (64.9) | 714 (67.8) | 354 (63.0) | |
| Secondary | 113 (32.1) | 120 (35.3) | 106 (29.4) | 83 (35.8) | 73 (40.1) | 52 (35.1) | 339 (32.2) | 208 (37.0) | |
| CHA2DS2-VASc, n (%) | 0.048 | ||||||||
| Score = 0 | 48 (13.6) | 50 (14.7) | 56 (15.5) | 39 (16.8) | 29 (15.9) | 28 (18.9) | 154 (14.6) | 96 (17.1) | |
| Score = 1 | 29 (8.2) | 38 (11.2) | 41 (11.4) | 30 (12.9) | 32 (17.6) | 13 (8.8) | 108 (10.3) | 75 (13.3) | |
| Score ≥2 | 275 (78.1) | 252 (74.1) | 264 (73.1) | 163 (70.3) | 121 (66.5) | 107 (72.3) | 791 (75.1) | 391 (69.6) | |
| Educational level, n (%) | 0.14 | ||||||||
| Basic or high school | 269 (76.4) | 267 (78.5) | 283 (78.4) | 184 (79.3) | 133 (73.1) | 101 (68.2) | 819 (77.8) | 418 (74.4) | |
| Higher education | 83 (23.6) | 73 (21.5) | 78 (21.6) | 48 (20.7) | 49 (26.9) | 47 (31.8) | 234 (22.2) | 144 (25.6) | |
| Comorbidity, n (%) | |||||||||
| Vascular disease† | 31 (8.8) | 34 (10.0) | 36 (10.0) | 33 (14.2) | 27 (14.8) | 14 (9.5) | 101 (9.6) | 74 (13.2) | 0.034 |
| Cancer | 35 (9.9) | 28 (8.2) | 29 (8.0) | 33 (14.2) | 32 (17.6) | 24 (16.2) | 92 (8.7) | 89 (15.8) | <0.001 |
| Stroke | 24 (6.8) | 28 (8.2) | 27 (7.5) | 18 (7.8) | 13 (7.1) | 12 (8.1) | 79 (7.5) | 43 (7.7) | 0.99 |
| Heart failure | 13 (3.7) | 10 (2.9) | 16 (4.4) | 19 (8.2) | 12 (6.6) | 9 (6.1) | 39 (3.7) | 40 (7.1) | 0.004 |
| Diabetes mellitus | 19 (5.4) | 20 (5.9) | 22 (6.1) | 25 (10.8) | 10 (5.5) | 11 (7.4) | 61 (5.8) | 46 (8.2) | 0.08 |
| Hypertension | 172 (48.9) | 153 (45.0) | 177 (49.0) | 104 (44.8) | 76 (41.8) | 66 (44.6) | 502 (47.7) | 246 (43.8) | 0.15 |
| Chronic kidney disease | 9 (2.6) | 13 (3.8) | 8 (2.2) | 9 (3.9) | 3 (1.6) | 5 (3.4) | 30 (2.8) | 17 (3.0) | 0.96 |
| COPD | 16 (4.5) | 32 (9.4) | 14 (3.9) | 18 (7.8) | 12 (6.6) | 9 (6.1) | 62 (5.9) | 39 (6.9) | 0.47 |
| Gastrointestinal bleeding | 10 (2.8) | 16 (4.7) | 10 (2.8) | 6 (2.6) | <3 | 5 (3.4) | 36 (3.4) | 13 (2.3) | 0.28 |
| Hospital setting, n (%) | <0.0001 | ||||||||
| Outpatient | 127 (36.1) | 125 (36.8) | 148 (41.0) | 72 (31.0) | 41 (22.5) | 50 (33.8) | 400 (38.0) | 163 (29.0) | |
| Hospitalized | 219 (62.2) | 202 (59.4) | 208 (57.6) | 138 (59.5) | 112 (61.5) | 79 (53.4) | 629 (59.7) | 329 (58.5) | |
| Virtual contact‡ | 6 (1.7) | 13 (3.8) | 5 (1.4) | 22 (9.5) | 29 (15.9) | 19 (12.8) | 24 (2.3) | 70 (12.5) | |
| Outcomes, n (%) | |||||||||
| Related event§ | 23 (6.5) | 23 (6.8) | 13 (3.6) | 13 (5.6) | 15 (8.2) | 16 (10.8) | 59 (5.6) | 44 (7.8) | 0.10 |
| Stroke | 18 (5.1) | 17 (5.0) | 10 (2.8) | 11 (4.7) | 8 (4.4) | 10 (6.8) | 45 (4.3) | 30 (5.3) | 0.40 |
| All-cause death | 5 (1.4) | 6 (1.8) | 3 (0.8) | <3 | 7 (3.8) | 6 (4.1) | 14 (1.3) | 15 (2.7) | 0.08 |
Dark grey, lockdown period 2020 (from 12 March to 1 April); Light grey, reference period 2019.
IQR, interquartile range; COPD, chronic obstructive pulmonary disease.
P-values referring to the comparison of the combined 3 weeks of lockdown with the three corresponding weeks in 2019.
The term vascular disease covers both ischaemic heart disease and peripheral artery disease.
No physical contact, but a hospital contact with a physician, e.g. via phone or video call.
Related event refers to ischaemic stroke 1 week before or 1 week after first-time AF diagnosis, the latter including death from all causes.
Hospital setting
The proportion of inpatient and outpatient new-onset AF diagnosis was similar comparing the lockdown with the corresponding weeks (Take home figure). The number of patients receiving their new-onset AF diagnosis through virtual hospital contact was higher during the lockdown (n = 70, 12.5%) than during the corresponding weeks (n = 24, 2.3%).
Related events
Proportions of ischaemic stroke or all-cause death related to registered new-onset AF seemed to increase following lockdown (Take home figure), though no statistically significant difference was present (Table 1). High age and CHA2DS2-VASc score were associated with increased odds of suffering a related event, though being diagnosed during lockdown vs. the corresponding weeks was not (Figure 2). Proportions of all-cause death, ischaemic stroke, heart failure, or vascular disease separately related to new-onset AF were also not increased during lockdown (Supplementary material online, Table S3 and Figure S1).
Discussion
Our primary finding was that the diagnosis of new-onset AF decreased by 47% during the first 3 weeks of the national COVID-19 lockdown in Denmark compared with the same period the previous year. The lowest IRRs were found 3 weeks into the lockdown compared with the corresponding weeks (Figure 1), suggesting a progression in the drop in new AF diagnoses. Patients diagnosed with new-onset AF during the lockdown were younger and with a lower CHA2DS2-VASc score; hence, we speculate that patients with better physical resources had an increased chance of being diagnosed during a lockdown. Moreover, during the lockdown, the elderly in particular were encouraged to stay at home and refrain from social contact including routine visits to their GP, thus limiting the possibility of early AF detection as well. In contrast, history of cancer, heart failure, and vascular disease (ischaemic heart disease and peripheral artery disease) were more prevalent among the AF patients diagnosed during lockdown, suggesting that these patient categories could be subject to more surveillance and increasing chances of undergoing physical exam or ECG. The non-significant slight increase in all-cause death could be due to the higher comorbidity burden overall among new-onset AF patients diagnosed during lockdown.
One can speculate about the reasons behind the steep decline in new-onset AF diagnoses.
First, the symptomatic presentation of AF spans from silent AF to a variety of mild to moderate symptoms (including palpitations, dizziness, chest discomfort, etc.) and to haemodynamic instability. The former is often discovered through opportunistic screening or a routine ECG in general practice, which has been proven highly effective to catch AF.15 Both activities reduced following lockdown, hence fewer patients would be referred for further evaluation. In contrast, those with more severe symptoms would be expected to seek medical attention, although the threshold for both patients contacting a physician and the physician agreeing to a consultation is arguably higher during a crisis where it is advised to postpone all non-acute matters for both the patient’s and the physician’s safety. Our data suggest that diagnoses were less frequently registered in all scenarios of AF presentations, since occurrence of new-onset AF in hospitalized patients and outpatients declined equally.
Secondly, with reduced hospital activities, especially concerning reduced hospital resources for non-urgent evaluation, many patients undergoing follow-up for suspected AF would have their appointment postponed; however, the fraction being hospitalized (59%) is comparable with that found in the literature (55%)16 (Table 1). With an increased risk and fear of contracting COVID-19 during a hospitalization, the threshold and demand for admission were arguably higher for physicians and patients, respectively.17 Interestingly, 12% of new-onset AF contacts were virtual during the lockdown as compared with 2% during the corresponding weeks (Table 1), suggesting a durable way of evaluating patients suspected of having AF when all physical contact should be minimized. For future health crisis management, telemedicine along with a targeted screening process should probably play a bigger role. The utilization of personal ECG devices as well as screening of high-risk individuals taking biomarkers and patient characteristics into account have proven effective in diagnosing AF.18,19 Thus to prepare for future situations like this, hospitals and GPs could look into implementing such procedures.
Finally, the possibility exists that the true incidence of AF simply is lower during major societal incidents such as the COVID-19 pandemic. Reports show that the incidence of influenza (and potentially other infectious conditions) has reached an all-time low following social distancing and improved personal hygienic measures.20 On the other hand, reports also suggest that the social isolation has led to more food and alcohol consumption and less physical activity, both of which are risk factors for AF occurrence, suggesting that an even larger group of AF patients remain undiagnosed.21,22
Thus, the observed decrease of new-onset AF may be partially explained by a true decrease of incident AF due to societal changes, while other factors suggest an opposite trend to what was observed, namely an increase in incidence.
Little is yet known about the collateral healthcare effects following a nationwide lockdown. Within the cardiovascular area, there have been reports of a decrease in ST-segment elevation myocardial infarction (STEMI) volume at invasive centres, but descriptions of the most common condition, AF, are missing.2,3 Our data support a significant impact on registered AF diagnoses, and may indicate a large group of patients who are undiagnosed and not properly evaluated. The proportion of patients with ischaemic stroke or death in relation to new-onset AF was not significantly increased during the lockdown, but we express concerns of appropriate management of AF patients since the proportions seem to increase stepwise during the weeks of lockdown, despite these patients being younger and with lower CHA2DS2-VASc scores (Table 1). We did see a non-significant increase in all-cause death as well, which could be explained by the history of cancer, heart failure, and vascular disease being more prevalent among the patients diagnosed during the lockdown (Table 1). Related hospitalizations with heart failure and vascular disease were, however, comparable (Supplementary material online, Table S3).
We found no statistically significant evidence of higher odds of suffering a related event during lockdown, although we would encourage further investigations regarding the progression of AF at the point of diagnosis. As expected, higher age and CHA2DS2-VASc score were associated with increased odds of suffering a related event, emphasizing the need for caution when evaluating these patients (Figure 2).
Strengths and limitations
As a nationwide study including all patients with new-onset AF, the risk of inclusion and selection bias is minimal. New-onset diagnosis of AF has a positive predictive value of 95%,23 and it is reassuring that extending the comparison to the corresponding weeks in 2017 and 2018 showed the same decline (Supplementary material online, Table S2). However, the diagnoses used have not been specifically validated during this epidemic. Some of the diagnoses in the registries may or may not have a different accuracy during a public health crisis, which should be kept in mind when evaluating the results. Furthermore, the registration of virtual outpatient contact was implemented in the beginning of 2019 and was available for all patient contacts registered during the lockdown in 2020 and corresponding weeks in 2019; however, the accuracy of this registration has not been validated. Information on redeemed prescriptions was only available until 31 January 2020, putting some limitations on our analysis. Our definition of hypertension, which is an important risk factor for AF and stroke, was based on prescription data. However, we did not find any difference of prevalence of hypertension; thus, we are confident that our definition captured long-standing hypertension. Additionally, we lacked information on subsequent prescriptions of oral anticoagulants (OACs) during the lockdown, making it hard to speculate on whether unregistered AF patients were diagnosed by GPs; diagnoses given outside of hospital contacts, such as at general practices, are not registered in our databases. Thus, patients diagnosed in general practice or any other healthcare contact outside the hospital system, who commenced relevant treatment and were referred to a department of cardiology, but then this was postponed by the cardiologist due to low risk and correct treatment, are not registered in our databases. This is a limitation, since these patients most probably account for some of the decline in the registration of new-onset AF, although we think it unlikely to have a major impact on the observed decline.
Another limitation is that the type of AF is not reliably registered in the registries. We used any AF diagnostic code and did not differentiate between paroxysmal, persistent, and permanent AF, nor valvular or non-valvular AF. The proportion of valvular AF has previously been reported to be ∼13% among Danish AF patients, and we do not believe our results would differ by excluding this group.16
Finally, we risk the possibility of a type 2 error, since we lacked up to 7 days of follow-up time among the AF patients suffering a related event during the lockdown. However, this would draw our estimates towards the null hypothesis.24
Clinical perspectives
We present the first evidence of a 47% drop in new-onset AF diagnoses following the COVID-19 pandemic public health crisis and we hope our findings can support measures to optimize care for patients with AF. This should be possible using already established screening methods, personal devices, and virtual hospital contacts. Lives saved by the necessary and immediate lockdown might result in poorer short- and long-term prognoses among other patient categories, hence focus should also be directed towards preventing probable collateral damage of the COVID-19 pandemic.
Conclusions
Following a national lockdown in Denmark, a 47% drop in registered new-onset AF cases was observed. In the event of prolonged or subsequent lockdowns, the risk of undiagnosed AF patients developing complications could potentially translate into poorer outcomes in patients with AF during the COVID-19 pandemic.
Funding
A.H. is supported by grants from ‘Snedkermester Sophus Jacobsen og Hustru Astrid Jacobsens Fond’ and ‘Ib Mogens Kristiansens Almene Fond’.
Conflict of interests: none declared.
Supplementary Material
References
- 1.The Danish Health Authority, COVID-19 outbreak in Denmark. https://www.sst.dk/da/corona-eng/FAQ (20 May 2020).
- 2. De Filippo O, D’Ascenzo F, Angelini F, Bocchino PP, Conrotto F, Saglietto A, Secco GG, Campo G, Gallone G, Verardi R, Gaido L, Iannaccone M, Galvani M, Ugo F, Barbero U, Infantino V, Olivotti L, Mennuni M, Gili S, Infusino F, Vercellino M, Zucchetti O, Casella G, Giammaria M, Boccuzzi G, Tolomeo P, Doronzo B, Senatore G, Grosso Marra W, Rognoni A, Trabattoni D, Franchin L, Borin A, Bruno F, Galluzzo A, Gambino A, Nicolino A, Truffa Giachet A, Sardella G, Fedele F, Monticone S, Montefusco A, Omedè P, Pennone M, Patti G, Mancone M, De Ferrari GM.. Reduced rate of hospital admissions for ACS during Covid-19 outbreak in Northern Italy. N Engl J Med 2020;doi: 10.1056/NEJMc2009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Solomon MD, McNulty EJ, Rana JS, Leong TK, Lee C, Sung S-H, Ambrosy AP, Sidney S.. The Covid-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med 2020;doi: 10.1056/NEJMc2015630. [DOI] [PubMed] [Google Scholar]
- 4. Heeringa J, Van Der Kuip DAM, Hofman A, Kors JA, Van Herpen G, Stricker BHC, Stijnen T, Lip GYH, Witteman JCM.. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J 2006;27:949–953. [DOI] [PubMed] [Google Scholar]
- 5. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJL.. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 7. Schmidt M, Schmidt SSAJ, Sandegaard JLJ, Ehrenstein V, Pedersen L, Sørensen HT.. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmidt M, Pedersen L, Sørensen HT.. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol 2014;29:541–549. [DOI] [PubMed] [Google Scholar]
- 9. Kildemoes HW, Sorensen HT, Hallas J.. The Danish National Prescription Registry. Scand J Public Health 2011;39:38–41. [DOI] [PubMed] [Google Scholar]
- 10. Jensen VM, Rasmussen AW.. Danish education registers. Scand J Public Health 2011;39:91–94. [DOI] [PubMed] [Google Scholar]
- 11. Olesen JB, Lip GYH, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, Selmer C, Ahlehoff O, Olsen A-MS, Gislason GH, Torp-Pedersen C.. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. Br Med J 2011;342:d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM, Andresen D, Camm AJ, Davies W, Capucci A, Olsson B, Aliot E, Cobbe S, Le Heuzey JY, Santini M, Vardas P, Manini M, Bramley C, Laforest V, Taylor C, Del Gaiso S, Huber K, De Backer G, Sirakova V, Cerbak R, Thayssen P, Lehto S, Blanc JJ, Delahaye F, Kobulia B, Zeymer U.. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 13. Friberg L, Rosenqvist M, Lip GYH.. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: The Swedish Atrial Fibrillation cohort study. Eur Heart J 2012;33;1500–1510. [DOI] [PubMed] [Google Scholar]
- 14.R Core Team. R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/ (20 May 2020).
- 15. Freedman B. Screening for atrial fibrillation. Circulation 2017;135:1851–1867. [DOI] [PubMed] [Google Scholar]
- 16. Mikkelsen AP, Hansen ML, Olesen JB, Hvidtfeldt MW, Karasoy D, Husted S, Johnsen SP, Brandes A, Gislason G, Torp-Pedersen C, Lamberts M.. Substantial differences in initiation of oral anticoagulant therapy and clinical outcome among non-valvular atrial fibrillation patients treated in inpatient and outpatient settings. Europace 2016;18:492–500. [DOI] [PubMed] [Google Scholar]
- 17. Lazzerini M, Barbi E, Apicella A, Marchetti F, Cardinale F, Trobia G.. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc Health 2020;4642:2019–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kotecha D, Breithardt G, Camm AJ, Lip GYH, Schotten U, Ahlsson A, Arnar D, Atar D, Auricchio A, Bax J, Benussi S, Blomstrom-Lundqvist C, Borggrefe M, Boriani G, Brandes A, Calkins H, Casadei B, Castellá M, Chua W, Crijns H, Dobrev D, Fabritz L, Feuring M, Freedman B, Gerth A, Goette A, Guasch E, Haase D, Hatem S, Haeusler KG, Heidbuchel H, Hendriks J, Hunter C, Kääb S, Kespohl S, Landmesser U, Lane DA, Lewalter T, Mont L, Nabauer M, Nielsen JC, Oeff M, Oldgren J, Oto A, Pison L, Potpara T, Ravens U, Richard-Lordereau I, Rienstra M, Savelieva I, Schnabel R, Sinner MF, Sommer P, Themistoclakis S, Van Gelder IC, Vardas PE, Verma A, Wakili R, Weber E, Werring D, Willems S, Ziegler A, Hindricks G, Kirchhof P.. Integrating new approaches to atrial fibrillation management: The 6th AFNET/EHRA Consensus Conference. Europace 2018;20:395–407. [DOI] [PubMed] [Google Scholar]
- 19. Chua W, Purmah Y, Cardoso VR, Gkoutos GV, Tull SP, Neculau G, Thomas MR, Kotecha D, Lip GYH, Kirchhof P, Fabritz L.. Data-driven discovery and validation of circulating blood-based biomarkers associated with prevalent atrial fibrillation. Eur Heart J 2019;40:1268–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The US Center for Disease Control and Prevention, COVIDView, Pneumonia and influenza mortality Report. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/pdf/covidview.pdf (20 May 2020).
- 21. Lippi G, Henry BM, Bovo C, Sanchis-Gomar F.. Health risks and potential remedies during prolonged lockdowns for coronavirus disease 2019 (COVID-19). Diagnosis 2020;7:85–90. [DOI] [PubMed] [Google Scholar]
- 22. Wingerter R, Steiger N, Burrows A, Estes NAM.. Impact of lifestyle modification on atrial fibrillation. Am J Cardiol 2020;125:289–297. [DOI] [PubMed] [Google Scholar]
- 23. Sundbøll J, Adelborg K, Munch T, Frøslev T, Sørensen HT, Bøtker HE SM.. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry. BMJ Open 2016;6:e012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sedgwick P. Pitfalls of statistical hypothesis testing: type I and type II errors. BMJ 2014;349:g4287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.