Abstract
Coronavirus disease 2019 (COVID-19) is an emerging infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We investigated the serum cytokine and chemokine levels in asymptomatic, mild, moderate, severe, and convalescent SARS-CoV-2–infected cases. Proinflammatory cytokine and chemokine production induced by SARS-CoV-2 were observed not only in symptomatic patients but also in asymptomatic cases, and returned to normal after recovery. IL-6, IL-7, IL-10, IL-18, G-CSF, M-CSF, MCP-1, MCP-3, IP-10, MIG, and MIP-1α were found to be associated with the severity of COVID-19. Moreover, a set of cytokine and chemokine profiles were significantly higher in SARS-CoV-2–infected male than female patients. The serum levels of MCP-1, G-CSF, and VEGF were weakly and positively correlated with viral titers. We suggest that combinatorial analysis of serum cytokines and chemokines with clinical classification may contribute to evaluation of the severity of COVID-19 and optimize the therapeutic strategies.
Keywords: SARS-CoV-2, cytokine, chemokine, disease severity
The serum levels of IL-6, IL-7, IL-10, IL-18, G-CSF, M-CSF, IP-10, MCP-1, MCP-3, MIG, and MIP-1α were associated with the severity of COVID-19. Men had higher levels of cytokines/chemokines than women. Some cytokines/chemokines were weakly correlated with the viral load.
In December 2019, a cluster of patients presented with emerging viral pneumonia in Wuhan, China, and were found to be infected with a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1–3]. As of 20 March 2020, SARS-CoV-2 has led to 326 558 confirmed cases worldwide, including 14 267 deaths. The clinical spectrums of confirmed SARS-CoV-2 infection appears to be variable, ranging from asymptomatic infection, mild upper respiratory tract illness, moderate lung infection with radiological abnormalities, to severe viral pneumonia with respiratory failure and in some cases death [4–6]. Epidemiological and clinical characteristics of patients with coronavirus disease 2019 (COVID-19) have been reported but the pathogenesis of SARS-CoV-2 infection has not been well defined [7, 8].
Virus-induced aberrant and excessive cytokine production (also known as hypercytokinemia) has been linked to morbidity and mortality in several viral infections [9–13]. Also, it has been reported that hyperinduction of proinflammatory cytokine plays an important role in the disease progression and the ultimate death of patients infected with SARS-CoV [14, 15], Middle East respiratory syndrome coronavirus (MERS-CoV) [12], and SARS-CoV-2 [8, 16] infection. However, the cytokine and chemokine profiles in SARS-CoV-2–infected cases with different degrees of disease severity remain unclear. In the present study, we investigated the serum cytokine and chemokine levels in asymptomatic, and mild, moderate, severe, and convalescent SARS-CoV-2–infected cases in Jiangsu Province, China. Furthermore, the relationships between sex and viral load and the levels of serum cytokines and chemokines in SARS-CoV-2–infected patients were explored. The findings of this research will further our understanding of the immunoregulatory events in disease progression and the pathogenesis of SARS-CoV-2 infection.
METHODS
Population and Samples
A total of 70 SARS-CoV-2–infected patients, diagnosed by real-time reverse transcription polymerase chain reaction (RT-PCR) or next-generation sequencing, and 4 convalescent cases in Jiangsu Province, China were included in this study. Of the 70 patients, 4 were asymptomatic and 66 were symptomatic. Serum samples of symptomatic cases were collected 1 to approximately 11 days after disease onset. According to the “Novel Coronavirus Pneumonia Diagnosis and Treatment Guidance” (version 5) published by the National Health Commission of China [17], the 66 symptomatic cases can be categorized into different clinical groups. The 22 mild cases had slight clinical symptoms and no radiological changes, the 36 moderate cases had fever, respiratory tract symptoms, and radiological abnormalities, and the 8 severe cases (including 1 critical cases) had at least 1 of the following: (1) respiratory distress with respiration rate ≥ 30 times/min, (2) oxygen saturation at rest ≤ 93%, (3) arterial Pao2/Fio2 ≤ 300 mmHg, and (4) respiratory failure, shock, or other organ failure requiring intensive care unit treatment. Additionally, 4 convalescent cases and 4 age- and sex-matched healthy controls who had undergone routine physical examinations in Jiangsu Province were also included. The exclusion criteria for healthy control individuals were as follows: acute or chronic infectious disease, any clinically significant disorder, and any medication with known influence on immunological factors. All procedures conducted in this study involving human materials were approved by the Jiangsu Provincial Center for Disease Control Ethics Committee.
Cytokine and Chemokine Assay
A total of 48 serum cytokines and chemokines were measured in a multiplex biometric immunoassay containing fluorescent microspheres conjugated with target-specific monoclonal antibodies according to the manufacturer’s instructions (Bio-Plex Pro Human Cytokine Screening 48-plex panel; Bio-Rad) and were detected on the Luminex 200 system (Luminex). According to their functions, the cytokines/chemokines were grouped into 6 categories: interleukins (IL-1α, IL-1β, IL-1ra, IL-2, IL-2Rα, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-16, IL-17, and IL-18), colony stimulating factors (G-CSF, GM-CSF, M-CSF, and stem cell factor [SCF]), interferons (IFN-α2 and IFN-γ), tumor necrosis factor (TNF-α, TNF-β, and TNF-related apoptosis inducing ligand [TRAIL]), growth factors (basic fibroblast growth factor [FGF], nerve growth factor-β [NGF-β], hepatocyte growth factor [HGF], leukemia inhibitory factor [LIF], platelet-derived growth factor [PDGF-BB], vascular endothelial growth factor [VEGF], and stem cell growth factor-β [SCGF-β]), and chemokine family (cutaneous T-cell-attracting chemokine [CTACK], eotaxin, growth related oncogene-α [GRO-α], IFN-γ inducible protein-10 [IP-10], monocyte chemoattractant protein-1 [MCP-1], MCP-3, migration inhibitory factor [MIF], monokine induced by interferon-γ [MIG], macrophage-inflammatory protein [MIP-1α], MIP-1β, regulated upon activation normal T-cell expressed and secreted [RANTES], and stromal cell-derived factor-1α [SDF-1α]).
SARS-CoV-2 Viral Load Detection
The SARS-CoV-2 viral loads of respiratory specimens were detected by real-time RT-PCR. The viral N gene was detected with a commercial kit according to the manufacturer’s instructions (SARS-CoV-2 Nucleic Acid test kit; BioGem). Human ribonucleases P (Rnasep) was selected as the internal reference gene. The relative viral load of each patient was calculated by using the equation 2−△Ct, in which △ cycle threshold value (Ct) = Ctviral N gene−Ct RnaseP. Data was translated to log2 (relative viral load), which was equivalent to −△Ct values [18].
Statistical Analysis
All statistical analyses were performed with SPSS (version 20.0), and the figures were prepared using GraphPad Prism (version 8.0). Normally distributed quantitative data is presented as mean ± standard deviation (SD), while nonnormally distributed data is presented as median (25th–75th percentile). The nonparamentic Mann-Whitney U test was used to compare the levels of serum cytokines between 2 groups. To explore the correlations between cytokines and the viral load, Spearman correlation analyses were performed because the distributions of variables were not normal; heat map analyses of the correlation coefficients were then conducted. The results were considered statistically significant if the P values were < .05.
RESULTS
Serum Cytokine and Chemokine Levels in SARS-CoV-2–Infected Asymptomatic, Symptomatic, and Convalescent Cases
We first investigated the serum cytokine and chemokine levels in asymptomatic, symptomatic, and convalescent cases and healthy controls. Compared with healthy controls, the serum IL-7, IL-10, and IP-10 levels were significantly higher in asymptomatic cases. The serum IL-1β, IL-1ra, IL-2, IL-2Rα, IL-6, IL-7, IL-8, IL-9, IL-10, IL-13, IL-15, IL-17, IL-18, G-CSF, M-CSF, IFN-α2, IFN-γ, TNF-α, TRAIL, basic FGF, HGF, PDGF-BB, VEGF, GRO-α, IP-10, MCP-1, and MIG levels were higher in symptomatic patients (Table 1). The eotaxin level was lower in symptomatic cases than healthy controls. Further comparison between asymptomatic and symptomatic cases showed that serum concentrations of IL-1ra, IL-1β, IL-6, and IP-10 were lower in asymptomatic cases than symptomatic cases. As expected, all of the cytokine and chemokine levels in convalescent cases were similar to asymptomatic cases and healthy controls. However, the serum levels of IL-1β, IL-1ra, IL-2, IL-6, IL-8, IL-9, IL-10, IL-12 (p40), IL-13, IL-15, IL-18, G-CSF, M-CSF, IFN-α2, IFN-γ, TNF-α, TRAIL, basic FGF, NGF-β, VEGF, CTACK, GRO-α, IP-10, MCP-1, MCP-3, MIF, and MIG in convalescent cases were significantly lower than those in symptomatic cases. Moreover, there were no statistically significant differences in the serum levels of IL-1α, IL-3, IL-4, IL-5, IL-12(p70), IL-16, GM-CSF, SCF, TNF-β, LIF, SCGF-β, MIP-1α, MIP-1β, RANTES, and SDF-1α between any of the groups, as shown in Table 1.
Table 1.
Characteristics and Serum Cytokine and Chemokine Levels of SARS-CoV-2–Infected Asymptomatic, Symptomatic, and Convalescent Cases and Healthy Controls (n = 78)
| Characteristic | Control (n = 4) | Asymptomatic (n = 4) | Symptomatic (n = 66) | Convalescent (n = 4) |
|---|---|---|---|---|
| Age, y, mean ± SD | 47.75 ± 7.85 | 42.00 ± 10.98 | 43.24 ± 14.76 | 40.25 ± 16.09 |
| Men, No. (%) | 2 (50) | 1 (25) | 37 (56) | 2 (50) |
| Women, No. (%) | 2 (50) | 3 (75) | 29 (44) | 2 (50) |
| Time, d, mean ± SD | 4.41 ± 3.47 | 35.75 ± 5.68 | ||
| No. of deaths | 0 | 0 | 0 | 0 |
| Serum cytokines, pg/mL, median (25th–75th percentile) | ||||
| IL-1α | 22.30 (10.83–30.47) | 24.19 (17.74–66.16) | 18.73 (14.14–26.15) | 18.00 (11.19–20.21) |
| IL-1β | 4.73 (4.06–5.61) | 6.78 (4.69–13.33) | 13.07 (8.55–21.97)b,d | 4.47 (2.82–6.49)e |
| IL-1ra | 389.80 (341.40–489.50) | 512.40 (298.60–1159.00) | 1441.00 (771.40–2255.00)b,d | 405.40 (156.40–1022.00)c |
| IL-2 | 3.93 (2.77–5.48) | 7.46 (2.48–13.02) | 9.69 (5.09–15.22)a | 2.99 (1.00–3.59)c |
| IL -2Rα | 97.08 (65.54–103.80) | 113.00 (68.77–184.20) | 189.68 (150.76–224.33)d | 99.33 (47.09–186.00) |
| IL -3 | 0.77 (0.40–1.26) | 0.61 (0.47–1.72) | 0.68 (0.32–1.01) | 0.61 (0.32–0.83) |
| IL -4 | 8.81 (8.42–10.58) | 8.11 (5.34–10.19) | 9.31 (7.48–11.01) | 7.46 (5.83–9.16) |
| IL -5 | 12.69 (5.38–21.80) | 11.48 (6.12–16.65) | 15.15 (8.62–78.05) | 10.60 (6.31–40.54) |
| IL -6 | 6.53 (5.17–10.47) | 6.44 (2.34–13.94) | 15.30(7.70–22.64)a,b | 2.51 (1.18–4.86)e |
| IL -7 | 14.39 (12.04–17.56) | 29.15 (21.26–33.79)a | 28.84 (22.41–35.30)d | 22.69 (13.56–27.58) |
| IL -8 | 11.28 (8.88–14.65) | 17.79 (12.18–33.72) | 33.29 (17.58–44.74)a | 4.18 (2.88–9.78)e |
| IL -9 | 259.70 (234.60–323.00) | 351.80 (334.60–375.10) | 391.77 (339.97–422.54)d | 274.80 (266.60–340.80)e |
| IL -10 | 2.57 (2.10–3.60) | 7.80 (6.42–8.17)a | 9.60 (5.64–13.24)a | 2.37 (0.83–5.53)c |
| IL -12 (p40) | 160.50 (146.20–181.50) | 246.10 (131.70–374.60) | 246.10 (138.20–333.10) | 56.29 (22.43–140.60)c |
| IL -12 (p70) | 6.11 (4.05–8.33) | 8.06 (4.22–13.62) | 6.68 (6.05–9.80) | 5.16 (3.18–6.06) |
| IL -13 | 6.24 (3.88–13.24) | 6.23 (2.73–21.15) | 19.95 (8.91–50.67)a | 4.71 (2.30–7.55)e |
| IL -15 | 337.80 (315.80–408.80) | 353.00 (166.00–575.90) | 560.25 (452.68–700.58)d | 243.50 (157.20–317.00)e |
| IL -16 | 130.80 (119.30–142.70) | 84.79 (50.40–170.60) | 135.50 (100.30–178.80) | 90.59 (57.76–127.90) |
| IL -17 | 11.35 (8.51–13.54) | 21.17 (11.90–24.68) | 16.63 (13.38–20.54)a | 15.17 (10.48–19.84) |
| IL -18 | 76.37 (66.95–94.23) | 75.93 (26.14–141.10) | 129.94 (99.26–165.04)a | 79.55 (46.63–109.80)c |
| G-CSF | 83.54 (70.97–98.15) | 78.14 (24.90–176.00) | 153.56 (117.73–243.29)a | 69.94 (48.21–103.50)c |
| GM-CSF | 9.54 (4.23–13.75) | 5.49 (0.91–16.48) | 7.83 (5.69–14.92) | 8.02 (7.08–11.51) |
| M-CSF | 23.68 (21.07–27.11) | 26.18 (13.88–49.79) | 47.82 (31.91–64.80)d | 17.51 (12.60–32.65)e |
| SCF | 129.60 (114.80–136.50) | 91.15 (48.13–184.40) | 121.70 (86.10–157.80) | 72.65 (59.76–134.10) |
| IFN-α2 | 19.67 (15.17–21.19) | 19.37 (15.77–29.36) | 28.21 (22.97–36.17)d | 16.45 (13.02–21.90)e |
| IFN-γ | 33.91 (27.84–40.03) | 48.21 (27.81–73.78) | 57.96 (44.02–72.15)d | 34.17 (27.54–42.97)c |
| TNF-α | 129.50 (98.75–151.60) | 151.50 (141.20–191.30) | 185.40 (164.80–213.70)d | 127.70 (102.60–152.30)e |
| TNF-β | 483.70 (423.30–554.80) | 515.70 (487.20–533.10) | 498.50 (444.10–559.50) | 427.70 (399.10–487.40) |
| TRAIL | 101.80 (94.15–105.90) | 107.80 (102.70–183.50) | 160.60 (129.20–204.50)d | 94.34 (83.14–102.10)e |
| Basic FGF | 63.94 (61.88–73.58) | 80.50 (53.65–106.00) | 90.24 (76.07–99.97)d | 59.38 (52.36–66.95)e |
| NGF-β | 4.47 (3.66–5.80) | 3.53 (1.47–6.98) | 5.58 (4.26–7.26) | 2.39 (0.14–5.14)c |
| HGF | 451.90 (378.20–667.40) | 562.00 (460.30–904.00) | 801.20 (601.80–1034.00)a | 597.70 (363.50–719.10) |
| LIF | 113.90 (66.46–168.70) | 95.43 (59.16–181.20) | 155.10 (96.78–222.70) | 67.37 (32.76–150.60) |
| PDGF-BB | 1179.00 (802.40–1834.00) | 2478.00 (1582.00–3309.00) | 2159.00 (1745.00–3068.00)a | 1524.00 (695.60–2034.00) |
| VEGF | 325.90 (254.00–474.90) | 517.00 (120.80–776.40) | 766.24 (597.48–1012.05)d | 356.60 (118.60–576.80)c |
| SCGF-β | 154 524.00 (143 555.00–161 369.00) | 174 667.00 (138 947.00–243 362.00) | 184 009.00 (157 334.00–223 500.00) | 149 084.00 (117 874.00–164 752.00) |
| CTACK | 925.60 (841.50–1007.00) | 958.70 (688.50–1517.00) | 1060.00 (822.40–1551.00) | 581.60 (367.50–838.60)e |
| Eotaxin | 36.21 (26.47–53.73) | 35.14 (16.24–59.31) | 15.45(11.61–25.51)a | 29.28 (18.62–52.00) |
| GRO-α | 1001.00 (979.30–1067.00) | 1129.00 (963.40–1286.00) | 1326.00 (1190.00–1439.00)a | 832.30 (796.40–1026.00)e |
| IP-10 | 110.30 (103.00–131.20) | 594.40 (397.00–846.20) a | 1655.00 (833.20–2458.00)d,b | 279.90 (144.20–369.50)e |
| MCP-1 | 72.61 (49.26–86.25) | 68.16 (42.12–182.80) | 128.70 (86.21–197.40)a | 41.75 (19.27–51.44)e |
| MCP-3 | 3.79 (3.01–5.09) | 2.20 (1.04–7.97) | 3.89 (2.30–5.69) | 1.07 (0.26–3.71)c |
| MIP-1α | 2.82 (1.94–3.16) | 3.01 (2.33–5.39) | 3.12 (2.69–4.00) | 2.81 (2.00–3.08) |
| MIP-1β | 319.90 (293.80–346.00) | 347.30 (299.40–430.70) | 330.00 (292.60–367.50) | 291.40 (273.10–327.90) |
| MIF | 2118.00 (1917.00–2705.00) | 2572.00 (681.10–5435.00) | 3318.00 (1963.00–5421.00) | 1228.00 (652.40–1895.00)c |
| MIG | 131.00 (97.84–181.00) | 178.90 (157.30–266.60) | 233.40 (161.10–354.40)a | 150.60 (96.84–185.10)c |
| RANTES | 9057.00 (8137.00–12 137.00) | 10 361.00 (5512.00–22 377.00) | 7409.00 (4362.00–12 982.00) | 7776.00 (3468.00–8775.00) |
| SDF-1α | 484.90 (331.10–707.50) | 469.40 (320.90–920.00) | 338.90 (260.10–436.00) | 469.80 (251.30–628.20) |
Abbreviations: CSF, colony-stimulating factor; CTACK, cutaneous T cell-attracting chemokine; FGF, fibroblast growth factor; GRO-α, growth related oncogene-α; HGF, hepatocyte growth factor; IFN, interferon; IL, interleukin; IP-10, IFN-γ inducible protein-10; LIF, leukemia inhibitory factor MCP, monocyte chemoattractant protein; MIF, migration inhibitory factor; MIG, monokine induced by interferon-γ; MIP, macrophage-inflammatory protein; NGF-β, nerve growth factor-β; PDGF, platelet-derived growth factor; RANTES, regulated upon activation normal T-cell expressed and secreted; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SCF, stem cell factor; SCGF-β, stem cell growth factor-β; SDF-1α, stromal cell-derived factor-1α; TNF, tumor necrosis factor; TRAIL, TNF-related apoptosis inducing ligand; VEGF, vascular endothelial growth factor.
a P < .05 verses control.
b P < .05 verses asymptomatic.
c P < .05 verses symptomatic.
d P < .01 verses control.
e P < .01 verses symptomatic.
Serum Cytokine and Chemokine Levels in SARS-CoV-2–Infected Patients With Mild, Moderate, and Severe Disease
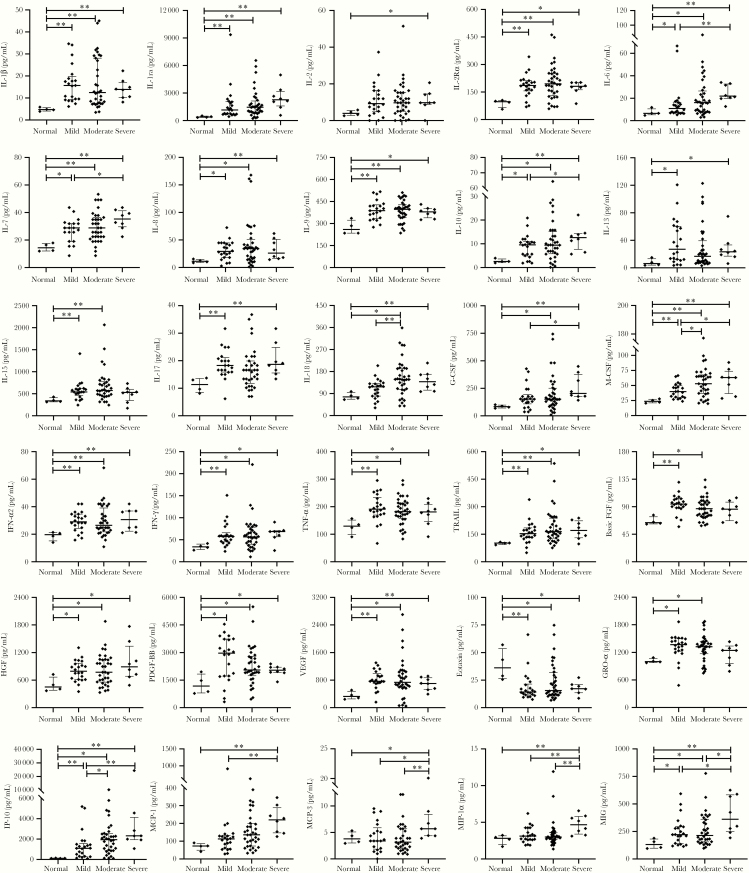
We further examined these cytokine and chemokine levels in symptomatic patients with different degrees of disease severity. The demographic and clinical characteristics of patients with different disease severity and healthy controls are shown in Table 2. There were no statistically significant differences in the serum levels of IL-1α, IL-3, IL-4, IL-5, IL-12(p40), IL-12(p70), IL-16, GM-CSF, SCF, TNF-β, basic FGF, LIF, SCGF-β, CTACK, MIP-1β, MIF, RANTES, and SDF-1α among overall groups. Compared with the healthy individuals, the mild cases had higher levels of serum IL-1β, IL-1ra, IL-2Rα, IL-6, IL-7, IL-8, IL-9, IL-10, IL-13, IL-15, IL-17, M-CSF, IFN-α2, IFN-γ, TNF-α, TRAIL, basic FGF, HGF, PDGF-BB, VEGF, eotaxin, GRO-α, IP-10, and MIG; the moderate cases had higher levels of serum IL-1β, IL-1ra, IL-2Rα, IL-6, IL-7, IL-8, IL-9, IL-10, IL-15, IL-18, G-CSF, M-CSF, IFN-α2, IFN-γ, TNF-α, TRAIL, basic FGF, HGF, PDGF-BB, VEGF, eotaxin, GRO-α, IP-10, and MIG; and the severe cases had higher levels of serum IL-1β, IL-1ra, IL-2, IL-2Rα, IL-6, IL-7, IL-8, IL-9, IL-10, IL-13, IL-17, IL-18, G-CSF, M-CSF, IFN-α2, IFN-γ, TNF-α, TRAIL, HGF, PDGF-BB, VEGF, eotaxin, IP-10, MCP-1, MCP-3, MIG, and MIP-1α. Compared with the mild cases, the severe cases had higher serum concentrations of IL-6, IL-7, IL-10, G-CSF, M-CSF, IP-10, MCP-1, MCP-3, MIG, and MIP-1α; and the moderate cases had higher serum concentrations of IL-18, IP-10, and M-CSF. Moreover, the severe cases also had higher levels of MCP-3, MIG, and MIP-1α than the moderate cases (Figure 1 and Supplementary Table 1).
Table 2.
Demographic and Clinical Characteristics of SARS-CoV-2–Infected Symptomatic Patients With Mild, Moderate, and Severe Disease
| Characteristics | Control (n = 4) | Mild (n = 22) | Moderate (n = 36) | Severe (n = 8) |
|---|---|---|---|---|
| Age, y, mean ± SD | 47.75 ± 7.85 | 43.32 ± 18.38 | 40.81 ± 11.80 | 54.00 ± 12.38 |
| Men | 2 (50) | 13 (59) | 19 (53) | 5 (63) |
| Women | 2 (50) | 9 (41) | 17 (47) | 3 (37) |
| Time from onset, d, mean ± SD | NA | 4.45 ± 4.76 | 4.42 ± 2.81 | 4.25 ± 2.05 |
| No. of deaths | 0 | 0 | 0 | 0 |
| Fever (temperature ≥ 37.3℃) | 0 | 16 (73) | 33 (92) | 8 (100) |
| Malaise | 0 | 6 (27) | 13 (36) | 2 (25) |
| Cough | 0 | 7 (32) | 15 (42) | 2 (25) |
| Sputum | 0 | 5 (23) | 9 (25) | 3 (38) |
| Pharyngalgia | 0 | 3 (14) | 10 (28) | 1 (13) |
| Headache | 0 | 4 (18) | 3 (8) | 1 (13) |
| Myalgia | 0 | 2 (9) | 8 (22) | 2 (25) |
| Emesis | 0 | 0 | 1 (3) | 0 |
| Diarrhea | 0 | 0 | 6 (17) | 0 |
| Comorbidities | 0 | 6 (27) | 8 (22) | 4 (50) |
| Hypertension | 0 | 3 (14) | 3 (8) | 4 (50) |
| Diabetes | 0 | 3 (14) | 1 (3) | 0 |
| Cardiocerebrovascular disease | 0 | 0 | 0 | 2 (25) |
| Chronic lung disease | 0 | 0 | 1 (3) | 1 (13) |
| Chronic kidney disease | 0 | 0 | 1 (3) | 0 |
| Chronic liver disease | 0 | 0 | 0 | 1 (13) |
| Other | 0 | 2 (9) | 4 (11) | 0 |
Data are No. (%) except where indicated.
Abbreviation: NA, not applicable; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Figure 1.
Serum cytokine and chemokine levels of SARS-CoV-2–infected symptomatic (mild, moderate, and severe) patients and healthy controls. Data are expressed as median with 25th and 75th percentiles. *P < .05, **P < .01. Abbreviations: CSF, colony-stimulating factor; FGF, fibroblast growth factor; GRO-α, growth related oncogene-α; HGF, hepatocyte growth factor; IFN, interferon; IL, interleukin; IP-10, IFN-γ inducible protein-10; MCP, monocyte chemoattractant protein; MIG, monokine induced by interferon-γ; MIP, macrophage-inflammatory protein; PDGF, platelet-derived growth factor; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TNF, tumor necrosis factor; TRAIL, TNF-related apoptosis inducing ligand; VEGF, vascular endothelial growth factor.
Then we analyzed the cytokine levels in symptomatic cases with the time since onset of symptom, and we found that most of the cytokines whose levels were associated with the severity of COVID-19 peaked at 6 to approximately 8 days (acute phase of disease) after onset (Supplementary Figure 1A). However, most of the other cytokines, except IL-1ra and MIF, did not have obvious peak time (Supplementary Figure 1B).
Comparison of Cytokine Levels between Male and Female COVID-19 Patients
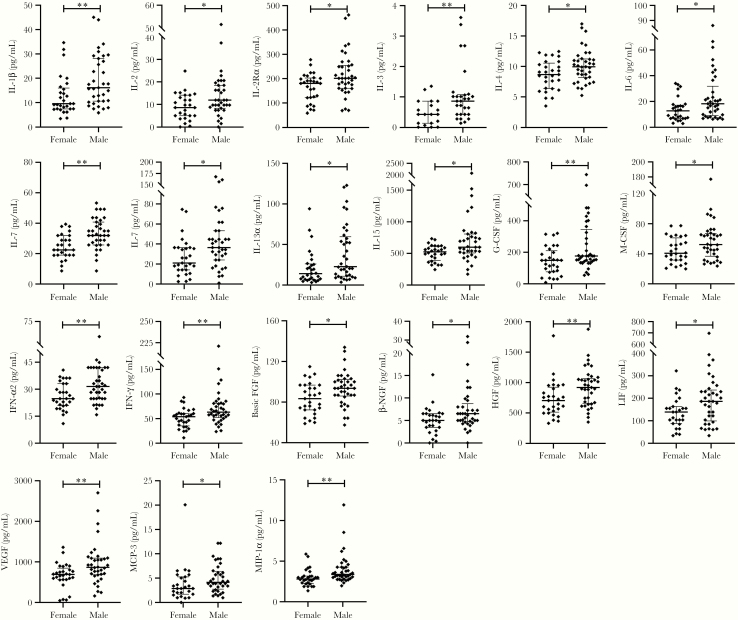
As it has been reported that COVID-19 is more likely to occur in men [7, 19], we investigate the effect of sex on the serum levels of cytokines and chemokines. The characteristics of SARS-CoV-2–infected male and female symptomatic patients are shown in Table 3. The male SARS-CoV-2–infected patients had higher serum concentrations of IL-1β, IL-2, IL-2Rα, IL-3, IL-4, IL-7, IL-8, IL-12 (p40), GM-CSF, IFN-α2, IFN-γ, basic FGF, NGF-β, HGF, LIF, VEGF, MCP-3, and MIP-1α than the female patients (Figure 2 and Supplementary Table 2).
Table 3.
Characteristics of SARS-CoV-2–Infected Male and Female Symptomatic Patients
| Characteristic | Male (n = 37) | Female (n = 29) |
|---|---|---|
| Age, y, mean ± SD | 41.78 ± 13.33 | 45.10 ± 16.46 |
| Time from onset, d, mean ± SD | 4.76 ± 4.20 | 3.97 ± 2.21 |
| Disease severity | ||
| Mild, No. (%) | 13 (35) | 9 (31) |
| Moderate, No. (%) | 19 (51) | 17 (59) |
| Severe, No. (%) | 5 (14) | 3 (10) |
| Deaths, No. (%) | 0 | 0 |
Figure 2.
Serum cytokine and chemokine levels in SARS-CoV-2–infected symptomatic male and female patients. Data are expressed as median with 25th and 75th percentiles. *P < .05, **P < .01. Abbreviations: CSF, colony-stimulating factor; FGF, fibroblast growth factor; HGF, hepatocyte growth factor; IFN, interferon; IL, interleukin; LIF, leukemia inhibitory factor; MCP, monocyte chemoattractant protein; MIP, macrophage-inflammatory protein; NGF-β, nerve growth factor-β; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VEGF, vascular endothelial growth factor.
Viral Loads Associated with Cytokine and Chemokine Concentrations
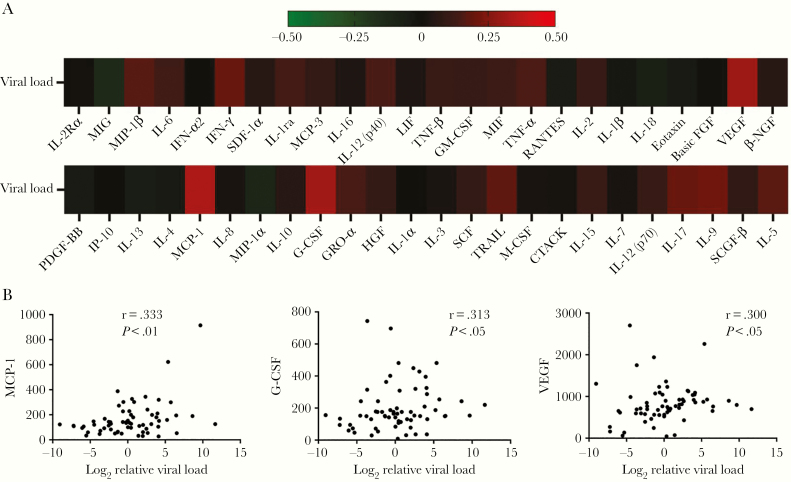
To explore the correlations between serum cytokine and chemokine levels and the viral load in SARS-CoV-2–infected patients, Spearman correlation analyses were performed. All the Spearman rank correlation coefficients are showed in a heatmap (Figure 3A). Statistical analysis demonstrated that the levels of MCP-1 (r = 0.333, P < .01), G-CSF (r = 0.245, P < .05), and VEGF (r = 0.300, P < .05) were weakly and positively correlated with the viral load (Figure 3B).
Figure 3.
Correlations between serum cytokine and chemokine levels and viral load in SARS-CoV-2–infected symptomatic patients. A, Heatmap showing the Spearman rank correlation coefficients between the levels of serum cytokines and the viral loads. The magnitude of correlation coefficients is shown on a color scale of red (strong) to black (intermediate) to green (weak) signal. B, Correlations between MCP-1, G-CSF, and VEGF levels and viral load. Abbreviations: CTACK, cutaneous T cell-attracting chemokine; FGF, fibroblast growth factor; G-CSF, granulocyte-colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor, GRO-α, growth related oncogene α; HGF, hepatocyte growth factor; IFN, interferon; IL, interleukin; IP-10, IFN-γ inducible protein-10; LIF, leukemia inhibitory factor; MCP-1, monocyte chemoattractant protein-1; MIF, migration inhibitory factor; MIG, monokine induced by interferon-γ; MIP, macrophage-inflammatory protein; NGF-β, nerve growth factor-β; PDGF, platelet-derived growth factor; r, correlation coefficient; RANTES, regulated upon activation normal T cell expressed and secreted; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SCF, stem cell factor; SCGF-β, stem cell growth factor-β; SDF-1α, stromal cell-derived factor-1α; TNF, tumor necrosis factor; TRAIL, TNF-related apoptosis-inducing ligand; VEGF, vascular endothelial growth factor.
DISCUSSION
The SARS-CoV-2 epidemic has affecting 189 countries worldwide and has been declared a public health emergency of international concern by the World Health Organization. Cytokine-mediated inflammatory responses have been associated with pulmonary inflammation and acute lung injury in SARS [14, 15], MERS-CoV [12], and in recently SARS-CoV-2 [8, 16] infection. In this study, we detected the serum levels of 48 cytokines and chemokines in a cohort of 74 patients including asymptomatic, mild, moderate, and severe cases with laboratory-confirmed COVID-19 in Jiangsu, China. Additionally, the relationship between sex, viral load, and serum cytokine and chemokine levels were explored in COVID-19 patients.
No previous studies had evaluated the serum cytokine and chemokine profiles among the asymptomatic, mild, and convalescent cases. Asymptomatic SARS-CoV-2–infected patients who had no symptoms but could still be contagious were identified [6, 20]. It is worth noting that asymptomatic and mild cases represent covert SARS-CoV-2 infections that could be seeding new outbreaks and should receive adequate attentions. Here, we found that circulating concentrations of 27 cytokines or chemokines (IL-1β, IL-1ra, IL-2, IL-2Rα, IL-6, IL-7, IL-8, IL-9, IL-10, IL-13, IL-15, IL-17, IL-18, G-CSF, M-CSF, IFN-α2, IFN-γ, TNF-α, TRAIL, basic FGF, HGF, PDGF-BB, VEGF, GRO-α, IP-10, MCP-1, and MIG) in symptomatic COVID-19 groups and 3 (IP-10, IL-10, and IL-7) in asymptomatic cases were increased compared to the healthy controls. These results indicated that IP-10, IL-10, and IL-7 may help to identify asymptomatic infections among suspected cases and close contacts. Also, we found that these 3 cytokines were of significant diagnostic value as analyzed by receiver operating characteristic curve (data not shown). The proinflammatory cytokines IL-1ra, IL-1β, IL-6, and chemokine IP-10 were significantly lower in asymptomatic cases than in symptomatic patients, suggesting these factors may be predictors of clinical symptoms. Also, in COVID-19 convalescent individuals, the serum cytokine and chemokine concentrations declined to normal levels. These results demonstrated that proinflammatory cytokines and chemokines production induced by SARS-CoV-2 were observed not only in symptomatic patients but also in the cases without any clinical symptoms, and returned to normal after recovery. These findings indicate that cytokines and chemokines may serve as “messengers” of SARS-CoV-2 infection and contribute to our understanding of the pathogenesis and outcomes throughout all stages of COVID-19.
We then examined these cytokine and chemokine levels in symptomatic patients with different disease severity. The symptomatic cases were categorized into 3 clinical groups, mild cases with slight upper respiratory tract symptoms and no radiological changes, moderate cases with pneumonia and radiological abnormalities, and severe cases with respiratory failure and in some cases death [17]. Severe COVID-19 patients had higher serum levels of IL-6, IL-7, IL-10, G-CSF, M-CSF, IP-10, MCP-1, MCP-3, MIG, and MIP-1α compared with mild cases, and higher levels of MCP-3, MIG, and MIP-1α compared with moderate cases. Moreover, the moderate COVID-19 patients had higher serum concentrations of IL-18, IP-10, and M-CSF than mild cases. Our results suggested that, in addition to IL-6, IL-7, IL-10, G-CSF, IP-10, MCP-1, and MIP-1α which have been previously reported to be associate with the severity of COVID-19 [8, 16], IL-18, MCP-3, M-CSF, and MIG might also be predictors of COVID-19 severity. These cytokines and chemokines associated with the severity of COVID-19 were mainly interleukins, chemokines, and colony stimulating factors.
As severe COVID-19 is more likely to occur in men [7, 19], we evaluated the serum cytokine and chemokine levels in female and male patients. Our results identified that SARS-CoV-2–infected male patients had significantly higher serum concentrations of IL-1β, IL-2, IL-2Rα, IL-3, IL-4, IL-7, IL-8, IL-12 (p40), GM-CSF, IFN-α2, IFN-γ, basic FGF, NGF-β, HGF, LIF, VEGF, MCP-3, and MIP-1α than female patients. These overproduced cytokine and chemokine profiles in male patients may function to clear pathogens but may also mediate destructive tissue inflammation, which may be interpreted as a reason why male COVID-19 patients are more likely to progress to severe disease. In addition, we found the serum levels of MCP-1, G-CSF, and VEGF were weakly and positively correlated with the viral titers.
The following limitations should be considered in interpretation of our findings. First, the number of subjects was limited due to the difficulty in obtaining samples from asymptomatic and convalescent cases. Second, the virus Ct values may be influenced by the low positive rate of SARS-CoV-2 RNA detection in serum and lack of quantitative viral RNA detection. Therefore, further research is needed to establish the exact involvement of viral and patient factors to verify these immunological responses. Nevertheless, this study demonstrated the circulating cytokine and chemokine profiles present at all stages of the progress of COVID-19.
In this study, we observed that SARS-CoV-2 infection was a potent inducer of proinflammatory cytokines and chemokines, which may be involved in defense against viral infections but may also mediate destructive lung injury. The abnormally elevated IP-10, IL-10, and IL-7 levels in asymptomatic cases may help to identify covert SARS-CoV-2 infections, which would be helpful to control new outbreaks. As disease progresses, an overwhelming induction of IL-6, IL-7, IL-10, IL-18, G-CSF, M-CSF, IP-10, MCP-1, MCP-3, MIG, and MIP-1α was found to be associated with the severity of COVID-19. The circulating cytokine and chemokine profiles returned to normal after recovery. Moreover, a set of cytokine and chemokine profiles were significantly higher in SARS-CoV-2–infected male than female patients. We believe that the cytokine and chemokine response pattern in different stage of the progress of COVID-19 could serve as biomarkers to evaluate the disease severity and outcome of COVID-19 patients. Furthermore, immunomodulatory treatment to regulate the cytokine responses accompanied by antiviral treatment could be an effective therapeutic strategy for SARS-CoV-2 infection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the healthy volunteers for their participation in the study; and Zhan Zhang for analytical suggestions and critical review of the manuscript.
Financial support. This work was supported by the National Natural Science Foundation of China (grant numbers 81601732, 31570926, 81871666, 31700035); Natural Science Foundation of Jiangsu Province (grant number BK20191489); the Key Research and Development Project of Jiangsu Province (grant numbers BE2019761, BE2017748); the Jiangsu provincial Medical Youth Talent (grant numbers QNRC2016537, JKRC2016018); and “The Six One” Project of Jiangsu Province (grant number LGY2017084).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Armstrong GL, MacCannell DR, Taylor J, et al. Pathogen genomics in public health. N Engl J Med 2019; 381:2569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020; 395:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 Novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ling Z, Xu X, Gan Q, et al. Asymptomatic SARS-CoV-2 infected patients with persistent negative CT findings. Eur J Radiol 2020; 126:108956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan MC, Cheung CY, Chui WH, et al. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir Res 2005; 6:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seo SH, Hoffmann E, Webster RG. Lethal H5N1 influenza viruses escape host anti-viral cytokine responses. Nat Med 2002; 8:950–4. [DOI] [PubMed] [Google Scholar]
- 11. Chi Y, Zhu Y, Wen T, et al. Cytokine and chemokine levels in patients infected with the novel avian influenza A (H7N9) virus in China. J Infect Dis 2013; 208:1962–7. [DOI] [PubMed] [Google Scholar]
- 12. Mahallawi WH, Khabour OF, Zhang Q, et al. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine 2018; 104:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu X, Zhang X, Zhao B, et al. Intensive cytokine induction in pandemic H1N1 influenza virus infection accompanied by robust production of IL-10 and IL-6. PLoS One 2011; 6:e28680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wong CK, Lam CW, Wu AK, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol 2004; 136:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Y, Li J, Zhan Y, et al. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect Immun 2004; 72:4410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China [published online ahead of print 12 March 2020]. Clin Infect Dis doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. China National Health Commission. Novel coronavirus pneumonia diagnosis and treatment guidance. Trial 5th ed.http://www.nhc.gov.cn/yzygj/s7653p/202002/d4b895337e19445f8d728fcaf1e3e13a/files/ab6bec7f93e64e7f998d802991203cd6.pdf Accessed 25 March 2020.
- 18. Ge Y, Zhao K, Qi Y, et al. Serum microRNA expression profile as a biomarker for the diagnosis of pertussis. Mol Biol Rep 2013; 40:1325–32. [DOI] [PubMed] [Google Scholar]
- 19. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med 2020; 382:1177–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.