Abstract
Background
Understanding the prevalence and clinical presentation of coronavirus disease 2019 in pediatric patients can help healthcare providers and systems prepare and respond to this emerging pandemic.
Methods
This was a retrospective case series of patients tested for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) across a pediatric healthcare network, including clinical features and outcomes of those with positive test results.
Results
Of 7256 unique children tested for SARS-CoV-2, 424 (5.8%) tested positive. Patients aged 18–21 years had the highest test positive rate (11.2%), while those aged 1–5 years had the lowest (3.9%). By race, 10.6% (226/2132) of black children tested positive vs 3.3% (117/3592) of white children. By indication for testing, 21.1% (371/1756) of patients with reported exposures or clinical symptoms tested positive vs 3.8% (53/1410) of those undergoing preprocedural or preadmission testing. Of 424 patients who tested positive for SARS-CoV-2, 182 (42.9%) had no comorbidities, 87 (20.5%) had asthma, and 55 (13.0%) were obese. Overall, 52.1% had cough, 51.2% fever, and 14.6% shortness of breath. Seventy-seven (18.2%) SARS-CoV-2–positive patients were hospitalized, of whom 24 (31.2%) required respiratory support. SARS-CoV-2-targeted antiviral therapy was given to 9 patients, and immunomodulatory therapy to 18 patients. Twelve (2.8%) SARS-CoV-2-positive patients required mechanical ventilation, and 2 patients required extracorporeal membrane oxygenation. Two patients died.
Conclusions
In this large cohort of pediatric patients tested for SARS-CoV-2, the rate of infection was low but varied by testing indication. The majority of cases were mild and few children had critical illness.
Keywords: COVID-19, coronavirus, testing, epidemiology
Originally identified in Wuhan, China, in December 2019, the novel coronavirus, known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become a global pandemic [1–3]. More than 3 million cases have been reported worldwide, with more than 200 000 deaths due to its related disease, coronavirus disease 2019 (COVID-19) [4].
While the prevalence and clinical manifestations of COVID-19 in adults have been reported in detail, our understanding of the epidemiology and outcomes of COVID-19 in children remains poorly understood. In the first detailed report of pediatric cases, mild infection was noted in the majority of 171 children infected with SARS-CoV-2 [5]. A subsequent analysis of 2143 children with laboratory-confirmed or suspected COVID-19 reported critical illness in 10% of infants, though a majority of these infant cases (293/379) were suspected rather than confirmed cases; thus, these children may have, in fact, been infected with other seasonal respiratory viruses [6]. In contrast, a systematic review of 18 published case series including 1065 children with confirmed SARS-CoV-2 infection in China and Singapore reported only 1 critically ill patient and no deaths [7], similar to reports from the first 2 weeks of the pandemic in Spain [8] and Italy [9], 2 of the most severely affected countries in Europe. In the United States, the Centers for Disease Control and Prevention (CDC) summarized 2572 pediatric COVID-19 cases reported to state health departments [10]. Hospitalization data were only available for 745 cases (29%), of whom 147 were hospitalized, 15 in the intensive care unit, with 3 deaths.
Regional differences in testing availability and strategies limit our understanding of the prevalence and clinical presentation of COVID-19, particularly in children. Most reports lack the denominator of patients tested and/or describe cohorts for which testing was restricted to patients with more severe disease. At our institution, a laboratory-derived assay was developed early in the pandemic, and testing capacity was rapidly expanded. This allowed for liberalization of testing criteria to include all admitted patients and all patients undergoing procedures regardless of symptomatology or exposure history. To help understand the epidemiology and outcomes of COVID-19 in pediatric patients, we describe results from the first 12 weeks of SARS-CoV-2 testing, including positive test rates and the clinical features and outcomes of all positive cases among children who presented to a large, integrated pediatric healthcare network.
METHODS
Study Design and Setting
This retrospective case series describes the epidemiology of pediatric SARS-CoV-2 infection across the Children’s Hospital of Philadelphia (CHOP) Care Network. The CHOP Care Network is located in southeastern Pennsylvania and southern New Jersey. It includes a 580-bed acute care hospital; there are more than 30 000 admissions and 100 000 emergency department (ED) encounters annually. The ambulatory care network includes 31 primary care practices, 4 urgent care centers, 10 specialty care centers, and 3 ambulatory surgical facilities with more than 1 million outpatient encounters per year. All sites use a common electronic health record (EHR; Epic Systems, Verona, WI). This study was approved by the CHOP Institutional Review Board.
Test Characteristics
The Infectious Disease Diagnostics Laboratory at CHOP developed a real-time TaqMan polymerase chain reaction (PCR) assay intended for qualitative detection of RNA from SARS-CoV-2 in respiratory specimens. The assay consists of a primer/probe set that amplifies and detects the N2 gene of the SARS-CoV-2 virus multiplexed with a primer/probe set that amplifies and detects the human β-actin gene as an internal control. The sequences for the N2 primers and probe used in this assay are identical to the N2 primer/probe sequences used in the SARS-CoV-2 molecular assay developed by the CDC and authorized by the US Food and Drug Administration [11]. The limit of detection for this assay is approximately 20 000 copies/mL, and the specificity for the virus was determined to be 100% through wet testing against common respiratory pathogens and in silico analysis.
The Xpert Xpress SARS-CoV-2 assay from Cepheid Inc. (Sunnyvale, CA) was also used in the CHOP Infectious Disease Diagnostics Laboratory. This test targets the E and N2 genes of SARS-CoV-2, contains an exogenous processing control, and has a limit of detection of 0.01 plaque-forming units/mL [12].
Network clinicians could also order SARS-CoV-2 PCR testing through commercial laboratories, including Laboratory Corporation of America (Burlington, NC) and Quest Diagnostics (Secaucus, NJ). The LabCorp assay detects the N1, N2, and N3 genes of SARS-CoV-2 as well as RNase P as an internal control [13]. It has a limit of detection of 6250 copies/mL. The Quest assay targets the N1 and N3 genes and has an exogenous RNA amplification control [14]. It has a stated limit of detection of 136 copies/mL.
Testing Environment
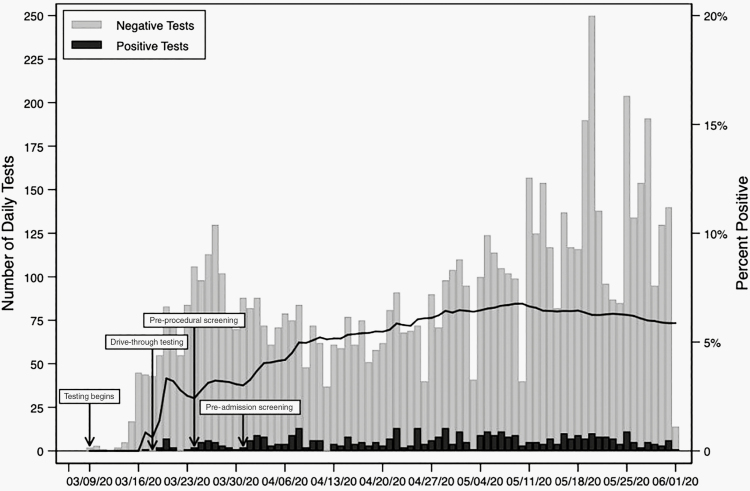
Hospital-based SARS-CoV-2 PCR testing began on 9 March 2020, with the first positive test on 16 March. Drive-through testing (all of which were sent to and tested by the CHOP laboratory) for ambulatory patients began on 18 March, followed by preprocedural testing (for all children undergoing aerosol-generating procedures) on 24 March and preadmission testing (for all admitted patients) on 1 April. Prior to 24 March, testing was selectively performed on patients with 1 or more symptoms suggestive of SARS-CoV-2 infection and/or with a high-risk exposure, including contact with a known or suspected SARS-CoV-2–positive individual or residence in or travel to a high-prevalence geographic region. After 1 April, the indication for testing was recorded in the EHR for tests performed in the ED, main hospital, and primary care/urgent care sites. Indication for testing was not recorded for drive-through tests or tests sent to commercial laboratories. After 23 April, rapid testing using the Xpert Xpress SARS-CoV-2 assay was also performed in the Infectious Disease Diagnostics Laboratory to allow for rapid preprocedural or preadmission testing.
Study Cohort
EHR data were extracted weekly for all patients aged ≤21 years with a valid test result for a PCR test for SARS-CoV-2 from 9 March 2020 through 1 June 2020. Patients were only enrolled once in the cohort. Parent/caregiver and adult household contacts of positive patients, exposed healthcare workers, and pregnant women followed at CHOP were excluded.
Data Collection
Once patients were identified for inclusion in the cohort, test characteristics, demographic data, and the indication for testing were extracted from the EHR for all tested patients. Manual chart review was then performed for all patients who tested positive for SARS-CoV-2 to obtain data on exposures, comorbidities, symptomatology, clinical severity, and treatment information. Patients were considered to have a potential exposure to SARS-CoV-2 if they reported a household contact or nonhousehold close contact with confirmed or suspected SARS-CoV-2 infection, contact with a healthcare worker or other patient with confirmed SARS-CoV-2 infection, residence in a long-term care or behavioral health residential facility where there were confirmed positive cases in patients or staff, or residence in or travel to an area with high levels of local transmission.
Data Analyses
Descriptive statistical analyses were performed using STATA 15.0 (StataCorp LP, College Station, TX).
RESULTS
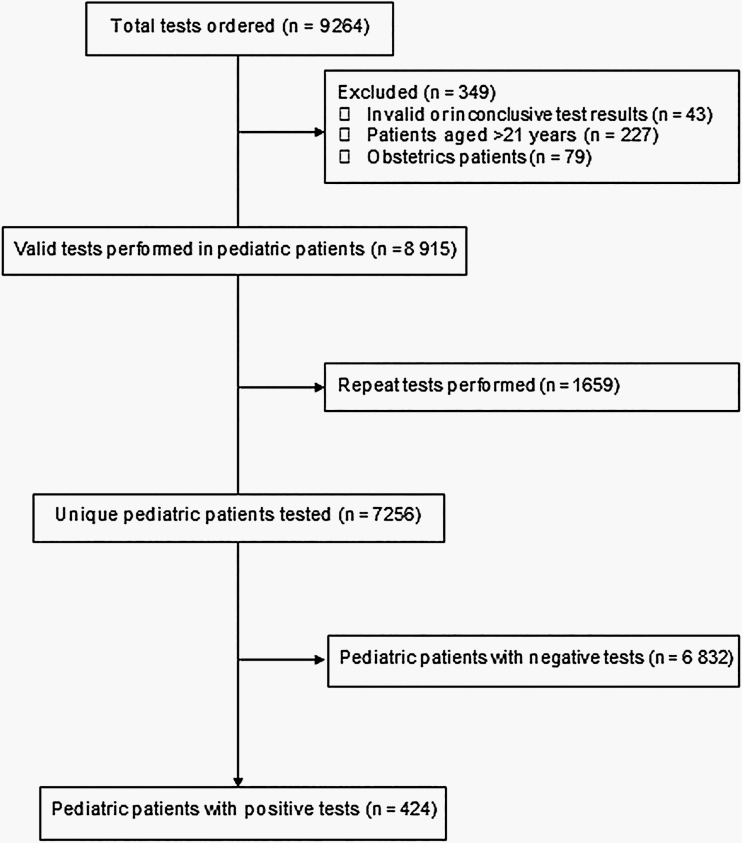
More than 9000 SARS-CoV-2 tests were ordered for network patients during the study period. After excluding tests with invalid results, tests performed on patients aged >21 years, and repeated tests in the same patients, a cohort of 7256 unique pediatric patients was identified (Figure 1). Overall, 6488/7256 (89.4%) patients were tested by the CHOP Infectious Disease Diagnostics Laboratory using either the laboratory-developed PCR or the rapid commercial test, with the remaining (768/7256, 10.6%) performed by commercial laboratories. The majority of tests were performed at the drive-through testing sites (2846/7256, 39.2%) and the ED (2311/7256, 31.8%), followed by the outpatient clinics (1108/7256, 15.3%), inpatient hospital (759/7256, 10.5%), and urgent care centers (232/7256, 3.2%). Nasopharyngeal (NP) swab or aspirate samples were collected for testing in 6961/7256 (95.9%) children; <1% of samples were from the oropharynx or lower respiratory tract, and specimen source was not specified for 3.4% of children.
Figure 1.
Flow chart of the study population.
A total of 424 of 7256 (5.8%) patients tested positive for SARS-CoV-2 during the study period (Figure 2). The median age of tested children was 5.9 years (interquartile range [IQR], 1.7–13.2; Table 1). Patients aged between 18 and 21 years had the highest test positive rate (11.2%), while those aged between 1 and 5 years had the lowest (3.9%). By race, 10.6% (226/2132) of black children tested positive vs 3.3% (117/3592) of white children. Patients with commercial insurance tested positive in 132/3903 tests (3.4%), while 265/2847 (9.3%) tests in patients with governmental or public insurance were positive. When the indication for testing was specified, tests performed for a potential exposure or concerning symptoms were positive in 21.1% (371/1756) of instances compared with 3.8% (53/1410) of tests performed for preprocedural or preadmission testing. All patients with positive test results had a documented indication for testing.
Figure 2.
Severe acute respiratory syndrome coronavirus 2 testing experience at the Children’s Hospital of Philadelphia during the study period 9 March 2020 through 1 June 2020.
Table 1.
Demographic Characteristics of All Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Testing Through 1 June 2020
| Characteristic | Total Tests | Positive Tests | Percent Positive (%) |
|---|---|---|---|
| Number, n | 7256 | 424 | 5.8 |
| Age, median (interquartile range), y | 5.9 (1.7–13.2) | 10.0 (2.6–15.6) | – |
| Age group | |||
| 0–12 months, n (%) | 1193 (16.4) | 70 (16.5) | 5.9 |
| 1–5 years, n (%) | 2456 (33.8) | 96 (22.6) | 3.9 |
| 6–11 years, n (%) | 1535 (21.2) | 77 (18.2) | 5.0 |
| 12–17 years, n (%) | 1651 (22.8) | 134 (31.6) | 8.1 |
| 18–21 years, n (%) | 421 (5.8) | 47 (11.1) | 11.2 |
| Male sex, n (%) | 3929 (54.3) | 215 (50.7) | 5.5 |
| Race | |||
| White, n (%) | 3592 (49.8) | 117 (27.6) | 3.3 |
| Black or African American, n (%) | 2132 (29.6) | 226 (53.3) | 10.6 |
| Asian or Asian Indian, n (%) | 230 (3.2) | 9 (2.1) | 3.9 |
| Multiracial, n (%) | 260 (3.6) | 10 (2.4) | 3.8 |
| Other race or unknown, n (%) | 997 (13.8) | 62 (14.6) | 6.2 |
| Ethnicity | |||
| Not Hispanic or Latino, n (%) | 6373 (87.8) | 364 (85.8) | 5.7 |
| Hispanic or Latino, n (%) | 739 (10.2) | 55 (13.0) | 7.4 |
| Not specified, n (%) | 144 (2.0) | 5 (1.2) | 3.5 |
| Insurance status | |||
| Commercial insurance, n (%) | 3903 (53.8) | 132 (31.1) | 3.4 |
| Government or public insurance, n (%) | 2847 (39.2) | 265 (62.5) | 9.3 |
| Self-pay, n (%) | 88 (1.2) | 10 (2.4) | 11.4 |
| Other or unknown, n (%) | 418 (5.8) | 17 (4.0) | 4.1 |
| Primary care network patient, n (%) | 3749 (51.7) | 272 (64.2) | 7.3 |
| Reason for testinga | |||
| Indication not specified, n (%)b | 4090 (56.4) | 0 (0.0) | 0.0 |
| Prior exposure or symptomatic patient, n (%)c | 1756 (24.2) | 371 (87.5) | 21.1 |
| Preprocedure or preadmission testing, n (%) | 1410 (19.4) | 53 (12.5) | 3.8 |
aPrior to 24 March, all testing was performed for those with clinical indication. After 1 April, the indication for testing performed in the emergency department and hospital was recorded in the electronic health record.
bDrive-through tests, tests at the ambulatory sites, and tests at commercial laboratories do not carry an indication for testing.
cIncludes testing of symptomatic patients and those with an exposure
Of SARS-CoV-2–positive patients, 42.9% (182/424) had no known comorbid medical conditions (Supplementary Table 1). Asthma (87/424, 20.5%), obesity (55/424, 13.0%), and mental health disorders (38/424, 9.0%) were the most prevalent comorbid conditions. Malignancy or other immunocompromising state (14/424, 3.3%), hypertension (3/424, 0.7%), or diabetes mellitus (5/424; 1.2%) were noted infrequently. Of symptoms reported at the time of testing (Table 2, Supplementary Table 2), 52.1% (221/42) had cough, 51.2% (217/424) fever, and 14.6% (62/424) shortness of breath; however, 25.0% (106/424) of SARS-CoV-2–positive patients reported no fever, cough, or shortness of breath. Of patients with positive tests, 54/424 (12.7%) were asymptomatic at the time of testing for SARS-CoV-2.
Table 2.
Reported Symptoms at Time of Testing for Patients With a Positive Severe Acute Respiratory Syndrome Coronavirus 2 Test
| Symptom | Total (n = 424) |
|---|---|
| Fever or cough or shortness of breath, n (%)a | 318 (75.0) |
| Fever, n (%) | 217 (51.2) |
| Cough, n (%) | 221 (52.1) |
| Shortness of breath, n (%) | 62 (14.6) |
| Congestion or rhinorrhea, n (%) | 133 (31.4) |
| Headache, n (%) | 76 (17.9) |
| Gastrointestinal symptoms, n (%)b | 74 (17.5) |
| Sore throat, n (%) | 59 (13.9) |
| Myalgias, n (%) | 57 (13.4) |
| Fatigue, n (%) | 26 (6.1) |
| Ageusia, n (%) | 24 (5.7) |
| Anosmia, n (%) | 24 (5.7) |
| Chest pain, n (%) | 24 (5.7) |
| Chills, n (%) | 16 (3.8) |
| Asymptomatic, n (%) | 54 (12.7) |
aShortness of breath includes any report of dyspnea, as well as the presence of retractions or tachypnea in children too young to report shortness of breath.
bIncludes abdominal pain, nausea, vomiting, or diarrhea.
Overall, 274/424 (64.6%) patients with positive SARS-CoV-2 tests were considered to have a potential exposure. This included 188/424 (44.3%) patients with a household contact with confirmed or suspected SARS-CoV-2 infection and 44/424 (10.4%) patients with a nonhousehold contact. Contact with a healthcare worker with SARS-CoV-2 infection was reported in 6/424 (1.4%) positives, all providers were outside of the care network. Residing in or visiting an area with widespread local SARS-CoV-2 transmission was identified in 42/424 (9.9%) positive cases, and 20 (4.7%) cases had been exposed to a congregant living facility (inpatient psychiatric facility, juvenile detention center, homeless shelter). White patients reported a potential exposure in 79/117 cases (67.5%) compared with 147/226 (65.0%) black patients. A potential exposure was reported in approximately half (25/54, 46.3%) of asymptomatic patients.
Overall, 77/424 (18.2%) SARS-CoV-2–positive patients were hospitalized. COVID-19, however, was not considered the primary reason for hospitalization in 26/77 (33.8%) of these patients (Supplementary Table 3). Only 24/424 (5.7%) patients required any respiratory support (Table 3). SARS-CoV-2–targeted antiviral therapy was given to 9 patients, and immunomodulatory therapy to treat COVID-19 was provided to 18 patients. Of note, 8 patients received intravenous immunoglobulin to treat a clinical condition compatible with multisystem inflammatory disease in children (MIS-C) [15]. Decisions regarding the administration of specific treatment modalities were guided by recently published recommendations [16, 17]. Twelve SARS-CoV-2–positive patients developed critical illness that required mechanical ventilation; their ages ranged from 2 months to 18 years.
Table 3.
Characteristics, Clinical Course, and Severity of Hospitalized Patients With a Positive Severe Acute Respiratory Syndrome Coronavirus 2 Test
| Characteristics | Patients (n = 77) |
|---|---|
| Documented comorbidities, n (%) | 49 (64) |
| Age, median (IQR), y | 9.9 (1.1–15.4) |
| Race | |
| White, n (%) | 28 (36.4) |
| Black or African American, n (%) | 32 (41.6) |
| Asian or Asian Indian, n (%) | 2 (2.6) |
| Multiracial, n (%) | 0 (0.0) |
| Other race or unknown, n (%) | 15 (19.5) |
| Length of stay, median (IQR),a days | 4 (1–11) |
| Need for intensive care, n (%) | 25 (32.9) |
| Need for respiratory support | |
| None, n (%) | 53 (68.8) |
| Supplemental oxygen, n (%) | 3 (3.9) |
| High-flow nasal cannula, n (%) | 5 (6.5) |
| Noninvasive ventilation, n (%) | 4 (5.2) |
| Mechanical ventilation, n (%) | 12 (15.6) |
| Extracorporeal membrane oxygenation, n (%)b | 2 (2.6) |
| Vasopressor support, n (%) | 13 (16.9) |
| Received severe acute respiratory syndrome coronavirus 2–directed therapy | |
| Remdesivir, n (%) | 6 (7.8) |
| Hydroxychloroquine, n (%) | 3 (3.9) |
| Azithromycin, n (%) | 1 (1.3) |
| Lopinavir/ritonavir, n (%) | 0 (0.0) |
| Received immunomodulatory therapy | |
| Steroids, n (%) | 15 (19.5) |
| Convalescent serum, n (%) | 4 (5.2) |
| Tocilizumab, n (%) | 3 (3.9) |
| Other immunomodulators, n (%)c | 8 (10.4) |
| Death, n (%) | 2 (2.6) |
Abbreviation: IQR, interquartile range.
aIncludes only patients who have been discharged from the hospital through 30 April 2020.
bThe patient who required extracorporeal membrane oxygenation cannulation also required mechanical ventilation.
cIncluding intravenous immunoglobulin and interleukin-1 blockade.
Two patients died during this study. One patient with multiply-relapsed leukemia developed gram-negative bacteremia and septic shock and subsequently died. It was noted during intubation that that patient’s respiratory status was perhaps worse than would be expected with septic shock, but the role of SARS-CoV-2 infection in the patient’s death has not been clearly defined. The second patient, an 18-year-old with obesity, hypertrophic obstructive cardiomyopathy, hypertension, and type 2 diabetes mellitus developed acute respiratory distress syndrome (ARDS) and myocarditis, required extracorporeal membrane oxygenation, and ultimately died of a malignant arrhythmia.
DISCUSSION
From 9 March 2020 through 1 June 2020, more than 9000 SARS-CoV-2 tests were performed on children across the largest pediatric healthcare network in the United States. Although approximately 6% of children tested positive, rates varied by testing indication. The majority of cases were mild, and only 77 patients were hospitalized. Few children had critical illness. Two deaths occurred; 1 in a patient with a life-limiting underlying condition and gram-negative bacteremia at the time of death and another in a woman with multiple comorbidities who developed ARDS and myocarditis. These data are consistent with prior reports that suggested a lower frequency of infection and severity of COVID-19 among children compared with adults.
Although the epidemiology of SARS-CoV-2 infection in children remains incompletely described, some features are emerging. Most notably, infection with SARS-CoV-2 has been identified less frequently in children than in adults. Children accounted for 2% of infections in a recent report from China [18]. In the United States, children accounted for 1.7% of cases despite making up 22% of the population [10]. These reports, however, were not based on population-based sampling and, therefore, could be biased by local approaches to testing and/or a generally milder clinical presentation in children.
The largest population-based data, including children from Iceland and Korea, suggest lower rates of infection in children compared with adults at times of moderate COVID-19 community transmission [19, 20]. However, screening took place after social distancing policies were put in place, including school closures. In contrast, in a recent report of the epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China, there was no difference seen in attack rates in young children compared with older adults [21]. Population-based surveillance using highly specific serologic testing is needed to better establish the prevalence and transmission dynamics of SARS-CoV-2.
Of those with confirmed SARS-CoV-2 infection, clinical outcomes appear to be less severe in children than adults. In our cohort, although 18.2% of patients tested were hospitalized, one-third of these patients were hospitalized for another indication. Only 12/424 patients (2.8%; 15.6% of those hospitalized) required mechanical ventilation. In contrast, in a cohort of adults from a large, integrated healthcare network in northern California, 29% of SARS-CoV-2–positive patients required inpatient care and 7% (29% of those hospitalized) received invasive ventilation [22]. Similarly, of 5700 SARS-CoV-2–infected patients in metropolitan New York, 21% of hospitalized patients died, but there were no reported deaths in children [23].
A clinical condition known as multisystem inflammatory syndrome in children (MIS-C) has been associated with SARS-CoV-2 infection and has been postulated to be a post-infectious inflammatory condition [24]. Notably, this condition has been reported in patients with negative SARS-CoV-2 PCR assays [25], including several patients at our institution [26]. Entry into this patient cohort requires a positive PCR assay; therefore, any description of the epidemiology of MIS-C using this patient cohort would be incomplete.
These data revealed concerning racial and socioeconomic differences in SARS-CoV-2–positive patients, even when accounting for the number tested in each racial group. Specifically, while only 3.3% of white children tested for SARS-CoV-2 were positive, 10.6% of black children tested were positive. Although unadjusted, these estimates are consistent with prior reports in US adults, suggestive of a disparity in the attack rate of SARS-CoV-2 [27]. Similarly, a higher proportion of patients with government or public insurance tested positive than those with commercial insurance. Further research is urgently needed to explore the social determinants of health driving these disparities.
This study has important strengths. The early development, validation, and high capacity of SARS-CoV-2 testing enabled us to develop a relatively early snapshot of the epidemic as it emerged in this region. While most available data have focused on adults, the present study leverages an integrated pediatric healthcare network linked by a common EHR with more than 1 million outpatient visits, 100 000 ED visits, and 30 000 inpatient admissions annually to describe the epidemiology of SARS-CoV-2 infection in children. Furthermore, the network serves a racially and socioeconomically diverse population across urban, suburban, and rural southeastern Pennsylvania and southern New Jersey.
This study has notable limitations. We did not conduct population-based sampling, and our cohort represents a composite of symptomatic/exposed patients tested from ambulatory sites, in the ED, or while hospitalized, as well as asymptomatic patients screened per hospital infection-prevention protocol. Despite its breadth and diversity, this pediatric healthcare network might not represent childhood infection with SARS-CoV-2 in other regions. Because patient follow-up to assess outcomes depended on documentation of encounters in the EHR, we could not collect data on patients who sought care outside of this network. Although the test characteristics of our laboratory’s validated SARS-CoV-2 PCR assay are robust, variability in NP sample collection can impact clinical sensitivity, and additional sites (lower respiratory tract, stool) were rarely used for the initial SARS-CoV-2 test specimen. Additionally, our ability to specifically characterize potential exposures is limited to what was elicited by treating clinicians and documented in the chart, as this was a retrospective chart review and not prospective contact tracing. Similarly, our classification of testing indication was limited by the data present in the EHR, and we were not able to identify testing indication for patients with negative SARS-CoV-2 tests prior to 1 April (when testing indication was added to the EHR) or in outpatients tested at drive-through locations. Therefore, the percent positive rates by testing indication are most likely an overestimation. Last, as inclusion in our cohort required a positive SARS-CoV-2 PCR test, we were not able to assess the prevalence of MIS-C or accurately describe the clinical epidemiology of that condition, which has been associated with SARS-CoV-2 infection.
In conclusion, this large cohort of pediatric patients tested for SARS-CoV-2 revealed that the overall frequency of infection was low but varied by testing indication and patient demographics. Most children with SARS-CoV-2 infection were asymptomatic or only mildly ill, and few children required intensive care. Two patients died with SARS-CoV-2 infection. These data build on data from prior reports, mostly smaller cohorts from other countries, suggesting a lower prevalence and severity of COVID-19 in children compared with adults.
Supplementary Material
Notes
Acknowledgments. We are grateful for the incredible work of the entire healthcare team at Children’s Hospital of Philadelphia, in particular, the members of the Department of Infection Prevention and Control and the Infectious Diseases Diagnostics Laboratory.
Financial support. This work was supported, in part, by a Eunice Kennedy Shriver National Institute of Child Health and Human Development grant to W. R. O. (T32 HD060550-10), a National Children’s Research Centre, Dublin, Ireland Clinical Research Fellowship to S. G. (D/19/6), and a National Institute of General Medical Science T32 grant to L. C. P. (T32 GM75766-13).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382: 727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. Coronavirus disease (COVID-19) pandemic. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed May 1, 2020. [Google Scholar]
- 5. Lu X, Zhang L, Du H, et al. SARS-CoV-2 infection in children. N Engl J Med 2020; 382: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics 2020; 145(6):e20200702. [DOI] [PubMed] [Google Scholar]
- 7. Castagnoli R, Votto M, Licari A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr 2020. In press. [DOI] [PubMed] [Google Scholar]
- 8. Tagarro A, Epalza C, Santos M, et al. Screening and severity of coronavirus disease 2019 (COVID-19) in children in Madrid, Spain. JAMA Pediatr 2020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parri N, Lenge M, Buonsenso D, Coronavirus Infection in Pediatric Emergency Departments Research Group Children with Covid-19 in pediatric emergency departments in Italy. N Engl J Med 2020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. CDC COVID-Response Team. Coronavirus disease 2019 in Children—United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 422–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. CHOP Infectious Diseases Diagnostics Laboratory. Children’s Hospital of Philadelphia (CHOP) SARS-CoV-2 RT-PCR test. U.S. Food and Drug Administration (FDA) accelerated emergency use authorization (EUA) summary. Available at: https://www.fda.gov/media/136656/download. Accessed 1 May 2020. [Google Scholar]
- 12. Cepheid Inc. Xpert Xpress SARS-CoV-2 test. U.S. Food and Drug Administration (FDA) accelerated emergency use authorization (EUA) instructions for use (IFU). Available at: https://www.fda.gov/media/136314/download. Accessed May 1, 2020. [Google Scholar]
- 13. Laboratory Corporation of America. COVID-19 RT-PCR test (LabCorp Laboratory Test Number: 139900). U.S. Food and Drug Administration (FDA) accelerated emergency use authorization (EUA) summary. Available at: https://www.fda.gov/media/136151/download. Accessed May 1, 2020. [Google Scholar]
- 14. Quest Diagnostics. SARS-CoV-2 RNA, qualitative real-time RT-PCR (test code 39433). U.S. Food and Drug Administration (FDA) accelerated emergency use authorization (EUA) package insert. Available at: https://www.fda.gov/media/136231/download. Accessed May 1, 2020. [Google Scholar]
- 15. Centers for Disease Control and Prevention. Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). Available at: https://www.cdc.gov/mis-c/hcp/. Accessed June 2, 2020. [Google Scholar]
- 16. Chiotos K, Hayes M, Kimberlin DW, et al. Multicenter initial guidance on use of antivirals for children with COVID-19/SARS-CoV-2. J Pediatric Infect Dis Soc 2020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis 2020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020. In press. [DOI] [PubMed] [Google Scholar]
- 19. Gudbjartsson DF, Helgason A, Jonsson H, et al. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med 2020; 382(24):2302–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Covid-19 National Emergency Response Center, Epidemiology, Case Management Team, Korea Centers for Disease Control Prevention. Coronavirus disease-19: the first 7755 cases in the Republic of Korea. Osong Public Health Res Perspect 2020; 11: 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bi Q, Wu Y, Mei S, et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis 2020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA 2020; 323(21):2195–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323(20):2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mahase E. Covid-19: concerns grow over inflammatory syndrome emerging in children. BMJ 2020; 369:m1710. [DOI] [PubMed] [Google Scholar]
- 25. Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020; 395:1607–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chiotos K, Bassiri H, Behrens EM, et al. Multisystem inflammatory syndrome in children during the COVID-19 pandemic: a case series. J Pediatric Infect Dis Soc 2020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yancy CW. COVID-19 and African Americans. JAMA 2020; 323(19):1891–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.