Abstract
Background
Hong Kong (HK) is a densely populated city near the epicentre of the coronavirus disease 2019 (COVID-19) outbreak. Stringent border control together with aggressive case finding, contact tracing, social distancing and quarantine measures were implemented to halt the importation and spread of the virus.
Methods
We performed an epidemiological study using government information covering the first 100 confirmed cases to examine the epidemic curve, incidence, clusters, reproduction number (Rt), incubation period and time to containment.
Results
A total of 93 of the 100 cases were HK residents (6 infected in Mainland China, 10 on the Diamond Princess Cruise). Seven were visitors infected in Mainland China before entering HK. The majority (76%) were aged ≥45 years, and the incidence increased with age (P < 0.001). Escalation of border control measures correlated with a decrease in the proportion (62.5% to 0%) of cases imported from Mainland China, and a reduction in Rt (1.07 to 0.75). The median incubation period was 4.2 days [95% confidence interval (CI), 4.0–4.5; 5th and 95th percentiles: 1.3 and 14.0). Most clusters with identifiable epidemiological links were households involving 2–4 people. Three medium-spreading events were identified: two from New Year gatherings (6–11 people), and another from environmental contamination of a worship hall (12 people). Despite intensified contact tracing, containment was delayed in 78.9% of cases (mean = 5.96 days, range = 0–24 days). An unusual transmission in a multi-storey building via faulty toilet plumbing was suspected with >100 residents evacuated overnight. Our analysis indicated that faulty plumbing was unlikely to be the source of this transmission.
Conclusions
Timely stringent containment policies minimized the importation and transmission of COVID-19 in HK.
Keywords: COVID-19, novel coronavirus, containment policy, epidemic curve, social distancing measures
Key Messages
Few studies have examined the epidemiological characteristics of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) in areas with stringent containment strategies.
Stringent border control measures implemented early in a city near the epicentre correlated with a drastic decrease in imported cases.
Social distancing measures including school closures and working from home, together with intensified case finding and contract tracing, enhanced surveillance and diagnostic services were able to break community transmission and curtail the reproductive rate of this novel pathogen to which the human population has naïve immunity.
Stringent all-round containment measures should be implemented in a timely fashion to restrict transmission and reduce disease burden.
Introduction
The severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) have caused epidemics with high mortality.1 SARS was first reported in Guangdong, China, giving rise to 8098 cases of human infection and 774 fatalities in 2003.2 MERS-CoV was first identified in Saudi Arabia and is responsible for 2494 human infections and 858 deaths since 2012.3
Cases of pneumonia of unknown aetiology were reported in Wuhan, China from 8 Dec 2019.4 The pathogen was subsequently identified as a novel coronavirus (SARS-CoV-2) by the Chinese Centre for Disease Control and Prevention. On 30 January, the World Health Organization (WHO) declared the SARS-CoV-2 outbreak a Public Health Emergency of International Concern.4 On 11 March, WHO declared coronavirus disease 2019 (COVID-19) a pandemic.5
Hong Kong (HK) is one of the most densely populated cities in the world.6 HK faces a high risk of imported COVID-19 cases due to its geographic proximity to and high cross-border flows with Mainland China. In 2003, HK was hard hit by SARS, resulting in 1755 infected cases and 299 deaths.2 Several super-spreading events occurred in the community, including at the Hotel Metropole and Amoy Gardens.7 The cluster in Amoy Gardens (a multi-storey building estate) highlighted the possibility of widespread dissemination via faulty sewage drainage. The HK Government implemented a comprehensive and coordinated approach to contain the spread of COVID-19.8–10 The key measures included: (i) emergency arrangements according to a Preparedness and Response Plan that stipulated the Government’s actions against novel infectious diseases, (ii) mandatory quarantine for people at risk of carrying the infection, (iii) promoting ‘social distancing’ including a work-from-home policy and school closure, (iv) implementing border control, (v) increasing the supply of surgical masks, and (vi) transparent communication with the public.11 The aims of this study were to describe the epidemiological characteristics of the first 100 confirmed COVID-19 cases in HK and to assess the effectiveness of the implemented policies. These findings will guide implementation of public health control measures in other metropolitan areas.
Methodology
Source of data
A retrospective observational study of the first 100 laboratory-confirmed COVID-19 cases was performed. In HK, all cases were diagnosed by reverse transcription polymerase chain reaction (RT-PCR) assays targeting two different regions of the RdRp gene in SARS-CoV-2. These assays were independently performed by local hospitals and the Public Health Laboratory. Environmental sampling of the Buddhist worship hall was conducted by the outbreak investigation team of the Centre for Health Protection (CHP), and the detection of SARS-CoV-2 RNA using RT-PCR was performed by the Public Health Laboratory. A patient database was constructed based on information provided by the CHP of the HK Government Department of Health.12–14 We analysed demographic information including the source of infection (whether locally acquired or imported), time from exposure to symptom onset, time to first healthcare consultation and isolation, and method of case identification. We used mid-2019 population census data provided by the HK Census and Statistics Department to analyse sex- and age-specific incidence rates of SARS-CoV-2 infection.
Data analysis
Sex–age-specific incidence
The incidence rate of COVID-19 stratified by age (≤24; 25–44; 45–64; ≥65 years) and gender was computed by the number of infected individuals divided by the population in each subgroup. Chi-square tests and Fisher’s exact tests were used to compare incidence rates according to age and gender.
Incubation period
Exposure and illness onset dates of each case were used to determine the incubation period. To estimate the length of incubation, we fitted parametric models including log-normal, Weibull, and gamma distributions to account for censored information.15 Model fitness between distributions was compared using the Akaike information criterion (AIC). We followed the method of Cowling et al. to calculate the 95% confidence interval (CI) using a parametric bootstrapping method with 1000 resamples over 100 iterations.15 The posterior percentiles and their corresponding CIs are presented.
Reproduction number
A time-varying reproduction number (Rt), defined as the average number of secondary infections generated by a single case in a population at time t, is a parameter for assessing whether control measures are sufficient to interrupt local disease transmission.16–18 If Rt is below unity, an epidemic may be regarded as being under control at time t. Because COVID-19 was seeded by imported cases in HK, we employed estimation equations to account for a risk from imported cases as previously described (see the Supplementary Appendix, available as Supplementary data at IJE online for technical details).19–21 By adapting an early local estimate,22 a lognormal-distributed serial interval of 4.4 days with a standard deviation (SD) of 3 days was assumed in the estimation. The median Rt and corresponding 95% CIs across different epidemic periods were computed.
Time to containment
In this analysis, ‘time to containment’ was defined as the interval in days between the date of symptom onset and the date of isolation of individual patients. We considered this as a continuous outcome variable in a multiple linear regression analysis. We controlled for age (in four age strata: 0–24, 25–44, 45–64 and ≥65 years), gender, source of infection (local vs imported), case identification (self vs by others) and whether the patient had attended any healthcare services before admission to hospital. In addition, we also controlled for the date of confirmation with reference to the first case, as we wanted to understand whether time to containment could be increased or decreased as the outbreak progressed. Bivariate analyses were first performed, and covariates with P < 0.20 were included in the multivariate regression analysis.
Results
Socio-demographic characteristics of patients
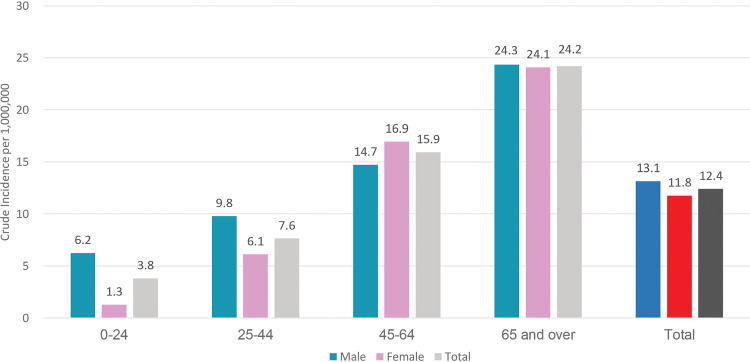
Among the 100 cases examined, 93 were local residents and the remaining 7 were from Mainland China. Most patients were aged between 45 and 64 years (40.9%) and ≥65 years (34.4%). There was no gender difference (Figure 1; Supplementary Table 1, available as Supplementary data at IJE online), whereas a marked age difference was observed (chi-square tests for trend <0.001). At the time of writing, 91 patients had been discharged, five were still hospitalized and four had died. A pet dog of a confirmed case had five consecutive nasal and oral swabs which tested positive for SARS-CoV-2 RNA, and a serological response was demonstrated.23
Figure 1.
Crude incidence rate by age and sex of the first 100 persons with confirmed COVID-19 in Hong Kong Special Administrative Region, China. Crude incidence rate per 1 000 000 population by age and sex. The age groups of 45–64 years and ≥65 years have higher crude incidence than the overall population.
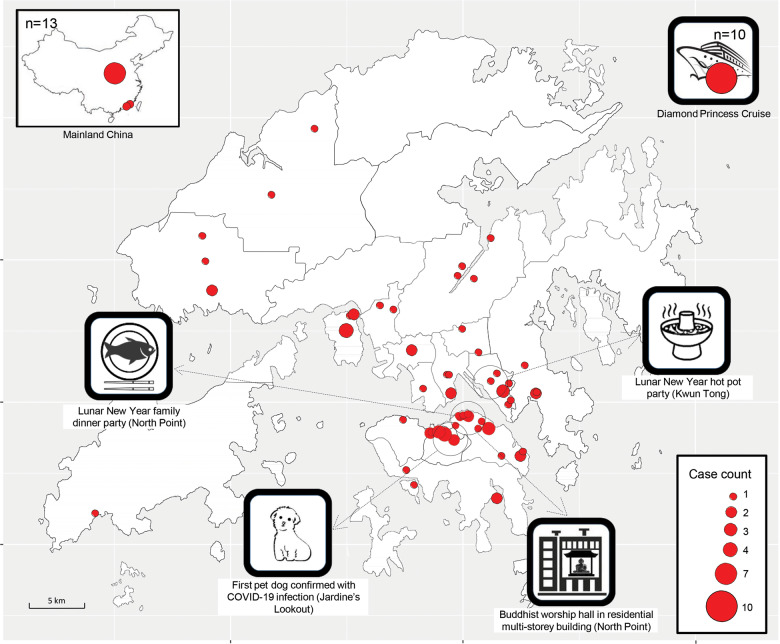
The geographical distribution of subjects is shown in Figure 2. Ten patients acquired infection from the Princess Diamond Cruise and 13 from Mainland China. The remaining 77 were local or ‘possibly local’ where patients had a travel history, but this was deemed unlikely as a source of infection according to the CHP investigation team.
Figure 2.
Geographical distribution of the first 100 persons with confirmed COVID-19 in Hong Kong Special Administrative Region, China. Geographic distribution of confirmed COVID-19 cases by residential address. The size of the circles is proportional to case counts. Nine cases were imported from Mainland China with the majority from Wuhan, Hubei. Ten cases were imported from the Diamond Princess Cruise ship. Dotted lines represent areas with special events. Medium-spreading events consisted of a Lunar-New-Year family dinner party at a seafood restaurant in North Point, a Lunar-New-Year hot pot party in Kwun Tong, and a Buddhist worship hall in a multi-storey residential building in North Point. Persons infected in the Buddhist worship hall had symptom onset from 8 to 29 February, spreading over 21 days.
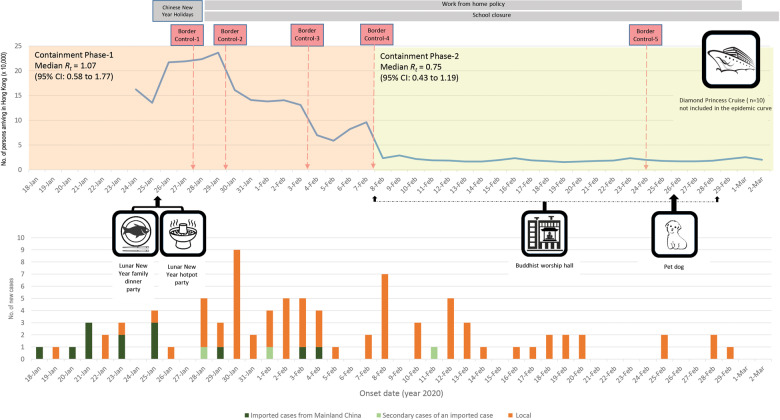
Epidemic curve
The first case (#1) confirmed on 23 January was a visitor from Mainland China identified via active fever screening at a land border control checkpoint. The last case (#100) included in this study was confirmed on 1 March (Figure 3). Thirteen patients were imported from Mainland China (12 from Wuhan, 1 travelled through Zhuhai and Macao) with the last confirmed on 4 February. These imported cases further spread to 3 people referred to as ‘imported secondary’ cases. Sixty-six patients presented themselves to healthcare services with clinical symptoms. Thirty-three patients were identified by active case finding through contact tracing and border screening, and another 1 was diagnosed while hospitalized for orthopaedic problems. Ten HK residents who acquired infection on board the Diamond Princess Cruise were excluded from the epidemic curve as they were evacuated and isolated upon arrival.
Figure 3.
Epidemic curve, case clusters and relationship to border control measures for the first 100 confirmed COVID-19 cases in Hong Kong Special Administrative Region, China. Upper panel: number of persons arriving in HK and the time line of border control and social distancing measures. Border Control-1: flight and high-speed train services from Wuhan in Hubei Province suspended. With the exception of HK residents, residents of Hubei Province and persons who had visited Hubei Province in the past 14 days upon arrival in HK were not permitted to enter HK. Border Control-2: further border control measures including: suspension of the HK section of the Guangzhou–Shenzhen–Hong Kong Express Rail Link and the Intercity Through Train, reducing flight services between HK and Mainland China to about half the original numbers, suspension of all cross-boundary ferry services, reducing land-based cross-boundary transport, and closure of six border control checkpoints between HK and Mainland China. Border Control-3: suspension of an additional four border control checkpoints between HK and Mainland China. Border Control-4: all people entering HK from Mainland China subjected to mandatory quarantine for 14 days upon arrival in HK. Persons entering HK from other places were also subjected to the 14-day mandatory quarantine if they had visited Mainland China in the past 14 days. Border Control-5: non-HK residents arriving from Korea restricted from entering HK, HK residents returning to HK who had been to Daegu and Gyeongsangbuk-do in Korea in the past 14 days required to stay in quarantine centres. HK residents returning from other cities and provinces in Korea were required to undergo medical surveillance for 14 days. Lower panel: epidemic curve by symptom onset date. Containment Phase 1 (18 January– 7 February), Containment Phase 2 (8 February–2 March). Ten persons who acquired infection from the Diamond Princess Cruise were not included as they were quarantined upon arrival and thus did not contribute to transmission in the HK community (6 had symptoms with onset dates between 4 and 24 February, 4 were asymptomatic). Medium-spreading events consisting of a Lunar-New-Year family dinner party at a seafood restaurant, a Lunar-New-Year hot pot party, a Worship hall in a residential multi-storey building, and the Diamond Princess cruise ship.
Progression of the COVID-19 epidemic in HK correlated with border control measures implemented by the Hong Kong Special Administrative Region (HKSAR) Government (Figure 3). Border Control-1 was implemented on 27 January, introducing suspension of flights and high-speed train services from Wuhan, and persons who had visited Hubei Province in the past 14 days were prohibited from entering HK. By that time, there had been 16 cases in HK (according to symptom onset), including 10 imported ones (all from Wuhan). Border Control-2 and -3 measures (Figure 3) to suspend most public transportation, and introduce land-based cross-border control points brought the daily numbers of people entering HK from 236 350 (30 January) to 95 852 (7 February). During this period of Containment Phase-1, only 2 direct imports and 1 secondary case from Mainland China were identified.
Border Control-4 measures were introduced on 8 February. All people entering HK from Mainland China were subjected to mandatory quarantine for 14 days upon arrival. The 14-day quarantine was also applicable to people entering HK from other locations but who had visited Mainland China in the 14 days prior to arrival in HK. These measures marked the start of Containment Phase-2 and kept the daily number of arrivals below 30 000. Only one of the 33 cases identified in the following 24 days had acquired infection from an imported case, and no new imported cases were detected. Social distancing policies including working from home, and school closures were implemented immediately following the Lunar-New-Year holidays (25–27 January), with the former lasting until 1 March and the latter still ongoing at the time of writing (Figure 3).
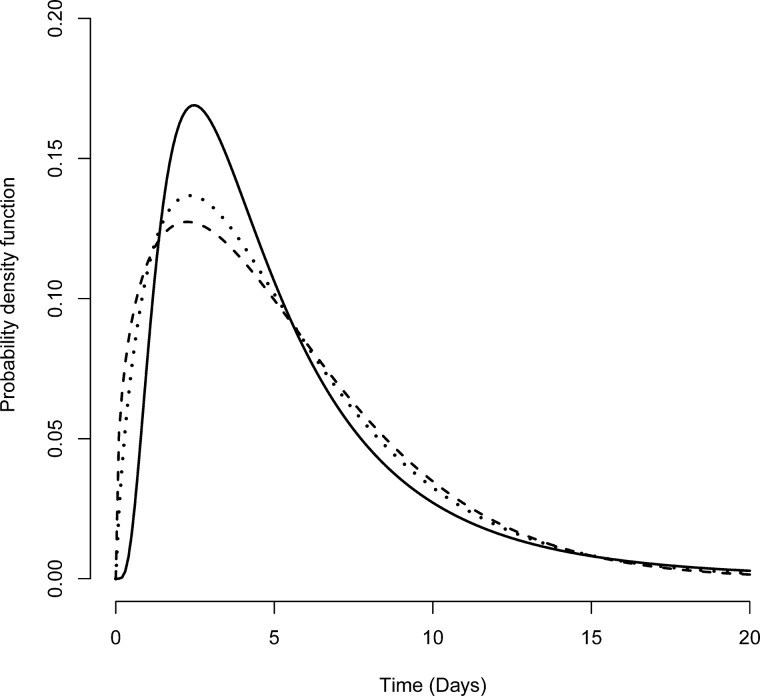
Incubation period
The distributional assumption for incubation periods was robust to different parametric distributions (Figure 4; Supplementary Table 2, available as Supplementary data at IJE online). By fitting to the lognormal distribution, we observed a median incubation period of 4.2 days (95% CI, 4.0–4.5). We estimated that <5% of infected individuals had an incubation period of <1.3 days (95% CI, 1.1–1.4), whereas the 95th percentile of the distribution was 14.0 days (95% CI, 13.1–15.3). Three parametric distribution methods showed a similar model fitness to the data based on AIC values.
Figure 4.
Probability distributions of the incubation period. Available serial intervals (in days) from 40 patients were fitted with parametric distributions: lognormal (solid line), Weibull (dashed line) and gamma (dotted line). Left, right, and interval censoring were accounted for in the likelihood of probability distributions. The AIC values for these three distributions were 87.4, 86.0 and 86.2, respectively.
Reproduction number
Rt peaked on 30 January (1.49, 95% CI, 0.92–2.27) when the number of entrants from Mainland China was still high (Figure 3). The median Rt was 1.07 (95% CI, 0.58–1.77) during Containment Phase-1 (Figure 3) before Border Control-4 policy was implemented (18 January–7 February). Median Rt dropped to 0.75 (95% CI, 0.43–1.19) after implementation of Containment Phase-2 (8–29 Feb).
Clusters
We defined clusters as two or more patients with an epidemiological link. There were 28 identifiable clusters with sizes ranging from 2 to 11 people, involving a total of 78 individuals (Table 2). Most of the clusters (71.4%, 20/28) were families of 2–4 people corresponding to the small family size in HK. Three workplace clusters consisted of 2 patients each. One suspected housing estate cluster (Estate H) consisted of 4 people. Three clusters were regarded as ‘medium-spreading events’ (MSE) involving 6–11 people. Another 10 people were part of the Diamond Princess Cruise outbreak. No healthcare workers were infected and no healthcare facility-associated clusters were detected.
Table 2.
Size of clusters with epidemiological link of transmission
| Clustera | Occurrence of cluster | No. of people infected in each cluster |
|---|---|---|
| Hot pot party | 1 | 11 |
| Worship hall | 1 | 11 |
| Cruise ship | 1 | 10 |
| Dinner party | 1 | 6 |
| Estate H | 1 | 4 |
| Family | 20 | 2 to 4 |
| Workplace | 3 | 2 |
Clusters were defined as the persons directly related to the event, secondary cases were not counted. Clusters were observed in the Lunar-New-Year hot pot party, Worship hall, Diamond Princess Cruise, and Lunar-New-Year dinner party, affecting 6–11 people. Smaller cluster sizes were observed in families, affecting 2–4 people.
Medium-spreading events
Lunar-New-Year family dinner party (n = 6 + 2)
On 26 January (the second day of the Lunar New Year), 29 people attended a dinner gathering in a seafood restaurant dining hall in North Point, HK. The party lasted >5 h. While no person at the dinner was identified as having respiratory symptoms, 6 close relatives attending the party (#46, 48, 49, 52, 53 and 54) showed symptoms with onset 4–13 days afterwards. After the index case (#46) was detected, contact tracing revealed 18 close contacts and 115 other contacts. The infection further spread to another family member (#42) in one household and a domestic helper (#61) in another household. The attack rate was 20.7%.
Lunar-New-Year hot pot party (n = 11 + 2)
Also on 26 Jan, 19 people gathered at a hot pot dinner in a party room of around 1400 square feet in Kwun Tong, HK. Participants were close relatives including 2 from Guangdong, China, 1 of whom had a cough. The infection status of these 2 relatives could not be verified as they left HK after the gathering. Subsequently, 11 family members (#27, 29–37 and 41) were infected, with illness onset 2–13 days after the gathering. Contact tracing revealed 46 close contacts and 166 other contacts. The infection further spread to 2 patients in the workplaces of 2 dinner participants. The attack rate was estimated to be 64.7% after excluding the 2 Mainland visitors whose infection status could not be verified.
Buddhist worship hall (n = 12)
A Buddhist worship hall located in a multi-storey residential building in North Point, HK was identified as the site of exposure for 11 patients (#65, 70, 73, 74, 76, 77, 83, 86, 92, 93 and 98). Visitors chanted sutras and shared meals during worship. Symptom onset dates ranged from 8–29 February, spreading over 21 days, suggesting that a persistent source remained throughout this duration. There were no other epidemiological links among the infected persons. Environmental samples from sutra cloths, kneeling pads and faucets in washroom facilities in the worship hall were positive for SARS-CoV-2 RNA. After we had censored data for the current analysis, the monk (#102) who lived at the worship hall was confirmed to be infected. The monk had travelled to Mainland China in January. Contact tracing for this event revealed 47 close contacts and 170 other contacts.
Estate H-toilet plumbing
On 10 Feb, members of two households living in the same block (Block Y) of a 34-multistorey estate (Estate H) in HK were tested positive for SARS-CoV-2. More than 100 residents living in the same block were evacuated overnight to isolation quarters as a precautionary measure. The patient with an earlier symptom onset date lived on a higher floor (13/F), and 2 siblings living on 3/F developed symptoms 8–11 days after the onset of symptoms in the patient living on 13/F. The infection was initially suspected to have spread via toilet drainage from leaking vent pipes and/or U-traps. Subsequent investigations indicated that all vent pipes and U-traps were functioning properly, although nine units in block Y, including the 3/F with 3 infected family members had their toilet vent pipes altered. It was noted that the 2 siblings had attended the medium-sized spreading event mentioned above (Lunar-New-Year dinner party in North Point, HK) on 4 and 7 days before their illness onset, respectively. Their mother who did not join the dinner also developed symptoms and was confirmed to be infected. No further cases were detected from other residents living in the same block, supporting the inference that the siblings were infected via the dinner party.
Time to containment
The distribution of time to containment, defined as the time from symptom onset to patient isolation, is shown in Figure 5. We excluded 5 patients who were asymptomatic. Cases that were identified by border control (n = 2), those already in quarantine centres (n = 12) and patients admitted for isolation on the same day of symptom onset (n = 7) were considered to have 0 days to containment. Four patients were identified after admission into general wards. These patients were put under standard precautions and droplet precautions upon admission. At that time, all public hospitals in HK were practicing enhanced strict infection control measures, which included personal protective equipment for all healthcare workers, and visitors to wards were not allowed. Furthermore, these hospitalized patients did not result in secondary cases among nearby patients and healthcare workers. Therefore, for the purpose of analysis in this study, we regarded these patients as ‘contained’ upon admission to hospital.
Figure 5.
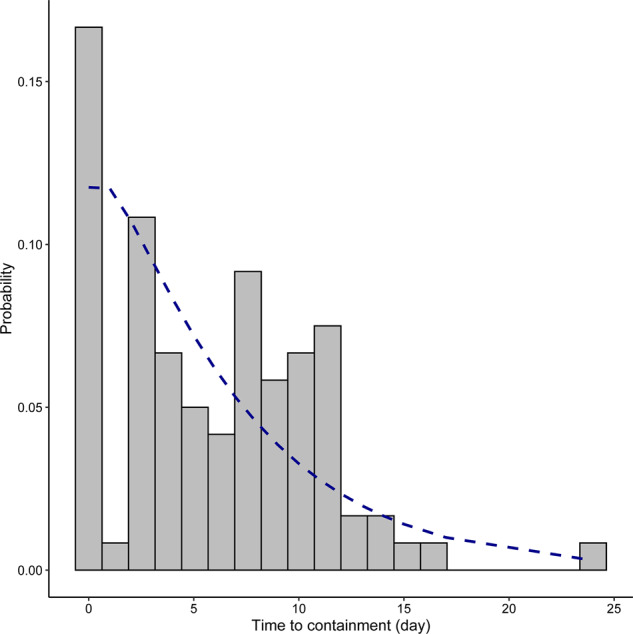
Probability distribution of time to containment (days from symptom onset to isolation). The blue line shows a fitted negative-binomial curve with a mean of 5.96 days and SD of 4.84. Mean and SD were calculated from actual figures.
Among the 95 eligible patients, 75 people (78.9%) had a containment delay. The average days to containment was 5.96 days (range 0–24 days, S.D. 4.84). Local cases [adjusted beta coefficient (β) for ‘imported cases’ = −4.23, 95% CI, −6.41 to −2.06, P < 0.001] and prior visits to medical facilities (adjusted β = 3.87, 95% CI, 1.99–5.76, P < 0.001) were associated with higher likelihood of longer time to containment (Table 1).
Table 1.
Factors associated with containment delay in days using linear regression analysis (n = 95); the covariates controlled for in the linear regression analysis include age, gender, date of diagnosis confirmation, classification of cases (imported vs local), case identification (self-identified vs not) and previous attendance at healthcare services before hospital admission
| Variables | Bivariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|---|
| n a (%) | n b (%) | Coef.c (95% CI) | P-value | Coef. (95% CI) | P-value | ||
| Age (years) | 0–24 | 5 (5) | 3 (4) | Refd | Ref | ||
| 25–44 | 18 (19) | 17 (23) | 3.24 (−1.60, 8.09) | 0.19 | 1.39 (−2.70, 5.48) | 0.50 | |
| 45–64 | 38 (40) | 29 (39) | 2.09 (−2.47, 6.65) | 0.37 | 0.17 (−3.76, 4.09) | 0.93 | |
| ≥65 | 34 (36) | 26 (35) | 3.65 (−0.94, 8.24) | 0.12 | 2.39 (−1.53, 6.31) | 0.23 | |
| Gender | Male | 46 (48) | 38 (51) | Ref | 0.45 | Ref | |
| Female | 49 (52) | 37 (49) | 0.76 (−1.22, 2.74) | 1.10 (−0.60, 2.80) | 0.20 | ||
| Confirm datee (interval = 1) | Range = 1–40 | 95 (95) | 75(100) | 0.10 (0.01, 0.20) | 0.03 | <0.01 (−0.08, 0.09) | 0.88 |
| Mean = 22.66 | |||||||
| Our case classification | Local | 73 (77) | 65 (87) | Ref | <0.001 | Ref | |
| Import | 22 (23) | 10 (13) | −5.86 (−7.88, −3.84) | −4.23 (−6.41, −2.06) | <0.001 | ||
| Self-identifiedf | No | 28 (29) | 16 (21) | Ref | 0.12 | Ref | |
| Yes | 66 (69) | 59 (79) | 1.71 (−0.44, 3.85) | −0.96 (−3.00, 1.09) | 0.36 | ||
| Missing | 1 (1) | 0 (0) | |||||
| Attended healthcare before admission episode | No | 54 (57) | 31 (41) | Ref | <0.001 | Ref | |
| Yes | 46 (48) | 44 (59) | 4.34 (2.57, 6.11) | 3.81 (1.88, 5·75) | <0.001 | ||
All cases.
Cases with containment delay.
Coef., coefficients.
Ref = reference group
Confirm date refers to the report date of positive SARS-CoV-2 real time RT-PCR laboratory result.
Self-identified refers to patients who presented themselves to hospitals or clinics, in contrast to those who were detected by border screening or through active case finding in contact investigations.
Healthcare attendance before admission to hospital
Doctors were advised to refer suspected cases to public hospitals for isolation and testing. Diagnostic tests were not available outside of public hospitals during the period covered in this study. Forty-six patients attended healthcare services without being suspected of having COVID-19 before admission into hospitals. Healthcare visits included private practitioners, Chinese medical practitioners and private hospitals. The median duration to the first healthcare visit was 1 day [range: 0–10 days, interquartile range (IQR): 2]. The median number of attendances was 2 (range: 1–6, IQR: 1.75).
Discussion
Comprehensive epidemiological information covering the initial period of an outbreak is useful in subsequent public health control for any emerging diseases.24 HK is a high-population-density city near the COVID-19 epicentre, where experience in containing the spread of SARS-CoV-2 will be instrumental in formulating public health policies in other high-population-density cities. Given the experience with SARS-CoV-1 in 2003,24,25 the HK Government escalated its Preparedness and Response level soon after the outbreak in Wuhan had been recognised, with active case finding, enhanced surveillance and laboratory tests available free-of-charge. Border control policies minimized the number of imported cases. This together with social distancing measures, intensive contact tracing and quarantine led to a reduction of Rt to 0.75, below a theoretical limit (1.0) for an outbreak to spread in a population. Compared with the Rt of 2.2 observed in the first 425 cases in Wuhan, containment actions implemented in HK can be regarded as successful.
An accurate estimation of the incubation period is vital in establishing the optimal duration for quarantine. Our estimation of a median incubation period of 4.2 days is in line with other studies,26–28 which is also within the range reported for SARS-CoV-1 (3.8–6.9 days) and MERS CoV (5.0–6.9 days).29–31 Our observed 95th percentile of incubation periods of 14.0 days also supports the widely adopted quarantine period of 14 days.
We observed that under stringent containment policies, most of the COVID-19 clusters were small and confined to households. This observation together with reports of pre-symptomatic transmission and high level of virus shedding within the early days of symptom onset is akin to influenza, making efficient containment by contact tracing and quarantine difficult to achieve.32 This is in contrast to SARS-CoV-1 where virus shedding peaked during the second week of illness.33 During SARS 2003 in HK, delay in containment was shown to have shortened as time progressed, reflecting the effects of contact tracing.34 Such an association was not observed for COVID-19 possibly due to the early start of the infectious period.
Super-spreading events were characteristic of SARS 2003 in HK, with alarming numbers of infected persons.7 Factors constituting super-spreading events include environmental, behavioural, social and cultural factors,25,31 whereas the virus strain often plays a comparatively smaller role. We have yet to observe super-spreading events for COVID-19, however, coincidence with the Lunar New Year with large family gatherings led to two ‘medium-spreading events’. These examples highlight the importance of predicting and assessing any potential super-spreading events taking into account factors such as demographics (e.g. high-density housing estates) or festivities (e.g. Lunar-New-Year celebrations).
The SARS infection in 2003 highlights a possibility of widespread dissemination via faulty sewage drainage systems in multi-storey buildings.7 With COVID-19, a similar spreading mechanism was suspected in the Estate H incident, leading to overnight en masse evacuations. Subsequent investigations suggested this mode of spread was unlikely.
Our study has several limitations. Although we observed an increase in incidence with age, potential biases could not be excluded. First, all schools were closed which may have protected most children from infection. Nonetheless, children were found to have milder symptoms, as in SARS and MERS, or even to be asymptomatic. We also lacked clinical information to examine the effect of co-morbidity especially among elderly people. Whether SARS-CoV-2 mimics SARS-CoV-1 in causing milder infections in children remains to be investigated. Furthermore, we could not assess the true incidence of infection, as mild and asymptomatic infections were likely to be missed. Transmission dynamics were skewed by containment measures where lifestyle and behaviours were greatly altered, such as near-universal facemask wearing in public areas. Lastly, clinical outcomes and the medical backgrounds of patients were not available.
In conclusion, in support of the WHO early investigation protocols,35 timely stringent containment measures should be implemented to break transmission and minimize disease burden associated with COVID-19.
Supplementary Material
Acknowledgement
We would like to thank the Centre for Health Protection, Department of Health, Hong Kong Special Administrative Region, China that made information of confirmed COVID-19 cases publicly available, which enabled our detailed analysis.
Supplementary data
Supplementary data are available at IJE online.
Conflict of Interest
None declared.
References
- 1. de Wit E, van Doremalen N, Falzarano D, Munster VJ.. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol 2016;14:523–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. https://www.who.int/csr/sars/country/table2004_04_21/en/ (9 March 2020, date last accessed).
- 3.WHO. Middle East respiratory syndrome coronavirus (MERS-CoV), 2019. https://wwwwhoint/emergencies/mers-cov/en/ (9 March 2020, date last accessed).
- 4.WHO. Rolling updates on coronavirus disease (COVID-19), 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen (9 March 2020, date last accessed).
- 5.WHO Director-General's opening remarks at the media briefing on COVID-19, 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (15 March 2020, date last accessed).
- 6.Census and Statistics Department of the Hong Kong SAR. 2016 Population By-census. Main table A119a: population by age, year, sex and marital status, 2018. https://www.bycensus2016.gov.hk/en/bc-mt.html (9 March 2020, date last accessed).
- 7. Yu IT, Li Y, Wong TW. et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med 2004;350:1731–739. [DOI] [PubMed] [Google Scholar]
- 8.Government of Hong Kong SAR. Cross-boundary passenger traffic estimation and arrangements for Lunar New Year festive period. https://wwwinfogovhk/gia/general/201802/07/P2018020700291htm2018. (9 March 2020, date last accessed).
- 9. Kwok KO, Wong V, Wei VW, Wong SY, Tang JW, Novel coronavirus (2019-nCoV) cases in Hong Kong and implications for further spread. J Infect 2020;80:671–93. [DOI] [PMC free article] [PubMed]
- 10. Lai TH, Tang EW, Chau SK, Fung KS, Li KK.. Stepping up infection control measures in ophthalmology during the novel coronavirus outbreak: an experience from Hong Kong. Graefes Arch Clin Exp Ophthalmol 258:1049–055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brand Hong Kong. Hong Kong’s multi-pronged response to COVID-19, 2020. https://www.brandhk.gov.hk/uploads/brandhk/files/factsheets/Hong_Kong_Themes/Factsheet_COVID-19_Mar%205_E.pdf (9 March 2020, date last accessed).
- 12.The Hong Kong Special Administrative Region Government. COVID-19 case database. https://www.coronavirus.gov.hk/eng/index.html#Updates_on_COVID-19_Situation (9 March 2020, date last accessed).
- 13.Centre for Health Protection of the Hong Kong Special Administrative Region Government. CHP daily press releases. https://www.chp.gov.hk/en/media/116/index.html (9 March 2020, date last accessed).
- 14.Centre for Health Protection of the Hong Kong Special Administrative Region Government. Coronavirus disease (COVID-19) in HK. https://chp-dashboard.geodata.gov.hk/covid-19/en.html (9 March 2020, date last accessed)
- 15. Cowling BJ, Fang VJ, Riley S, Peiris JM, Leung GM.. Estimation of the serial interval of influenza. Epidemiology (Cambridge, Mass.) 2009;20:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chong KC, Zhang C, Zee BC. et al. Interpreting the transmissibility of measles in two different post periods of supplementary immunization activities in Hubei, China. Vaccine 2017;35:1024–029. [DOI] [PubMed] [Google Scholar]
- 17. Chong KC, Hu P, Lau S. et al. Monitoring the age-specificity of measles transmissions during 2009-2016 in Southern China. PLoS One 2018;13:e0205339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nishiura H, Chowell G, The effective reproduction number as a prelude to statistical estimation of time-dependent epidemic trends In: Chowell G, Hyman JM, Bettencourt LMA, Castillo-Chavez C (eds). Mathematical and Statistical Estimation Approaches in Epidemiology. Dordrecht: Springer, 2009, pp.103–21. [Google Scholar]
- 19. Chong KC, Cheng W, Zhao S. et al. Monitoring disease transmissibility of 2019 novel coronavirus disease in Zhejiang, China. Int J Infect Dis 2020; doi:10.1101/2020.03.02.20028704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chong KC, Cheng W, Zhao S, et al. Transmissibility of coronavirus disease 2019 (COVID-19) in Chinese cities with different transmission dynamics of imported cases. medRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 21. Cori A, Ferguson NM, Fraser C, Cauchemez S.. A new framework and software to estimate time-varying reproduction numbers during epidemics. Am J Epidemiol 2013;178:1505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao S, Gao D, Zhuang Z, et al. Estimating the serial interval of the novel coronavirus disease (COVID-19): a statistical analysis using the public data in Hong Kong from January 16 to February 15, 2020. medRxiv2020.
- 23.The Government of the Hong Kong Special Administrative Region. Press Releases, 2020. https://www.info.gov.hk/gia/general/202003/26/P2020032600756.htm (15 April 2020, date last accessed).
- 24. Anderson RM, Fraser C, Ghani AC. et al. Epidemiology, transmissiondynamics and control of SARS: the 2002-2003 epidemic. Philos Trans R Soc London B 2004;359:1091–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tomlinson B, Cockram C.. SARS: Experience at Prince of Wales Hospital, Hong Kong. Lancet 2003;361:1486–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Q, Guan X, Wu P. et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020;382:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Backer JA, Klinkenberg D, Wallinga J.. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20–28 January 2020. Euro Surveill 2020;25:2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Linton NM, Kobayashi T, Yang Y. et al. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: a statistical analysis of publicly available case data. J Clin Med 2020;9:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Virlogeux V, Park M, Wu JT, Cowling BJ.. Association between severity of MERS-CoV infection and incubation period. Emerg Infect Dis 2016;22:526–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park HY, Lee EJ, Ryu YW. et al. Epidemiological investigation of MERS-CoV spread in a single hospital in South Korea, May to. Euro Surveill 2015;20:21169. [DOI] [PubMed] [Google Scholar]
- 31. Nishiura H, Endo A, Saitoh M. et al. Identifying determinants of heterogeneous transmission dynamics of the Middle East respiratory syndrome (MERS) outbreak in the Republic of Korea, 2015: a retrospective epidemiological analysis. BMJ Open 2016;6:e009936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arons MM, Hatfield KM, Reddy SC. et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med 2020;382:2081–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peiris J, Guan Y, Yuen K.. Severe acute respiratory syndrome. Nat Med 2004;10:S88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Donnelly CA, Ghani AC, Leung GM. et al. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet 2003;361:1761–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. Coronavirus disease (COVID-19) technical guidance: The Unity Studies: Early investigations protocols. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/early-investigations (15 April 2020, date last accessed).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.