ABSTRACT
The evidence of long-term clinical dynamic on Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) RNA re-positive case are less. We performed a 108 days follow-up on dynamic clinical presentations in a case, who hospitalized three times due to the positive recurrence of SARS-CoV-2 RNA after discharge, to understand the prognosis of the 2019-Coronavirus disease (COVID-19). In this case, positive SARS-CoV-2 recurred even after apparent recovery (normal CT imaging, no clinical symptoms, negative SARS-CoV-2 on stool sample and negative serum IgM test) from COVID-19, viral shedding duration lasted for 65 days, the time from symptom onset to disappearance was up to 95 days. Erythrocyte-associated indicators, liver function and serum lipid metabolism presented abnormal throughout during the observation period. Awareness of atypical presentations such as this one is important to prompt the improvement of the management of COVID-19.
Keywords: coronavirus disease 2019, SARS-CoV-2 re-positive, recovered patients, long-term follow-up, erythrocyte
The evidence of long-term clinical dynamic on SARS-CoV-2 re-positive case are less and awareness of atypical presentations such as this one is important to prompt the improvement of the management of COVID-19.
INTRODUCTION
Potential infectivity of the recovered patients has been aroused great concern in many countries as the recurrence of positive SARS-CoV-2 during post-discharge surveillance (China Daily Website 2020; Lan et al. 2020; Yao et al. 2020). Nowadays, the virus is spreading rapidly around the world. There will be more and more discharged patients in the future with medical rescuing. However, the infectivity of such cases remains unknown, and the evidence of long-term clinical dynamic on SARS-CoV-2 RNA re-positive cases are less. The present report describes 3.5-months presentations of the virus, clinical symptom, laboratory finding and CT image in a case who was admitted to hospital three times due to SARS-CoV-2 RNA recurred positive after discharge.
METHODS
At our institution, Xixi Hospital (a designated infectious disease hospital of Zhejiang province, China), the negative pressure isolation ward was reserved for confirmed cases of COVID-19. Individual with respiratory symptoms but without suspicious exposure or travel history was placed solely in surveillance ward. The other general wards were all ceased operating. All patients were advised to avoid mingling and to wear a surgical mask at all times; visitors were not allowed. Each of healthcare workers used full personal protective equipment including face shields, N95 masks, gloves and isolation gowns; hands disinfecting before and after contact with patients. All of the hospital staff, who contacted with confirmed patients, were isolated in a designated hotel. The re-positive patient, who readmitted to the hospital, was treated as a confirmed case. Breathing apparatus, catheter, patient monitoring and other equipment for confirmed patients were all discarded and disposed of as biohazard waste after discharge. Air disinfection (twice a day) was performed by air-sterilizing machines in each ward for the prevention of COVID-19 transmission.
The case in this study was diagnosed as COVID-19 by the positive detection of SARS-CoV-2 RNA in nasopharyngeal swabs according to the manufacturer's instructions (Liferiver, Shanghai, China), which recommended by Zhejiang Center for Disease Control and Prevention. In brief, the target genes of reverse transcription-polymerase chain reaction (RT-PCR) were E gene (first line screen assay), RdRP gene (confirmatory assay) and N gene (additional confirmatory assay). The reaction condition started with 45°C for 10 min and 95°C for 3 min, followed by 45 cycles of amplification at 95°C for 15 s and 58°C for 30 s. The test results of SARS-CoV-2 were reported as positive (Cycle threshold; Ct ≤ 43) and negative (Ct > 43). A total of two consecutively RT-PCR tests were conducted on each respiratory sample during the period of discharge surveillance. Laboratory results were collected and standardized as a uniform table, and checked by two researchers of this study.
Fitness for discharge were improvement of chest radiographic evidence, SARS-CoV-2 negative in respiratory samples for two consecutive detections at least one day apart and abatement of fever for at least three days (National Health Commission of the People's Republic of China 2020). The patient was isolated in a single-occupancy room of the community hospital for the next 2 weeks, and evaluated with the results of SARS-CoV-2 RNA on days 7 and 14 after discharge, to determine whether he could be released from quarantine.
All Healthcare workers in our hospital and the community hospital underwent two nasopharyngeal swabs for the RT-PCR of SARS CoV-2 RNA, which were performed seven days apart. Each healthcare worker will be released from isolation after fulfilled the criteria of both SARS CoV-2 RNA detection presented negative. This study was approved by the Institutional Review Board of Xixi Hospital.
RESULTS
No hospital staff was infected during the observation period. The case was a 35-years-old man with an exposure history of Hubei province in China. He presented high fever (39°C), cough, sore throat and fatigue, and was admitted to hospital as positive detection of SARS-CoV-2 RNA on 30 January 2020. He denied other underlining diseases. The severity of disease was mild.
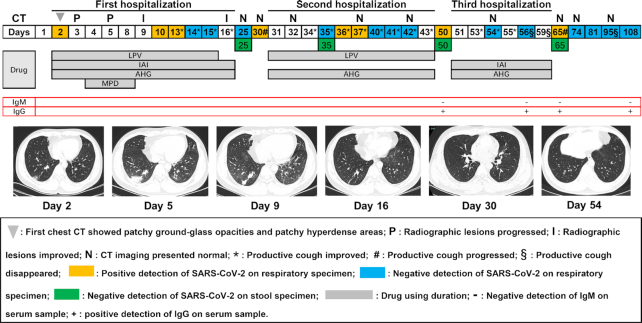
Time kinetics of CT presentation, clinical symptom (cough or sputum) and virus are described in Fig. 1. The day of symptom onset was defined as day 1. On day 2, CT images showed patchy ground-glass opacities and patchy hyperdense areas in the lower lunge zone.
Figure 1.
Time dynamics of virus, chest radiograph and clinical symptom on a case recovered from COVID-19 during the observation period. LPV: lopinavir; IAI: interferon α2b atomization inhalation; AHG: arbidol hydrochloride granules; MPD: methylprednisolone.
During the first hospitalized period, lopinavir (LPV) combined with interferon α2b atomization inhalation (IAI) and arbidol hydrochloride granules (AHG) was used for more than fourteen days, methylprednisolone (MPD) was added to the combined therapy for four days after the radiographic lesions progressed (days 3 and 5), the nasopharyngeal swab tests of SARS-CoV-2 RNA were positive on the days 2, 10 and 13 and were negative on days 14 and 15. Improvement of radiographic lesions and clinical symptoms (productive cough) occurred on days 9 and 13, respectively. Temperature decreased to normal (under 37.4°C) after five days of treatment (data not shown). He discharged on day 16 and quarantined under surveillance for 2 weeks. On day 25, the results of SARS-CoV-2 RNA from nasopharyngeal swab and stool sample were both negative; the opacities on radiography almost shrunk completely and maintained normal until the end of the observation period. However, the nasopharyngeal swab test of SARS-CoV-2 RNA revealed positive and clinical symptoms deteriorated on day 30; then, he was admitted to hospital again on day 31.
In the period of the second hospitalization, LPV and AHG were used throughout; the nasopharyngeal swab performed with a positive result for SARS-CoV-2 on days 36 and 37, a negative result on days 35, 40, 41 and 42. The clinical symptoms improved on day 34, after which there was a continuous improvement until the end of the second hospitalization. However, on day 50 that of under post-discharge surveillance, the SARS-CoV-2 RNA of nasopharyngeal swab revealed positive again, but negative on stool sample. The results of IgM for SARS-CoV-2 presented negative, and IgG showed positive.
During the third hospitalization, the case was treated on admission with combined therapy (IAI + AHG) throughout, had a mild symptom of white productive cough which improved continuously since the day 53 and completely disappeared on day 56. But, there was a sore throat following with fever on day 53, which receded significantly after antibiotics using. The test of respiratory tract samples revealed negative on days 54 (nasopharyngeal swab) and 56 (sputum), respectively. Chest radiography was repeated on day 54 and showed no abnormalities. He was discharged and isolated under surveillance for the next 2 weeks as before. On day 65, the test of RT-RCR for SARS-CoV-2 on sputum showed positive again, the virus test of stool specimen still presented negative, clinical symptoms that had disappeared before re-emerged, but just presented slight cough. Then, the cough disappeared on day 95 after two consecutively negative virus detections of sputum (on days 81 and 95). The serum test of IgM presented negative on days 56, 65 and 108. At the end of the observation, the detection of SARS-CoV-2 on sputum revealed negative.
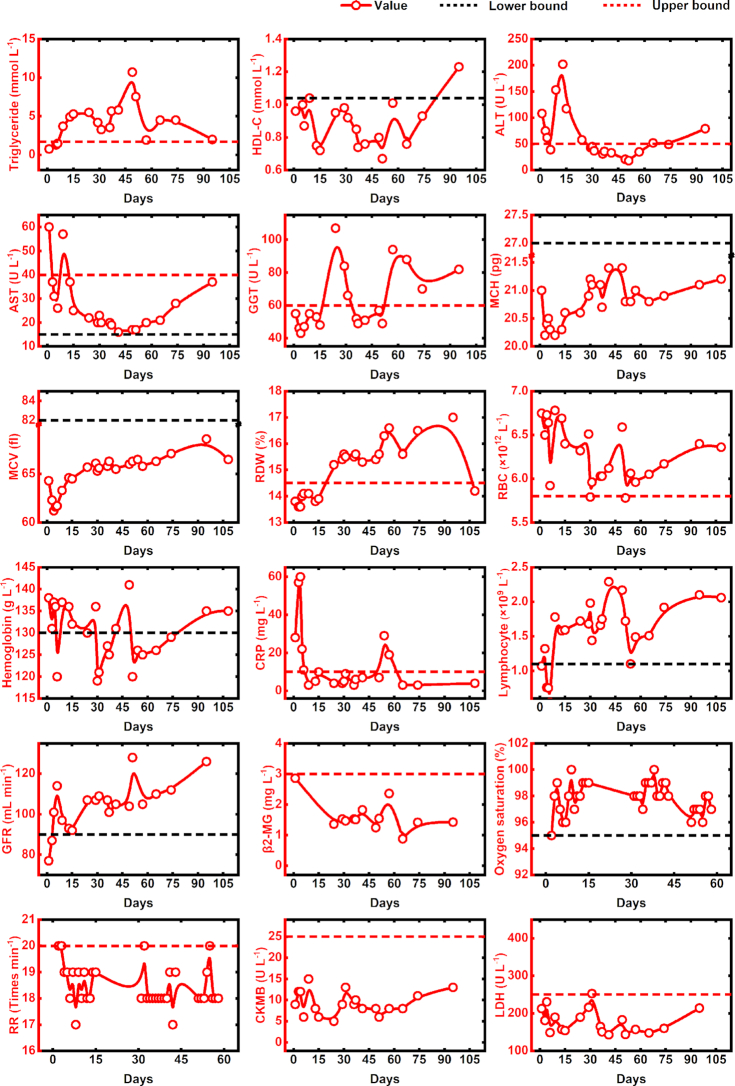
Figure 2 showed the changes over time in eighteen laboratory indicators during the observation period. As can be seen from the figure, most values of triglyceride, red cell distribution width (RDW), red blood cell count (RBC) were above the upper bound of normal range. The levels of high-density lipoprotein cholesterol (HDL-C), mean corpuscular hemoglobin (MCH) and mean corpuscular volume (MCV) were mainly under the lower threshold of normal range. About half of the levels of blood hemoglobin were under the lower threshold of normal range. Part of the values of gamma-glutamyltransferase (GGT) were greater than the upper bound of normal range. At the early stage of COVID-19, aspartate transaminase (AST), alanine transaminase (ALT) and rapid c-reactive protein (CRP) levels were high, the values of lymphocyte count and glomerular filtration rate (GFR) were low, but all returned to normal within 15 days after the symptom onset. Nearly all the values of β2-macroglobulin (β2-MG), respiratory rate (RR), creatine kinase isoenzyme (CKMB) and lactate dehydrogenase (LDH) were normal during the observation period.
Figure 2.
The temporal changes of laboratory indicators in a patient recovered from COVID-19. HDL-C: high-density lipoprotein cholesterol; ALT: alanine transaminase; AST: aspartate aminotransferase; GGT: gamma-glutamyltransferase; MCH: mean corpuscular hemoglobin; MCV: mean corpuscular volume; RDW: red cell distribution width; RBC: red blood cell count; CRP: rapid c-reactive protein; GFR: glomerular filtration rate; β2-MG: β2-macroglobulin; RR: respiratory rate; CKMB: creatine kinase isoenzyme; LDH: lactate dehydrogenase.
DISCUSSION
In our study, the observation period is 108 days, during which we described the whole dynamic progression of COVID-19 in a recovered patient whose SARS-CoV-2 RNA recurred positive over and over again after discharge. In accordance with the health authorities, this case fulfilled the criteria before each discharge (National Health Commission of the People's Republic of China 2020). But, viral detection all presented positive within 14 days after each discharge. This finding is consistent with the previous study reported by Lan et al. (2020), in which the recurrence of positive virus appeared 5 to 13 days after discharge. It is notable that the positive SARS-CoV-2 recurred (Fig. 1. Day 65) even after apparent recovery (normal CT imaging, no clinical symptoms, negative SARS-CoV-2 on stool sample and negative serum IgM test) from COVID-19. This extremely warrants us to investigate the shedding window of COVID-19 and reappraise the current criteria for hospital discharge and discontinuation of quarantine. Although false-negative SARS-CoV-2 RNA results could have occurred because of potential sampling error (Xie et al. 2020), the reexamination of sputum was consistent with that of previous decreased the possibility of false-results.
Interestingly, of this case, the erythrocyte-associated indicators presented abnormal throughout during observation period. It is difficult to find a reasonable explanation for this phenomenon. But, previous studies on HIV, reported that erythrocyte-associated indicator is related to virus dynamic during antiviral therapy (Motswaledi, Kasvosve and Oguntibeju 2013; Zhang et al. 2018). A previous study by Hess et al. (2002) reported that higher numbers of HIV-1 associated with erythrocytes were correlated with a long clinical stage of HIV-1 infection and erythrocyte-associated HIV-1 might serve as a marker for virological failure. In view of the drugs used to treat HIV, could also inhibit SARS-CoV-2 replication (Hung et al. 2020), the pathogenic mechanism of SARS-CoV-2 may exist similarities with that of HIV. Thus, we speculate that the abnormality of erythrocyte-associated indicators was associated with a poor clinical outcome of SARS-CoV-2 recurrence. It may be as a result of erythrocytes feed SARS-CoV-2. The values of hemoglobin, which is a prognostic factor in HIV infection (Obirikorang and Yeboah 2009), were all decreased to the lower bound during post-discharge surveillance, provides evidence on this hypothesis. The higher number of RBC and the lower level of MCH also give us a hint on this issue from another perspective. This may explain why recurrence of measurable SARS-CoV-2 RNA within days or weeks of cessation of antiretroviral therapy in a fraction of patients. Future study needs to define the pathophysiological role of erythrocytes in SARS-CoV-2 infection.
Of this mild case, the functionality of lungs, kidney and heart was substantially normal during the follow-up period. High CRP levels and low lymphocyte counts appeared during the initial period of COVID-19, but all returned to normal for 15 days of treatment. Similar findings were also observed in other mild COVID-19 infected patients (Chen et al. 2020). The levels of ALT were much higher than that of AST, indicates that acute liver damage occurred on this patient at the early stage of COVID-19. This might be directly caused by the viral infection of liver cells (Zhang, Shi and Wang 2020). The levels of ALT and AST both decreased to normal on the heels of the first negative SARS-CoV-2 RNA occurrence, which proves the possibility of viral exposure in the liver. However, the repetitive abnormal level of GGT, an index of liver dysfunction (Whitfield 2001), implicates that liver impairment has not yet recovered completely and liver dysfunction sustained during the follow-up period. The obvious abnormal lipid metabolism may result from prolonged liver dysfunction (Speliotes et al. 2018). Liver damage in mild cases of COVID-19 is often transient and can return to normal without any special treatment (Zhang, Shi and Wang 2020). But, in this case, dyslipidemia and liver dysfunction lasted more than 2 months. Some studies have reported that the abnormalities of lipid metabolism and liver dysfunction associate with the severity of COVID-19 (Cai et al. 2020; Ren et al. 2020). Given the case of this study presented a mild clinical symptom and a slight lesion of chest CT, it could be one of the underlining causes of the lingering characteristic of COVID-19. How different underlying liver conditions influence the relapse of COVID-19 needs to be meticulously evaluated in large-scale clinical trials.
Of this case, the time from symptom onset to disappearance was at least 95 days and the duration of viral shedding after COVID-19 onset lasted for 65 days which is longer than that of 37 days (Zhou et al. 2020). These issues present great difficulties and challenges for the screen of COVID-19 and for the prevention and control of this epidemic.
Our study has limitations; the inherent shortcomings due to a retrospective observational case study, the absence of RT-PCR detection of SARS-CoV-2 RNA on broncho-alveolar lavage fluid and the lack of pathological examination on liver and lung tissues make it difficult to reach a firm conclusion. All above limitations require further study; nonetheless, our primary results provided new and important insights for this topic.
In this study, no hospital staff was infected. Control measures including isolation, effective protection and social distancing, play pivotal roles for the prevention of SARS-CoV-2 infection. Nowadays, the epidemic is under control in China; most COVID-19 patients were discharged from the hospital. We believed that the implementation of isolation and surveillance for at least 14 days effectively prevents the second spread of COVID-19. However, how much time will be needed for isolation for recovered cases and the infectivity of them still require more clinical evidence to confirm. Awareness of atypical presentations such as this one is important to prompt the management of patient isolation and prevent inter-human transmission in the world.
ACKNOWLEDGEMENTS
We express our greatest respect and sincere wishes to the front-line workers in the struggle against COVID-19.
Contributor Information
Fang Liu, Medical Laboratory, Xixi Hospital of Hangzhou, Hangzhou Sixth People's Hospital, 2 Hengbu Road, Xihu District, Hangzhou, 310023, China.
Zhao-bin Cai, Department of Infectious Disease, Xixi Hospital of Hangzhou, Hangzhou Sixth People's Hospital, 2 Hengbu Road, Xihu District, Hangzhou, 310023, China.
Jin-song Huang, Department of Infectious Disease, Xixi Hospital of Hangzhou, Hangzhou Sixth People's Hospital, 2 Hengbu Road, Xihu District, Hangzhou, 310023, China.
Wen-yan Yu, Medical Laboratory, Xixi Hospital of Hangzhou, Hangzhou Sixth People's Hospital, 2 Hengbu Road, Xihu District, Hangzhou, 310023, China.
Hai-ying Niu, Medical Laboratory, Xixi Hospital of Hangzhou, Hangzhou Sixth People's Hospital, 2 Hengbu Road, Xihu District, Hangzhou, 310023, China.
Yan Zhang, Medical Laboratory, Xixi Hospital of Hangzhou, Hangzhou Sixth People's Hospital, 2 Hengbu Road, Xihu District, Hangzhou, 310023, China.
Dong-ming Sui, Medical Laboratory, Xixi Hospital of Hangzhou, Hangzhou Sixth People's Hospital, 2 Hengbu Road, Xihu District, Hangzhou, 310023, China.
Fei Wang, Medical Laboratory, Xixi Hospital of Hangzhou, Hangzhou Sixth People's Hospital, 2 Hengbu Road, Xihu District, Hangzhou, 310023, China.
Li-zhi Xue, Medical Laboratory, Xixi Hospital of Hangzhou, Hangzhou Sixth People's Hospital, 2 Hengbu Road, Xihu District, Hangzhou, 310023, China.
Ai-fang Xu, Medical Laboratory, Xixi Hospital of Hangzhou, Hangzhou Sixth People's Hospital, 2 Hengbu Road, Xihu District, Hangzhou, 310023, China.
AUTHOR CONTRIBUTIONS
LF, CZB and HJS conceived, designed and organized the study, interpreted the results and drafted the manuscript. XAF helped supervise the study. The other authors contributed to collect the data on site.
FUNDING
This work was supported by Hangzhou Research Project of 2019-Coronavirus Disease [grant number 20202013A01]. Zhao-bin Cai was in receipt of grants.
Conflicts of interest
The authors declare that they have no competing interests.
REFERENCES
- Cai Q, Huang D, Yu H et al. . COVID-19: abnormal liver function tests. J Hepatol. 2020, DOI:10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Sang L, Jiang M et al. . Longitudinal hematologic and immunologic variations associated with the progression of COVID-19 patients in China. J Allergy Clin Immunol. 2020, DOI:10.1016/j.jaci.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- China Daily Website Recovered patient re-hospitalized after positive test: Chinese and Beijing. http://ex.chinadaily.com.cn/exchange/partners/45/rss/channel/www/columns/2n8e04/stories/WS5e4fc442a31012821727963c.html(21 February2020, date last accessed). [Google Scholar]
- Hess C, Klimkait T, Schlapbach L et al. . Association of a pool of HIV-1 with erythrocytes in vivo: a cohort study. Lancet. 2002;359:2230–234. [DOI] [PubMed] [Google Scholar]
- Hung IF, Lung KC, Tso EY et al. . Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan L, Xu D, Ye G et al. . Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020;323:1502–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motswaledi MS, Kasvosve I, Oguntibeju OO. The role of red blood cells in enhancing or preventing HIV infection and other diseases. Biomed Res Int. 2013;2013:758682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health Commission of the People's Republic of China Diagnosis and treatment protocols of the novel coronavirus pneumonia (trial version 6): Chinese and Beijing. http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2/files/b218cfeb1bc54639af227f922bf6b817.pdf(19 February2020, date last accessed). [Google Scholar]
- Obirikorang C, Yeboah FA. Blood haemoglobin measurement as a predictive indicator for the progression of HIV/AIDS in resource-limited setting. J Biomed Sci. 2009;16:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Yang Y, Wang F et al. . Association of the insulin resistance marker TyG index with the severity and mortality of COVID-19. Cardiovasc Diabetol. 2020;19:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speliotes EK, Balakrishnan M, Friedman LS et al. . Treatment of dyslipidemia in common liver diseases. Clin Gastroenterol Hepatol. 2018;16:1189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38:263–355. [DOI] [PubMed] [Google Scholar]
- Xie X, Zhong Z, Zhao W et al. . Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020:200343, DOI:10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao XH, He ZC, Li TY et al. . Pathological evidence for residual SARS-CoV-2 in pulmonary tissues of a ready-for-discharge patient. Cell Res. 2020;30:541–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Chew GM, Shikuma CM et al. . Red blood cell distribution width as an easily measurable biomarker of persistent inflammation and T cell dysregulation in antiretrovirally treated HIV-infected adults. HIV Clin Trials. 2018;19:172–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Yu T, Du R et al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]