Abstract
Background
We aimed to evaluate the antiviral activity and safety of darunavir/cobicistat (DRV/c) in treating COVID-19 patients.
Methods
In this single-center, randomized, and open-label trial, mild patients with polymerase chain reaction (PCR)–confirmed COVID-19 were enrolled in Shanghai, China. Participants were randomized to receive DRV/c for 5 days on the top of interferon alpha 2b inhaling or interferon alpha 2b inhaling alone. The primary end point was the virological clearance rate of oropharyngeal swabs at day 7 after randomization in the intention-to-treat population (clinicaltrials.gov: NCT04252274).
Results
From January 30, 2020, to February 6, 2020, a total of 30 patients were enrolled, of whom 18 (60%) were male, aged 47.2 ± 2.8 years; 63.3% (19/30) of the participants had fever, and 46.7% (14/30) had cough at enrollment. The participants were randomized (range) at 4 (2–5) days after onset of symptoms. The proportion of negative PCR results at day 7 was 46.7% (7/15) and 60.0% (9/15) in the DRV/c and control groups (P = .72), respectively. The viral clearance rate at day 3 was 20% (3/15) in both study groups, while the number increased to 26.7% (4/15) in the DRV/c group and remained 20% (3/15) in the control group at day 5. Fourteen days after randomization, 1 participant in the DRV/c group progressed to critical illness and discontinued DRV/c, while all the patients in the control group were stable (P = 1.0). The frequencies of adverse events in the 2 groups were comparable.
Conclusions
Five days of DRV/c did not increase the proportion of negative conversion vs standard of care alone, although it was well tolerated.
Keywords: antiviral activity, COVID-19, darunavir, protease inhibitors, SARS-CoV-2
The outbreak of the novel coronavirus disease (COVID-19) caused by SARS-CoV-2 has already became a pandemic. Although most cases were mild in China, around 14% of patients were severely ill, while another 5% of patients were critical [1]. As of April 17, 2020, there were 1 991 562 confirmed cases and 130 885 deaths worldwide [2]. Unfortunately, a vaccine will not be available in the coming months. Therefore, there is an urgent need to explore drugs that have potent anti-SARS-CoV-2 activity.
Gene sequences of the SARS-CoV-2 show that it is closely related to SARS-CoV (about 79%) [3]. COVID-19 and SARS are also similar in terms of transmission route and clinical characteristics, while the currently reported mortality rate of COVID-19 is lower than that of SARS [4]. In retrospective case–control studies, a combination of lopinavir/ritonavir (LPV/r) and ribavirin was associated with reduced frequency of acute respiratory distress syndrome or mortality in SARS patients [5]. Meanwhile, lopinavir/ritonavir and interferon β were found to be effective for Middle East respiratory syndrome coronavirus (MERS-CoV) infection in animal experiments and case reports [6–9]. Therefore, it is reasonable to infer that LPV/r may also have anti-SARS-CoV-2 activity.
Darunavir (DRV) and LPV are both HIV-1 protease inhibitors that share a similar mechanism for inhibiting HIV replication. In phase III studies, DRV/r showed better efficacy and tolerability (less diarrhea and dyslipidemia and fewer adverse reactions) in people with HIV (PWH) compared with LPV/r [10].
We conducted this clinical trial aiming to evaluate the antiviral activity and safety of darunavir/cobicistat (DRV/c) for treating COVID-19.
METHODS
Study Population
This pilot study was a single-center, randomized, open-label trial conducted at Shanghai Public Health Clinical Center (SPHCC) to preliminarily investigate the efficacy and safety of DRV/c in treating pneumonia caused by the SARS-CoV-2. SPHCC is the only hospital in Shanghai to treat adult COVID-19 cases. The study aimed to enroll 30 participants, with a 1:1 randomization ratio (N = 15 subjects in each arm). All the participants had laboratory-confirmed SARS-CoV-2 infection and were willing to participate the study, as evidenced by signing an informed consent. Participants were excluded if they met any of the following criteria: hypersensitivity to darunavir, cobicistat, or any excipients; patients with severe liver injury (Child-Pugh Class C); patients receiving concomitant medications that are highly dependent on cytochrome P450 3A clearance, and for which the elevated plasma concentrations are associated with serious or life-threatening events; subjects considered to be unable to complete the study (eg, severely and critically ill patients) or not suitable for the study by researchers. Patients who met any of the following criteria were classified as severe cases: respiratory rate ≥30 times/min, pulse oxygen saturation ≤93% at resting, or ratio between partial pressure of oxygen in arterial blood and fraction of inspired oxygen (PaO2/FiO2) ≤300 mmHg. Critical illness was defined as respiratory failure that needed mechanical ventilation or shock or exacerbation of any comorbidity that required transfer to the intensive care unit. This study was approved by the Ethics Committee of SPHCC, and informed consent was obtained from patients. The study is registered at ClinicalTrials.gov (NCT04252274).
Procedure
After informed consent, participants were randomized to the DRV/c group or the control group depending on the parity of their medical record number. All the participants received interferon alpha 2b and standard of care as per guideline recommendation in China [11]. Herein, participants in experiment group received 1 pill of DRV/c (a single-tablet regimen containing 800 mg of darunavir and 150 mg of cobicistat) per day for 5 days, while participants in the control group did not receive oral antiviral drugs.
Laboratory confirmation of SARS-CoV-2 was achieved by the Chinese Center for Disease Prevention and Control (CDC). Subsequent test of oropharyngeal swabs for SARS-CoV-2 after admission was performed by both SPHCC and CDC based on the recommendation of the National Institute for Viral Disease Control and Prevention (China), as previously described [12].
End Points
The primary end point was viral clearance rate at day 7 after randomization. After randomization, respiratory samples were collected every 1–2 days until viral clearance. Viral clearance was defined as reverse transcriptase polymerase chain reaction (RT-PCR) negative on at least 2 consecutive oropharyngeal swabs collected at least 1–2 days apart. Secondary end points included viral clearance at day 3 and day 5, the critical illness rate of subjects during the 14 days after randomization, the mortality rate of subjects at day 14, and the number of participants with treatment-related adverse events.
Statistical Analysis
Depending on the distribution of the data, categorical variables were described as frequency rates and percentages, and continuous variables were described with mean, median, and interquartile range (IQR) values. For the primary end point, both intention-to-treat (ITT) and per-protocol (PP) analysis were used. The ITT population included all the participants who were randomized, while the PP population only included the participants who completed 5 days of DRV/c regimens. Variables were compared in the 2 study groups using t tests, the Mann-Whitney test, or the χ 2 test. The log-rank test was used to assess between-group differences in the duration of viral shedding. Hazard ratios and associated 95% confidence intervals were calculated with the use of a Cox proportional hazards model. All analyses were performed using STATA, version 12.0 (StataCorp, College Station, TX, USA).
RESULTS
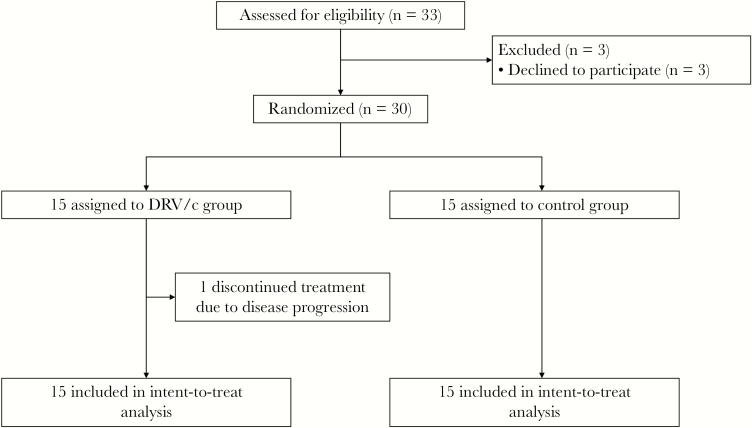
From January 30, 2020, to February 6, 2020, a total of 30 participants were enrolled, of whom 18 (60%) were male, with a mean age (SD) of 47.2 (2.8) years (Figure 1). Fever was the most common symptom of onset, occurring in 86.7% (26/30) of the participants, followed by cough, which was present in 46.7% (14/30) of the participants. Less common symptoms included sore throat (2 [6.7%]), nausea (2 [6.7%]), fatigue (1 [3.3%]), diarrhea (1 [3.3%]), and headache (1 [3.3%]). The participants were admitted (IQR) at 4 (2–5) days after onset of symptoms. On admission, 11 patients in the DRV/c group and 8 patients in the control group still presented with fever; 33.3% (10/30) of participants showed unilateral pneumonia, and the others presented with bilateral pneumonia via chest computed tomography. Despite a difference that was not significant, more patients in the DRV/c group showed bilateral pneumonia (80.0% vs 53.3%). The clinical characteristics and laboratory findings of the 2 groups were comparable (Table 1). All participants completed the study, except 1 patient in the DRV/c group who progressed to critical condition on day 4 and withdraw from the study.
Figure 1.
Trial profile. Abbreviation: DRV/c, darunavir/cobicistat.
Table 1.
Baseline Characteristics of the Intention-to-Treat Population
| DRV/c Group | Control Group | |
|---|---|---|
| Age, mean ± SD, y | 51.5 ± 12.2 | 42.9 ± 17.7 |
| Sex male, No. (%) | 9 (60) | 9 (60) |
| Days from 1 set of symptoms, median (IQR), d | 4 (2–5) | 4 (3–6) |
| Signs and symptoms at admission, No. (%) | ||
| Fever | 11 (80.0) | 8 (86.7) |
| Cough | 7 (47.7) | 7 (47.7) |
| Sore throat | 1 (6.7) | 1 (6.7) |
| Nausea | 1 (6.7) | 1 (6.7) |
| Diarrhea | 1 (3.3) | 0 |
| Fatigue | 0 (0) | 1 (6.7) |
| Chronic comorbidity | ||
| Cardiovascular diseases, No. (%) | 4 (26.7) | 4 (26.7) |
| Diabetes, No. (%) | 0 | 2 (13.3) |
| Radiological findings | ||
| Bilateral pneumonia | 12 (80) | 8 (53.3) |
| Unilateral pneumonia | 3 (20) | 7 (46.7) |
| Laboratory findings | ||
| White blood cells, mean ± SD, ×109/L | 4.6 ± 1.2 | 5.2 ± 1.6 |
| CD4 T-cell counts, mean ± SD, cells/μL | 495 ± 228 | 631 ± 365 |
| Alanine aminotransferase, median (IQR), U/L | 23 (14–38) | 21 (11–40) |
| Estimated glomerular filtration rate, median (IQR), mL/min/1.73 m2 | 99.5 (78.8–110.6) | 114.4 (106–141.7) |
| C-reactive protein, median (IQR), mg/L | 17 (6.4–33) | 9.6 (3–17.3) |
| D-dimer, median (IQR), μg/mL | 0.43 (0.31–0.8) | 0.32 (0.28–0.71) |
| Lactate, median (IQR), mmol/L | 1.3 (0.9–1.6) | 1.6 (1.1–3.1) |
Abbreviations: DRV/c, darunavir/cobicistat; IQR, interquartile range.
In the ITT analysis, the proportions of negative conservation of SARS CoV-2 at day 7 were 46.7% (7/15) and 60.0% (9/15) in the DRV/c and control groups (P = .72), respectively. In PP analysis, the proportions of negative conservation of SARS CoV-2 were 50.0% (7/14) and 60.0% (9/15) in the 2 groups (P = .72), respectively.
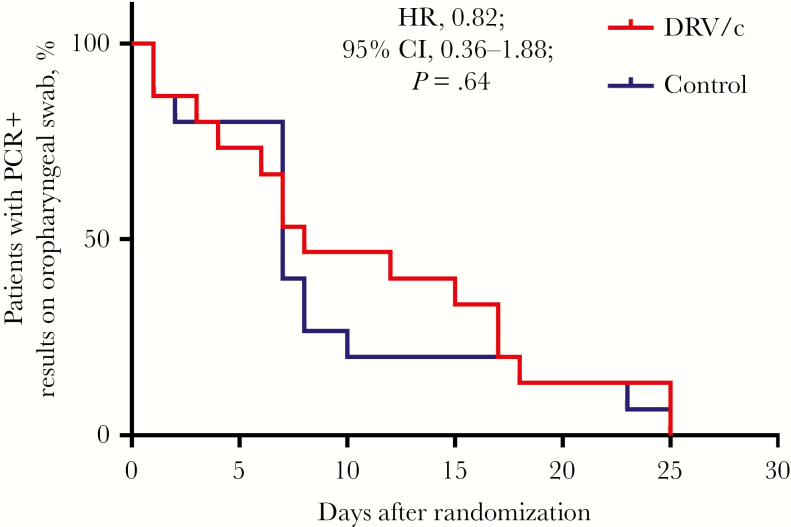
The viral clearance rate at day 3 was 20% (3/15) in both study groups. The proportion of negative PCR results was 26.7% (4/15) in the DRV/c group and 20% (3/15) in the control group at day 5. The median duration from randomization to confirmed negative PCR was 8 days and 7 days, respectively. DRV/c was not associated with faster clearance of SARS-COV-2 on oropharyngeal swab (hazard ratio, 0.82; 95% CI, 0.36–1.88) (Figure 2).
Figure 2.
Proportion of patients in each group with positive reverse transcription polymerase chain reaction results on oropharyngeal swabs during follow-up. Abbreviations: DRV/c, darunavir/cobicistat; HR, hazard ratio; PCR, polymerase chain reaction.
The 11 patients with fever in the DRV/c group defervesced at a median (IQR) of 4 (2–6) days, while 8 patients in the control groups defervesced (IQR) at 5 (2–6.8) days after randomization (P = .72). Computed tomography (CT) images from 7 patients in the DRV/c group and 4 patients in the control group showed worsening at day 7 (P = .45). Fourteen days after randomization, 1 participant in the DRV/r group progressed to critical illness (acute respiratory distress syndrome [ARDS]), while all the patients in the control group were stable (P = 1.0). This patient received mechanical ventilation on day 7. All patients were alive at day 14.
The frequencies of adverse events in the 2 groups were comparable. Diarrhea occurred in 20% (3/30) of participants in the DRV/c group and 13.3% (2/30) of participants in the control group, respectively. One patient in the DRV/c group developed anemia (hemoglobin levels dropped from 11.3 g/dL to 9.9 g/dL). Elevated transaminase levels, defined as >2-fold of the upper limit of the normal range, were observed in 13.3% (2/30) of patients in the DRV/c group and 26.7% (4/30) of patients in the control group. Renal dysfunction (defined as estimated glomerular filtration rate <90 mL/min/1.73 m2 in patients without chronic kidney diseases) occurred in 13.3% (2/30) of patients in the DRV/c group and 6.7% (1/30) of patients in the control group. All the adverse events were mild. No participants discontinued DRV/c due to these adverse events.
DISCUSSION
In this pilot study, we found that the viral clearance rate at day 7 in the DRV/c was comparable to that in the control group. In addition, the median duration of viral shedding from randomization was 8 days in the DRV/c group compared with 7 days in the control group, although there was no statistical significance. The only patient who progressed to ARDS and received mechanical ventilation was also in the DRV/c group. Taken together, our results do not suggest that 5 days of DRV/c could increase the proportion of negative conversion at day 7 vs standard of care alone. These findings are in line with the reported preliminary in vitro data of DRV only being active at 300 µM, a concentration that is much higher than what is usually achieved with oral administration of DRV/c [13]. Furthermore, it is consistent with the docking results where DRV is being virtually docked in viral proteins. The SARS-CoV-2 main protease popped up as 1 of 4 potential targets for DRV, and DRV did not come out as one of the best docked drugs [13].
To the best of our knowledge, this is the first randomized controlled trial conducted to evaluate the efficacy of DRV/c in treating COVID-19. The results in the current study are consistent with findings from a randomized controlled study that also failed to show the benefit of LPV/r treatment beyond standard care in hospitalized adult patients with severe COVID-19 [14]. Along this line, it is quite possible that HIV-1 protease inhibitors may not have clinically significant anti-SARS-CoV-2 activity.
The DRV/c was well tolerated in COVID-19 patients. Diarrhea occurred in a comparable proportion of participants in the 2 groups. It is likely that diarrhea in the DRV/c group was caused by SARS-CoV-2 rather than drug-related adverse events [15, 16]. Similarly, COVID-19 may also cause damage to other organs such as the liver, the kidneys, and the hematological system, which could explain the occurrence of adverse events including anemia, elevated transaminase levels, and kidney dysfunction observed in the current study [4, 17–19].
Our study was limited by the small sample size and open-label design. In this pilot study, we did not observe any trend of improvement in the DRV/c group compared with the control group. Second, we did not enroll severe/critical patients. The results may be not applicable to this population. Finally, the timing of antiviral administration also impacted the antiviral effects, and we do not know if earlier administration would have changed the results.
In conclusion, our pilot study does not suggest that 5 days of DRV/c could increase the proportion of negative conversion at day 7 vs standard of care alone, although it was well tolerated.
Acknowledgments
We thank all patients involved in the study. We thank Janssen for donating the DRV/c used in this study. We also thank Ling Gu and Danping Liu for their coordination.
Financial support. This work was supported by the Ministry of Science and Technology of China (2017ZX09304027); the Shanghai Science and Technology Committee (20411950200); Shanghai Major Projects on Infectious Diseases (shslczdzk01102); and the Shanghai “Rising Stars of Medical Talent” Youth Development Program, Specialist Program (No. 2019–72).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. J.C. and H.L. participated in the conceptualization of the study. J.C., L.X., L.L., Q.X., Y.L., D.H., H.W., S.S., S.X., and Y.S. were study investigators and participated in the conduct of the study, including the recruitment and follow-up of participants. J.C., Y.S., and H.L. were involved in formal data analysis, methodology, project administration, and supervision. J.C. and H.L. were responsible for funding acquisition. All authors participated in the drafting and review of the manuscript.
References
- 1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323:1239–42. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Situation report - 87 coronavirus disease 2019 (COVID-19) 2020 Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200416-sitrep-87-covid-19.pdf?sfvrsn=9523115a_2. Accessed 17 April 2020. [PubMed]
- 3. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020; 395:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan KS, Lai ST, Chu CM, et al. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med J 2003; 9:399–406. [PubMed] [Google Scholar]
- 6. Yao TT, Qian JD, Zhu WY, Wang Y, Wang GQ. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus—a possible reference for coronavirus disease-19 treatment option. J Med Virol 2020; 92(6):556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim UJ, Won EJ, Kee SJ, et al. Combination therapy with lopinavir/ritonavir, ribavirin and interferon-α for Middle East respiratory syndrome. Antivir Ther 2016; 21:455–9. [DOI] [PubMed] [Google Scholar]
- 8. Spanakis N, Tsiodras S, Haagmans BL, et al. Virological and serological analysis of a recent Middle East respiratory syndrome coronavirus infection case on a triple combination antiviral regimen. Int J Antimicrob Agents 2014; 44: 528–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Min CK, Cheon S, Ha NY, et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep 2016; 6:25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Orkin C, DeJesus E, Khanlou H, et al. Final 192-week efficacy and safety of once-daily darunavir/ritonavir compared with lopinavir/ritonavir in HIV-1-infected treatment-naïve patients in the ARTEMIS trial. HIV Med 2013; 14:49–59. [DOI] [PubMed] [Google Scholar]
- 11. National Health Commission of China. Diagnosis and treatment of pneumonia caused by 2019 new coronavirus (trial version 7) Available at: http://www.gov.cn/zhengce/zhengceku/2020-03/04/5486705/files/ae61004f930d47598711a0d4cbf874a9.pdf. Accessed 21 March 2020.
- 12. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020; 579:265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu C, Liu Y, Yang Y, et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharmaceutica Sinica B. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020; 382:1787–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen J, Qi T, Liu L, et al. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Meyer S, Bojkova D, Cinati J, et al. Lack of antiviral activity of darunavir against SARS-CoV-2. medRxiv 2020.04.03.20052548 [Preprint]. 8 April 2020. doi: 10.1101/2020.04.03.20052548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323(11):1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]