Abstract
Corona virus disease 2019 (COVID-19) patients with severe immune abnormalities are at risk of cytokine release syndrome (CRS). The definition, prevention, and treatment of symptoms of CRS in critically ill patients with COVID-19 are important problems. We report a single-center case series of 11 COVID-19 patients with acute respiratory distress syndrome from The First Affiliated Hospital of Guangzhou Medical University in China from 26 January 2020 to 18 February 2020. The termination date of follow-up was 19 February 2020. Eight patients were determined to have characteristics of CRS, including pulmonary inflammation, fever, and dysfunction of nonpulmonary organs. An increase in interleukin-6 in peripheral blood was the highest risk factor and an early indicator of CRS in COVID-19.
Keywords: COVID-19, cytokine release syndrome, IL-6, immunophenotype, severe/critical pneumonia
IL-6 was the highest risk factor and an early indicator of cytokine release syndrome in COVID-19.
In December 2019, a novel coronavirus, named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the International Committee on Taxonomy of Viruses, was discovered in Wuhan, Hubei province, China [1–3]. The disease spread rapidly to all provinces of China outside of Wuhan [4, 5]. By 17 February 2020, there were 70 636 confirmed cases and 1772 deaths in China [6]. Some of the critically ill coronavirus disease 2019 (COVID-19) patients with pneumonia had serious symptoms with no specific drug treatment indicated. Severe inflammatory reaction and respiratory distress syndrome can lead to rapid progression of the disease and may cause death. A guideline, “guidelines for the diagnosis and treatment of novel coronavirus (2019-nCoV) infection by the National Health Commission (trial version 5)” [7]. It suggested that immune factors should be examined in the therapy of COVID-19 patients with pneumonia. The release of inflammatory immune cytokines could increase inflammation of the lung and risk occurrence of acute respiratory distress syndrome (ARDS) [8]. ARDS may lead to hypoxia and lung injury, causing further release of inflammatory factors by multiple mechanisms [9–11]. Therefore, an excessive release of inflammatory factors may promote ARDS in COVID-19 patients with pneumonia.
Critically ill COVID-19 patients with pneumonia experience fever, pulmonary inflammation, respiratory distress, and other clinical signs. In published data, the proportion of patients requiring intensive care unit (ICU) treatment is 26.1% [12]. The average age of patients in the ICU is higher than that of non-ICU patients [12]. In addition, the incidence of acute heart injury and respiratory distress in ICU patients with COVID-19 is much higher than that in the general population [13]. Serious pulmonary inflammation is common in critically ill COVID-19 patients with pneumonia [14]. The large area of infection and inflammatory reaction causes many immunologic problems, such as cytokine release syndrome (CRS), which can rapidly lead to deterioration and death [15, 16]. In this study, after analysis of the clinical diagnosis and immunological characteristics of 11 critically ill COVID-19 patients with ARDS, we diagnosed 8 (72.7%) patients with features of CRS. Therefore, we defined this phenomenon as COVID-19 infection-related CRS. Our results demonstrate that IL-6 was an early indicator of CRS in COVID-19–associated pneumonia, and it is suggested that improving ventilation and controlling the area of pulmonary inflammation may be an effective method to treat COVID-19 pneumonia patients with CRS and ARDS. Our research provides experimental support and experience useful for the treatment of critically ill COVID-19 patients with pneumonia.
METHODS
Study Design and Participants
The patients enrolled in this single-center study were confirmed to have severe or critical COVID-19–associated pneumonia. The inclusion criteria were defined by the “guidelines for the diagnosis and treatment of novel coronavirus (2019-nCoV) infection by the National Health Commission (trial version 5)” [7]. Enrollment began on 26 January 2020. We obtained verbal consent from each patient. According to the results of monitoring (the date of transfer out of the ICU), the final follow-up date was 18 February 2020 for all COVID-19 patients participating in this study. Study of this series of cases was approved by the ethics committee of the First Affiliated Hospital of Guangzhou Medical University, China.
Data Collection
The team (X. L., S. C., Y. L., L. N., P. L., L. H., L. C., and Y. L.) at the First Affiliated Hospital of Guangzhou Medical University conducted an analysis of each patient’s medical records, including: (1) basic information on the patient: name, sex, age, hospitalization number, diagnosis, date of admission, condition, laboratory examination results, chest computed tomography (CT) scan, nursing care, antiviral treatment, corticosteroid treatment, and respiratory support (records were taken by a team of trained doctors and data were obtained from electronic medical records); (2) data generated by bedside monitors: heart rate, respiration, invasive/noninvasive blood pressure, blood oxygen saturation, and hemodynamic monitoring; and (3) medical order execution information: administration times for all medicines, drug name, dose, concentration, route, automatically generated quantity in and out (data were automatically collected by the ICU clinical information system).
The diagnoses of ARDS and shock were based on the World Health Organization Interim Guidelines for COVID-19. Hypoxemia was defined as arterial oxygen tension/inhaled oxygen fraction (Pao2/Fio2) below 300 mm Hg. Acute renal injury was detected according to the highest serum creatinine level or other standard urine test and classified according to the overall results of improvement in kidney disease. If the patient had clinical symptoms or signs of nosocomial pneumonia or bacteremia, it was diagnosed as a secondary infection and combined with positive culture of new pathogens from lower respiratory tract samples (including sputum, tracheal intubation suction fluid, bronchoalveolar lavage fluid, or blood samples taken ≥48 hours after admission). If the serum level of cardiac biomarkers (such as cardiac troponin I) was higher than 99% of the reference upper limit, or the electrocardiogram and echocardiography showed new abnormalities, cardiac injury was diagnosed.
RT-PCR Assay for COVID-19
Reverse transcription polymerase chain reaction (RT-PCR) was performed with a Bio-Rad CFX96 Real-Time PCR System (BioRad Laboratories) in a 20-μL reaction volumes containing 10 μL RNA, 5 μL ORF1ab/N primer provided with a COVID-19 kit (HuiruiBio), One-Step RT-PCR Master Mix Reagents from the kit, and 5 μL RNase-free H2O (TiangenBio). Thermal cycling was performed at 55°C for 15 minutes for reverse transcription, followed by 95°C for 5 minutes, 45 cycles at 95°C for 10 seconds, and then 55°C for 40 seconds. Fluorescence was recorded during the 55°C phase. Results of RT-PCR were recorded as the cycle threshold value. Samples with cycle threshold value ≥39.2 were considered negative; samples with cycle threshold value ≤35 were considered positive; and samples with cycle threshold value between 35 and 39.2 were retested.
Flow Cytometry Analysis
Ethylene diamine tetra acetic acid (EDTA) anticoagulated venous blood samples (2 mL) were collected from each of the patients with COVID-19. Peripheral blood mononuclear cells (PBMCs) were isolated using density gradient Ficoll-Paque Plus (Amersham Biosciences) centrifugation. Duplicate PBMCs (106 cells/tube) were stained with CD3-fluorescein isothiocyanate/ CD56+16-phycoerythrin/ CD45-peridinin chlorophyll-cyanine 5.5/ CD4-phycoerythrin cyanin 7/ CD19-allophycocyanin/ CD8-allophycocyanin-cyanine 7/ CD25-allophycocyanin/ CD127-phycoerythrin (Tongsheng Shidai Biote) in the dark at room temperature for 30 minutes. The cells were then fixed and permeabilized using a permeabilization solution (BD Biosciences). The mononuclear cells were gated using cell size and internal structure in a NovoCyte D3000 flow cytometer (Agilent Technologies, Palo Alto, USA). The frequency of various subsets of T cells was determined using FlowJo software version 10 (TreeStar). The number of different subsets of T cells was calculated and expressed as the number of cells per L for each patient.
Cytokine Detection
The plasma levels of interleukin-1β (IL-1β), IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12P70, IL-17A, interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and (IFN-α) were detected according to the 12 cytokine detection kit manufacturer’s instructions (Cell-Genebio). Briefly, EDTA anticoagulated blood samples were centrifuged at 800g for 20 minutes to collect the supernatant. Microspheres in buffer were added added at a volume equal to the supernatant and vortex mixed, incubated in darkness for 30 minutes, and again vortex mixed. A 25-µL volume of the microsphere mixture, with 25 µL of the supernatant and vortex mixed sample to be tested and fluorescent detection reagent, was incubated in the dark for 2.5 hours at room temperature. This was followed by the addition of 1 mL phosphate-buffered saline (PBS) and centrifugation at 200g for 5 minutes. The supernatant was discarded and the microspheres were resuspended in 110 µL PBS for flow cytometry. Fcap version 3.0 was used for data analysis; 5 replicates from each batch were analyzed giving a repeat coefficient of variation < 2%.
Statistical Analysis
The results of continuous measurements are presented as mean (SD) if they were normally distributed or median (interquartile range [IQR]) if they were not normally distributed. Categorical variables are given as count (%). For laboratory results, we assessed whether the measurements were outside the normal range. We used SPSS version 26.0 for all analyses.
Patient and Public Involvement
This was a single-center case series and no patients were involved directly in the study design, setting the research questions, or the outcome measures. No patients were asked to advise on interpretation or writing up of the results.
RESULTS
Clinical Characteristics and Laboratory Examination
Eleven critically ill COVID-19 patients with pneumonia were enrolled in the study and all were admitted to the ICU; 10 (90.9%) were male. The median age was 58 years (IQR, 49–72 years). The median time from first symptoms to respiratory distress was 10 days (IQR, 7–13 days). All patients had fever: the highest temperature was 40°C and the median was 38.5°C (IQR, 38–39.6°C). Four (36.4%) patients had muscle soreness in the early stage of fever and 9 (81.8%) had dry cough in the early stage of the disease. All patients had symptoms of hypoxia when they were admitted to the ICU. Among them, 5 (45.5%) patients had a history of cardiocerebrovascular disease and 4 (36.4%) patients had a history of type 2 diabetes. All the patients had symptoms of low oxygen saturation and metabolic acidosis (Table 1). After entering the ICU, all the patients had 1 or more manifestations of organ dysfunction or failure. These included 9 patients with cardiac dysfunction, 8 with renal dysfunction, 6 with liver dysfunction and coagulation dysfunction, 5 with coagulant function abnormality only, and 4 with multiorgan failure syndrome. Ten patients were treated with steroids and all patients had antiviral and anti-infective therapy (Table 2 and Table 3).
Table 1.
Demographics and Baseline Characteristics of COVID-19 Patients
| Characteristic | Value (n = 11) |
|---|---|
| Age, y, median (IQR) | 58 (49–72) |
| Sex | |
| Female | 1 (9.1) |
| Male | 10 (90.9) |
| Signs and symptoms at admission | |
| Fever | 11 (100.0) |
| Maximum temperate, °C, median (IQR) | 38.5 (38–39.6) |
| Fatigue | 5 (45.5) |
| Dry cough | 9 (81.8) |
| Productive cough | 1 (9.1) |
| Pharyngalgia | 1 (9.1) |
| Rhinorrhea | 0 (0.0) |
| Myalgia | 4 (36.4) |
| Diarrhea | 1 (9.1) |
| Headache | 0 (0.0) |
| More than 1 symptom or sign | 11 (100.0) |
| Exposure | |
| Close contact with Wuhan | 10 (90.9) |
| Close contact with patients in Guangzhou | 1 (9.1) |
| Chronic disease | |
| Cardiovascular and cerebrovascular diseases | 5 (45.5) |
| Hypertension | 3 (27.3) |
| Coronary heart disease | 2 (18.2) |
| Coronary atherosclerotic heart disease | 1 (9.1) |
| Diseases of digestive system | 2 (18.2) |
| Endocrine system diseases | 4 (36.4) |
| Malignant tumor | 1 (9.1) |
| Nervous system diseases | 0 (0.0) |
| Respiratory diseases | 2 (18.2) |
| Days from onset of symptom, median (IQR) | |
| Hospital admission | 5 (2–6) |
| ICU | 8 (4–13) |
| ARDS | 10 (7–13) |
Data are No. (%) except where indicated.
Abbreviations: ARDS, acute respiratory distress syndrome; ICU, intensive care unit; IQR, interquartile range.
Table 2.
Clinical Treatment of Patients With COVID-19 Pneumonia
| Comorbid Conditions and Treatments | Patients, No. (%) |
|---|---|
| Comorbid Condition | |
| Any | 11 (100.0) |
| ARDS | 11 (100.0) |
| Respiratory failure | 4 (36.4) |
| Acute renal injury | 4 (36.4) |
| Drug-induced liver injury | 2 (18.2) |
| Shock | 4 (36.4) |
| MODS | 4 (36.4) |
| Chest X-ray and CT findings | |
| Unilateral pneumonia | 0 (0.0) |
| Bilateral pneumonia | 11 (100.0) |
| Multiple mottling and ground-glass opacity | 11 (100.0) |
| Treatment | |
| Oxygen therapy | 11 (100.0) |
| Mechanical ventilation | 11 (100) |
| Invasive | 8 (72.7) |
| Noninvasive (ie, face mask) | 3 (27.3) |
| CRRT | 0 (0) |
| ECMO | 2 (18.2) |
| Antibiotic treatment | 11 (100.0) |
| Antifungal treatment | 10 (90.9) |
| Antiviral treatment | 11 (100.0) |
| Glucocorticoids | 9 (81.8) |
| Intravenous immunoglobulin therapy | 1 (9.1) |
Abbreviations: ARDS, acute respiratory distress syndrome; CRRT, continuous renal replacement therapy; CT, computed tomography; ECMO, extracorporeal membrane oxygenation; MODS, multiple organ dysfunction syndrome.
Table 3.
Laboratory Results of Patients With COVID-19 Pneumonia
| Analyte | Normal Range | Patients, Median (IQR) (n = 11) |
|---|---|---|
| Blood count | ||
| White blood cell count, ×109/L | 4.0–10.0 | 10.2 (6.5–12.4) |
| Neutrophil count, ×109/L | 1.8–8.0 | 9.6 (4.9–11.8) |
| Lymphocyte count, ×109/L | 0.9–5.2 | 0.3 (0.2–0.5) |
| Monocyte count, ×109/L | 0.16–1.0 | 0.4 (0.3–0.5) |
| Platelet count, ×109/L | 100–400 | 143 (113–210) |
| Coagulation function | ||
| Prothrombin time, s | 11–14.5 | 15.1 (14.8–15.6) |
| Activated partial thromboplastin time, s | 28–42.8 | 37.6 (35.2–52.6) |
| D-dimer, ng/mL FEU | 68–494 | 1318 (673–4757) |
| Liver function | ||
| Alanine aminotransferase, U/L | 5–40 | 24 (15.9–27.7) |
| Albumin, g/L | 35–55 | 33.6 (30.5–37.2) |
| Total bilirubin, μmol/L | 1.7–22.2 | 15.1 (11.2–20.4) |
| Direct bilirubin, μmol/L | 0–6 | 4.9 (4.1–5.7) |
| Kidney function | ||
| Serum glucose, mmol/L | 3.6–6.1 | 11.6 (7.72–16.03) |
| Serum urea nitrogen, mmol/L | 3.6–9.5 | 10.2 (7.4–17.3) |
| Serum creatinine, μmol/L | 57.0–111.0 | 86.1 (62–146.6) |
| Myocardial injury markers | ||
| Lactate dehydrogenase, U/L | 109–255 | 396.5 (357.6–529) |
| Creatine kinase, U/L | 10–190 | 154.5 (66.5–501.6) |
| Creatine kinase-myocardial band, U/L | 3–25 | 13 (8–21) |
| Myoglobin, g/L | <70 | 62.1 (37.25–389.85) |
| Troponin I, μg/L | 0–0.04 | 0.01 (0–0.04) |
| Serum B-type natriuretic peptide precursor, pg/mL | <300 | 364.8 (147.2–746.9) |
| Infection-related biomarkers | ||
| Procalcitonin, ng/mL | 0.0–5.0 | 0.32 (0.095–0.435) |
| C-reactive protein, mg/dL | 0–0.6 | 12.06 (6.24–13.75) |
| Cytokines | ||
| IL-2, pg/mL | 0–5.71 | 1.57 (1.09–1.96) |
| IL-4, pg/mL | 0–2.80 | 1.97 (1.64–2.12) |
| IL-6, pg/mL | 0–5.30 | 26.87 (14.26–92.2) |
| IL-10, pg/mL | 0–4.91 | 4.75 (3.83–11.19) |
| TNF-α, pg/mL | 0–2.31 | 1.7 (1.27–2.07) |
| IFN-γ, pg/mL | 0–7.42 | 1.46 (1.24–1.98) |
| Immunocyte detection and absolute count | ||
| CD3+CD45+, % | 50–84 | 66 (59.8–68.4) |
| CD3+CD4+, % | 26–61 | 44.1 (32.0–53.3) |
| CD3+CD8+, % | 15–44 | 18.3 (6.9–32.8) |
| CD4+/CD8+ | 1.4–2.0 | 2.09 (1.05–7.7) |
| B cell (CD3−CD19+), % | 5–18 | 28.2 (11.8–30.3) |
| NK cell (CD3−CD16+CD56+), % | 7–40 | 9.7 (3.7–12.4) |
| NK T cell (CD3+CD16+CD56+), % | 2–13 | 2.8 (0.7–5.3) |
| Treg cell (CD4+CD27+127−), % | 3–8 | 5.1 (3.3–7.8) |
| CD3+CD45+ absolute count, cells/μL | 955–2860 | 296 (169–355) |
| CD3+CD4+ absolute count, cell/μL | 550–1440 | 220 (101–308) |
| CD3+CD8+ absolute count, cells/μL | 320–1250 | 56 (33–141) |
| B cell (CD3−CD19+) absolute count, cells/μL | 90–560 | 81 (61–146) |
| NK cell (CD3−CD16+CD56+) absolute count, cells/μL | 150–1100 | 34 (8–72) |
| NK T cell (CD3+CD16+CD56+) absolute count, cells/μL | 40–300 | 10 (3–16) |
Abbreviations: FEU, fibrinogen equivalent units; IFN-γ, interferon-γ; IL, interleukin; IQR, interquartile range; NK cell, natural killer cell; TNF-α, tumor necrosis factor-α; Treg, T regulatory cell.
Analysis of Immunological Parameters
Flow cytometry was used to detect peripheral blood monocytes, including: CD3 (CD3+CD45+), CD4 (CD3+CD4+), CD8 (CD3+CD8+), natural killer (NK; CD3−CD16+CD56+), T regulatory (Tregs; CD4+CD25+CD127 low), and B lymphocytes (CD3−CD45+). On the first day after being admitted to the ICU, the absolute counts of CD3, CD4, CD8, and NK cells in the peripheral blood were lower than normal values: CD3 (IQR, 169–355; range, 50–635 cells/μL), CD4 (IQR, 101–308; range, 27–350 cells/μL), CD8 (IQR, 33–141; range, 21–277 cells/μL), and NK immune cells (IQR, 8–72; range, 5–170 cells/μL). Eight patients had a significant increase in the CD4/CD8 ratio (IQR, 1.05–7.7), which is an important index of autoimmune disease (systemic lupus erythematosus, type 1 diabetes, and rheumatoid arthritis) and transplant rejection [17] (Table 3). Three had a decrease in B lymphocytes and 2 patients had a slight increase in Tregs (Supplementary Figure 1). After treatment, the concentrations of CD4 in 2 patients and CD8 in 3 patients increased to normal levels. In addition, IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ were measured in all patients; IL-6 significantly increased in all (IQR, 14.26–99.2; range, 4.58–1182.91 ng/L) (Table 3). The level of IL-10 increased in 5 patients, while IL-4 and IFN-γ increased in 3 and 2 patients, respectively. After treatment, the concentrations of IL-6 in 3 patients, IL-10 in 4 patients, and IL-4 and IFN-γ in all patients returned to normal levels (Supplementary Figure 1).
Identification of CRS in Critically Ill COVID-19 Patients With Pneumonia
The main characteristics of CRS include a decrease in T cells and NK cells, an increase in IL-6, fever, organ and tissue dysfunction, and an abnormal coagulation function [18]. All of the 11 patients in the ICU had pulmonary infection and fever. All had a significant decrease in circulating T cells and NK cells, and an increase in IL-6 secretion in peripheral blood. In addition, all the patients had dysfunction or failure of 1 or more organs, and 7 patients had coagulation dysfunction. Eight patients showed a significant increase in the CD4/CD8 ratio, suggesting the possibility of an autoimmune response (Table 3 and Table 4). None of the patients had a known prior history of immune disease. The level of IL-6 in 3 of the patients returned to normal after treatment of the infection, and CT imaging showed that the pulmonary shadows had disappeared. These 3 patients had no evidence of extrapulmonary organ injury or blood coagulation dysfunction, and 2 of them were transferred out of the ICU. Eight of the 11 patients showed evidence of CRS features (Table 3 and Figure 1). After treatment, the levels of CD4, CD8, and NK cells were returned to normal. In addition, IL-6 and IFN-γ decreased. At the same time, the organ function of the patients improved (Table 3 and Supplementary Figure 1).
Table 4.
Severity of Illness Scores and Blood Gas Analysis in Patients With COVID-19 Pneumonia (n = 11)
| Score | Normal Range | Patients, Median (IQR) |
|---|---|---|
| Time from hospital admission to ICU admission | NA | 8 (6–10) |
| APACHE II | NA | 20 (7–22) |
| SOFA | NA | 9 (3–12) |
| pH | 7.35–7.45 | 7.38 (7.24–7.45) |
| Pao2, mm Hg | 83–108 | 106 (69.2–132) |
| Pao2:Fio2, mm Hg | 400–500 | 193 (115–210) |
| Paco2, mm Hg | 35–48 | 39.5 (37.9–46.7) |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; Fio2, fraction of inspired oxygen; ICU, intensive care unit; IQR, interquartile range; NA, not available; Paco2, partial pressure of carbon dioxide; Pao2, partial pressure of oxygen; SOFA, Sequential Organ Failure Assessment.
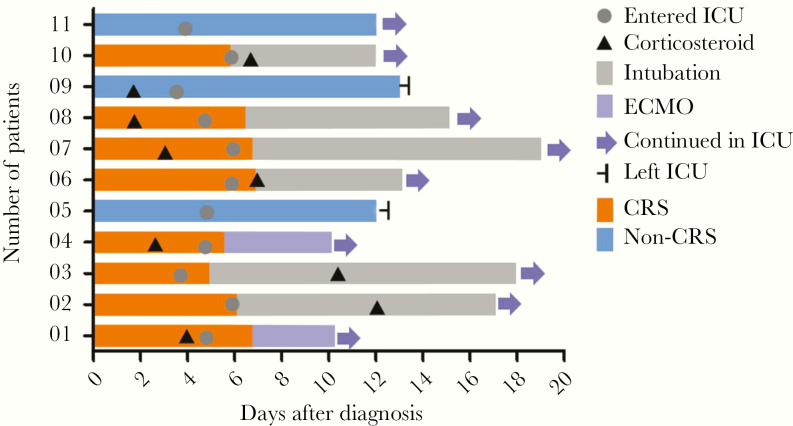
Figure 1.
Timeline of disease course according to days from definitive diagnosis of illness and days from entering ICU. Before entering the ICU, 5 patients received corticosteroid therapy. In the treatment and nursing processes of the ICU, 6 patients were intubated, 2 patients were given ECMO, and 8 patients had CRS symptoms; 2 patients who had non-CRS symptoms were transferred out of the ICU. Abbreviations: CRS, cytokine release syndrome; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit.
IL-6 Was a Sensitive Indicator for the Outcome of COVID-19 Pneumonia With CRS
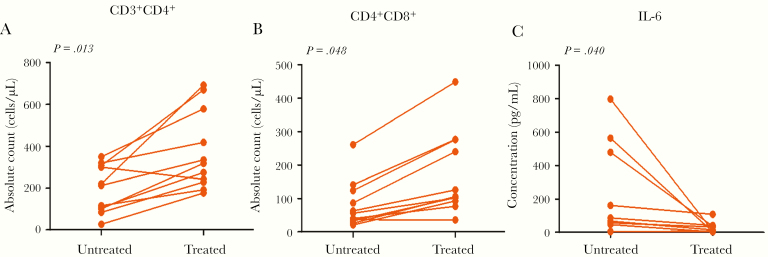
All 11 patients were admitted to the ICU because of fever and ARDS. Among them, 3 patients received noninvasive ventilation and 8 patients received invasive ventilation (including 2 given extracorporeal membrane oxygenation treatment). Four patients received drug treatment to reduce fever and 7 had physical hypothermy (ice compress). All of the patients were COVID-19 positive and received antiviral or anti-infective treatment, while 9 received hormone treatment. During the treatment period, in 9 patients there was increased peripheral CD3, in 9 patients increased CD4, in 9 patients increased CD8, and in 8 patients increased NK cells, while in 7 patients there was a decrease in CD3/CD8. After treatment, the absolute count of CD4 and CD8 T cells increased and the concentration of IL-6 decreased (Table 4 and Figure 2A–C). However, 3 patients (patient numbers 02, 03, and 07) were in a stable condition after initial ICU treatment, and their IL-6 levels showed a rapid increase (increased by 7.2 to 9.2 times) in 1 or 2 days (Supplementary Figure 2A and 2D); the absolute number of CD4 and CD8 cells decreased 1 day later than the increase in IL-6 in peripheral blood (Supplementary Figure 2B and 2C). The Pao2 and Pao2/Fio2 decreased within 1 day after the rise in IL-6 (Supplementary Figure 2D and 2E). Bedside chest radiography results showed disease progression. These cases indicate that IL-6 was an early indicator of CRS in COVID-19–associated pneumonia.
Figure 2.
The relationship between immunologic detection and CRS after coronavirus infection. A–C, Changes in immune cell subsets and cytokine IL-6 after effective treatment in the ICU. The absolute counts of CD4+ and CD8+ cells increased, while inflammatory cytokine IL-6 decreased. Abbreviations: CRS, cytokine release syndrome; ICU, intensive care unit; IL-6, interleukin-6.
Relationship of Pulmonary Inflammation and CRS Characteristics in COVID-19
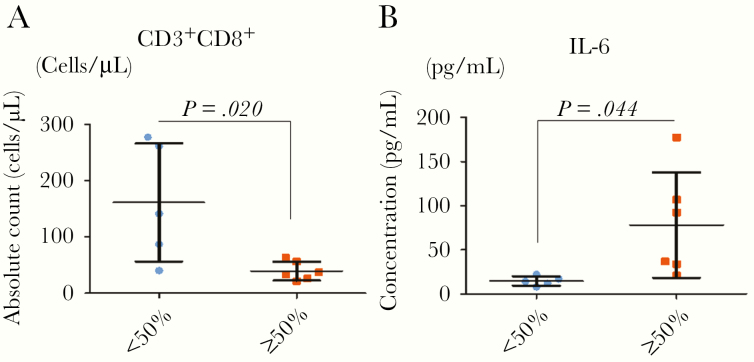
Serious pulmonary inflammation is a common symptom in critically ill COVID-19 patients with pneumonia [13]. The large area of infection and inflammatory reaction may cause CRS [14, 15]. In our study, according to the distribution of patchy shadows or ground-glass opacity in the chest CT images, we divided the patients into groups with <50% and ≥50% of the lungs affected (Supplementary Figure 2G). The numbers of CD8, CD4, and NK cells in the peripheral blood were higher in the <50% group than in the ≥50% group (Figure 3A and Supplementary Figure 2H and 2I). In addition, the level of IL-6 in the peripheral blood was lower in the patients with an area of inflammation ≥50% than in those with and area of <50% on CT imaging (Figure 3B).
Figure 3.
The relationship between pulmonary inflammation and CRS characteristics after coronavirus infection. A and B, The relationships between the shadowed area of lung and the absolute count of CD3+CD8+ and the level of IL-6 in plasma were analyzed. In patients with a shadowed area less than 50%, the absolute counts of CD8+ were higher than in patients with a shadowed area ≥50%, but the level of IL-6 showed the opposite relationship. Error bars indicate the mean ± SD. P < .001 in t test. Abbreviations: CRS, cytokine release syndrome; IL-6, interleukin-6.
DISCUSSION
This was a single-center study of 11 critically ill COVID-19 patients admitted to the ICU with persistent fever and ARDS. In addition to routine laboratory tests in the ICU, the number of immune cell subsets and cytokine levels in peripheral blood were analyzed in all patients. After analysis of the results, clinical characteristics, and diagnoses in all patients, we identified 8 (72.7%) COVID-19 critical patients with pneumonia who had the clinical characteristics of CRS.
Lung injury has a potential risk for CRS [19]. Cytokine release is common in immunotherapy, especially in cellular immunotherapy. The reasons for the occurrence of CRS are unclear, although the main mechanism is that inflammatory cells, such as effector T cells and macrophages, accumulate rapidly from peripheral blood in response to chemokines and release a large number of cytokines into the blood when they kill tumor cells, viruses, or bacteria [20]. CRS is characterized by a marked increase in specific cytokines (such as IL-6), persistent fever, and organ and tissue damage [18]. The chain reaction caused by CRS usually results in rapid immune-related organ injury and acute functional failure [21]. In our study, the patients with severe pneumonia showed an increase in IL-6, and the level of IL-6 was associated with the extent of pulmonary inflammation. It has been suggested that an increase in IL-6 is caused by severe pneumonia and the immune reaction in the lung. However, the decrease in IL-6 in patients during recovery from severe pneumonia was earlier than the resolution of the area of pulmonary shadowing. Together with findings from previous publications, our results indicate that IL-6 may act as a prognostic factor in COVID-19 patients with severe pneumonia.
From analysis of the clinical and immunological characteristics of 11 COVID-19 patients with severe pneumonia we found that these patients had different degrees of fever; peripheral blood CD4, CD8, NK, and other immune T cells decreased; CD4/CD8 and IL-6 increased significantly; multiple organ injuries were present; and coagulation dysfunction occurred. The concentration of IL-6 and the number of CD4, CD8, and NK immune cells in the peripheral blood can be significantly reduced by improving ventilation, lowering body temperature, and using anti-inflammatory and other supportive treatment. The function of organs and tissues will thus be improved, which demonstrates the importance of cytokines. COVID-19 patients with severe pneumonia have symptoms and manifestations similar to those of CRS. Therefore, we define this phenomenon as COVID-19–related CRS, which is characterized by: (1) a substantial increase in IL-6 cytokine in peripheral blood; (2) continuous fever; and (3) organ and tissue damage caused by the cytokine-related immune reaction and coagulation dysfunction. We found that 8 of 11 (72.7%) critically ill patients in our study had characteristics consistent with CRS.
Large-scale lung injury caused by viral pneumonia is the inducing factor for COVID-19–related CRS. Given that large-scale lung injury is a typical feature of critical patients, COVID-19–related CRS may be a common phenomenon in critically ill patients. We found that a large area of lung injury (≥50%) with a decrease in CD4 and CD8 cells (<50% minimum normal range) and an increase in IL-6 in peripheral blood were the most important risk factors for CRS. With COVID-19–associated pneumonia in critically ill patients, the change in IL-6 levels occurred 1 or 2 days earlier than the decreases in the absolute number of CD4 and CD8 cells in peripheral blood. This suggests that IL-6 may be an early indicator of CRS in COVID-19–related pneumonia.
This study has several limitations. First, we studied only 11 critical patients in a single center and we were unable to conduct a group comparison study. Additional data from critical patients elsewhere in China would be useful. Second, due to the strong infectivity of the virus, we were not able to construct an animal model of COVID-19–related pneumonia. A validated model could help clarify the molecular mechanism of CRS caused by viral pneumonia. Third, we have not carried out a trial on the treatment of COVID-19–associated pneumonia with IL-6 monoclonal antibody.
CONCLUSIONS
We identified and defined CRS in 11 critically ill patients with COVID-19–related pneumonia. IL-6 was an early indicator of CRS in COVID-19 pneumonia. We also found that reducing injury to the lung is a useful method to prevent and improve pneumonia-related CRS in critically ill patients with COVID-19.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. X. L. and Y. L., conceptualized the paper. W. W. and S. W. analyzed the data, with input from P. L., L. H., S. C., L. N., L. C., and Y. L. The initial draft was written by W. W. with all authors providing critical feedback and edits to subsequent revisions. All authors approved the final draft of the manuscript. J. H. is the guarantor.
Disclaimer. The funder played no role in the writing of this article.
Financial support. This work was supported by the National Natural Science Foundation of China (grant number 81972200); and Guangdong Province (grant numbers 2019A1515011937 Basic and Applied Research Fund; 201904010028 Science and Technology Plan; 2020A111128033 Special Fund for Science and Technology Innovation Strategy; and 2020B1111340002 Special Emergency Project of Science and Technology Department).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Huang C, Wang Y, Li X, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan JF, Yuan S, Kok KH, et al. . A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020; 395:514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu F, Zhao S, Yu B, et al. . A new coronavirus associated with human respiratory disease in China. Nature 2020; 579:265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li Q, Guan XH, Wu P, et al. . Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020; 382:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tan WJ, Zhao X, Ma XJ, et al. . A novel coronavirus genome identified in a cluster of pneumonia cases—Wuhan, China 2019–2020. China CDC Weekly 2020; 2:61–2. [PMC free article] [PubMed] [Google Scholar]
- 6. National Health Commission of the People’s Republic of China. http://www.nhc.gov.cn/. 2020. [DOI] [PMC free article] [PubMed]
- 7. Lin L, Li TS. Interpretation of “guidelines for the diagnosis and treatment of novel coronavirus (2019-nCoV) infection by the National Health Commission (trial version 5)” [in Chinese]. Zhonghua Yi Xue Za Zhi 2020; 100:E001. [DOI] [PubMed] [Google Scholar]
- 8. Huppert LA, Matthay MA, Ware LB. Pathogenesis of acute respiratory distress syndrome. Semin Respir Crit Care Med 2019; 40:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Butt Y, Kurdowska A, Allen TC. Acute lung injury: a clinical and molecular review. Arch Pathol Lab Med 2016; 140:345–50. [DOI] [PubMed] [Google Scholar]
- 10. Mokra D, Kosutova P. Biomarkers in acute lung injury. Respir Physiol Neurobiol 2015; 209:52–8. [DOI] [PubMed] [Google Scholar]
- 11. Paolone S. Extracorporeal membrane oxygenation (ECMO) for lung injury in severe acute respiratory distress syndrome (ARDS): review of the literature. Clin Nurs Res 2017; 26:747–62. [DOI] [PubMed] [Google Scholar]
- 12. Wang D, Hu B, Hu C, et al. . Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Panitchote A, Mehkri O, Hastings A, et al. . Factors associated with acute kidney injury in acute respiratory distress syndrome. Ann Intensive Care 2019; 9:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu X, Yu C, Zhang L, Luo L, Liu J. Imaging features of 2019 novel coronavirus pneumonia. Eur J Nucl Med Mol Imaging 2020; 47:1022–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao H, Chen H, Xiaoyin M, et al. . Autophagy activation improves lung injury and inflammation in sepsis. Inflammation 2019; 42:426–39. [DOI] [PubMed] [Google Scholar]
- 16. Tanaka T, Narazaki M, Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy 2016; 8:959–70. [DOI] [PubMed] [Google Scholar]
- 17. Carvajal Alegria G, Gazeau P, Hillion S, Daïen CI, Cornec DYK. Could lymphocyte profiling be useful to diagnose systemic autoimmune diseases? Clin Rev Allergy Immunol 2017; 53:219–36. [DOI] [PubMed] [Google Scholar]
- 18. Uciechowski P, Dempke WCM. Interleukin-6: a masterplayer in the cytokine network. Oncology 2020; 98:131–7. [DOI] [PubMed] [Google Scholar]
- 19. Kogure Y, Ishii Y, Oki M. Cytokine release syndrome with pseudoprogression in a patient with advanced non-small cell lung cancer treated with pembrolizumab. J Thorac Oncol 2019; 14:e55–7. [DOI] [PubMed] [Google Scholar]
- 20. Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. . Cytokine release syndrome. J Immunother Cancer 2018; 6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu E, Marin D, Banerjee P, et al. . Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med 2020; 382:545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.