Abstract
Background
Despite the ongoing spread of coronavirus disease 2019 (COVID-19), knowledge about factors affecting prolonged viral excretion is limited.
Methods
In this study, we retrospectively collected data from 99 hospitalized patients with coronavirus disease 2019 (COVID-19) between 19 January and 17 February 2020 in Zhejiang Province, China. We classified them into 2 groups based on whether the virus test results eventually became negative. Cox proportional hazards regression was used to evaluate factors associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) shedding.
Results
Among 99 patients, 61 patients had SARS-CoV-2 clearance (virus-negative group), but 38 patients had sustained positive results (virus-positive group). The median duration of SARS-CoV-2 excretion was 15 (interquartile range, 12–19) days among the virus-negative patients. The shedding time was significantly increased if the fecal SARS-CoV-2 RNA test result was positive. Male sex (hazard ratio [HR], 0.58 [95% confidence interval {CI}, .35–.98]), immunoglobulin use (HR, 0.42 [95% CI, .24–.76]), APACHE II score (HR, 0.89 [95% CI, .84–.96]), and lymphocyte count (HR, 1.81 [95% CI, 1.05–3.1]) were independent factors associated with a prolonged duration of SARS-CoV-2 shedding. Antiviral therapy and corticosteroid treatment were not independent factors.
Conclusions
SARS-CoV-2 RNA clearance time was associated with sex, disease severity, and lymphocyte function. The current antiviral protocol and low-to-moderate dosage of corticosteroid had little effect on the duration of viral excretion.
Keywords: SARS-CoV-2, viral excretion, disease severity, risk factors, lymphocyte function
Male sex, immunoglobulin use, APACHE II score, and lymphocyte count were independent factors associated with the duration of SARS-CoV-2 RNA shedding. The current antiviral protocol and low-to-moderate dosage of corticosteroid had little effect on the duration of viral excretion.
Since December 2019, a pneumonia of unknown cause sparked an outbreak in Wuhan, China, which spread rapidly to other provinces of China and then to other countries. The causative pathogen was identified as a novel β-coronavirus, which was named the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1], and the disease was named coronavirus disease 2019 (COVID-19). The basic clinical and epidemiologic characteristics of newly emerging COVID-19 had been reported in different cases [2, 3]. The SARS-CoV-2 infection causes severe respiratory symptoms, which indicates that the respiratory tract is the main source of human-to-human transmission. In addition, SARS-CoV-2 RNA has been detected in stool specimens as well as gastrointestinal tissue, which indicates that the fecal-oral route might be another nonnegligible transmission method [4]. So far, there is no vaccine or effective antiviral treatment for SARS-CoV-2.
Little information is available on the duration of SARS-CoV-2 excretion and the factors influencing viral shedding. It was reported that the RNA of Middle East respiratory syndrome coronavirus (MERS-CoV) could be detected in respiratory specimens for >1 month [5]. Severe acute respiratory syndrome coronavirus (SARS-CoV) RNA could be detected in sputum for 20 days and even >100 days in stool specimens from illness onset [6]. Prolonged viral shedding in the respiratory tract is associated with severe outcomes in patients with seasonal influenza and MERS-CoV [7, 8]. Features of viral shedding are useful for the evaluation of disease pathogenesis, disease course characteristics, and treatment effects for SARS-CoV-2 infection.
Hence, we conducted a study on 99 hospitalized patients with confirmed SARS-CoV-2 infection identified between 19 January and 17 February 2020. We compared the clinical characteristics between patients with negative virus detection during hospitalization and those with persistent positive virus detection to evaluate the impact of different factors on the duration of SARS-CoV-2 shedding.
PATIENTS AND METHODS
We performed a single-center, retrospective cohort study from 19 January to 17 February 2020 of the SARS-CoV-2 outbreak in Zhejiang Province at the First Affiliated Hospital of Zhejiang University. A total of 99 SARS-CoV-2–infected patients, including 15 patients transferred from other hospitals, were recruited in this study. All patients were observed for 28 days after admission unless they met the discharge criteria. COVID-19 was diagnosed on the basis of interim World Health Organization guidance [9]. Only patients with laboratory-confirmed infection, including 1 asymptomatic patient, were enrolled in this study. The asymptomatic patient was admitted to the hospital with his relatives and presented with fever and cough symptoms after admission for 2 days. The criteria for discharge were the absence of fever for at least 3 days, improvements in chest computed tomography and clinical symptoms, and 2 consecutive negative results for SARS-CoV-2 RNA in respiratory samples obtained at least 1 day apart. Respiratory specimens (nasopharyngeal swabs, sputum, or endotracheal aspirates) and stool specimens were collected daily to determine the amount of SARS-CoV-2 RNA by using real-time reverse-transcription polymerase chain reaction (RT-PCR), as previously described [3]. We defined COVID-19 patients as virus negative if the respiratory specimens were SARS-CoV-2 RNA negative across >2 intervals. Otherwise, we defined patients as virus positive if their specimens were persistently SARS-CoV-2 RNA positive during hospitalization.
Data Collection
The clinical data included demographic characteristics, medical comorbidities, laboratory findings, progression, and treatment of clinical illness. Documented medical comorbidities included hypertension, diabetes mellitus, cardiac diseases, chronic lung disease, pregnancy, and immunosuppressive disease. Complete blood counts, coagulation profiles, inflammatory indexes including C-reactive protein (CRP) and procalcitonin (PCT), and serum biochemical parameters including renal and liver function, creatine kinase, and lactate dehydrogenase were tested for all patients at admission.
This study was approved by the National Health Commission of China and the Ethics Commission of the First Hospital of Zhejiang Province. Written informed consent was obtained from all participating patients.
Shedding Duration
Shedding duration was defined as the time from illness onset to viral shedding cessation. Illness included, but was not limited to, the following symptoms: fever, sore throat, cough, runny nose, nausea and vomiting, diarrhea, and fatigue. Illness onset was defined as the first appearance of the symptoms or the first RT-PCR–positive result for the virus for asymptomatic patients. Shedding cessation was defined as the occurrence of 2 consecutive RT-PCR negative results of respiratory specimens in a 24-hour interval.
Statistical Analyses
For most variables, descriptive statistics such as median with interquartile range (IQR; for data with skewed distribution) and proportion (%) were calculated. The t test, Mann–Whitney U test, and Kruskal–Wallis test were used for continuous variables. The χ 2 test and Fisher exact test were used for categorical variables. To identify risk factors associated with a prolonged duration of SARS-CoV-2 RNA shedding, we performed a time-dependent Cox proportional hazards model adjusted for baseline covariates. Outcomes were defined as time to SARS-CoV-2 RNA negativity, and we censored patients if they never cleared SARS-CoV-2 RNA during hospitalization. Kaplan–Meier curves were used to estimate the cumulative SARS-CoV-2 RNA negativity rate and the stratified log-rank statistic to compare the differences in viral clearance between the 2 groups. Statistical analyses were performed using SPSS software, version 20.0. For all analyses, probabilities were 2-tailed, and a 2-tailed P value of < .05 was considered significant.
RESULTS
Patient Characteristics
A total of 99 patients with SARS-CoV-2 infection were included in this study from 19 January to 17 February 2020. During hospitalization, 61 patients (61.6%) had SARS-CoV-2 clearance (virus-negative group), but 38 patients (38.4%) still tested positive for SARS-CoV-2 RNA (virus-positive group). The demographic and clinical characteristics of COVID-19 patients are summarized in Table 1. The median age was 54 (IQR, 39–46) years, and 22.2% of the patients were aged >65 years. The median age of patients in the virus-positive group was significantly greater than that of those in the virus-negative group (61.5 [IQR, 50.3–72.3] years vs 50 [IQR, 38.5–59] years; P = .001). The number of elderly patients (>65 years old) in the virus-positive group was significantly higher than that in the virus-negative group (P = .006). A total of 61.6% of patients were male, but no significant difference in sex was found between the 2 groups. Hypertension (n = 36 [36.4%]) and diabetes mellitus (n = 16 [16.2%]) were the most common comorbidities among the COVID-19 patients. Additionally, a few patients had chronic lung disease, cardiac disease, immunosuppressive disease, and pregnancy, but there were no significant differences between the 2 groups (Table 1).
Table 1.
Clinical Characteristics of 99 Hospitalized Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection
| Characteristic | Total (N = 99) | Virus Negative (n = 61) | Virus Positive (n = 38) | P Value |
|---|---|---|---|---|
| Demographic | ||||
| Age, y, median (IQR) | 54 (39–64) | 50 (38.5–59) | 61.5 (50.3–72.3) | .001 |
| >65 y old | 22 (22.2) | 8 (13.1) | 14 (36.8) | .006 |
| BMI, kg/m2, median (IQR) | 24.2 (21.9–26) | 24.2 (21.7–26.0) | 24.0 (22.1–26.2) | .84 |
| Male sex | 61 (61.6) | 35 (57.4) | 26 (68.4) | .20 |
| Current smoking | 13 (13.1) | 8 (13.1) | 5 (13.2) | 1.00 |
| Comorbidity | ||||
| Hypertension | 36 (36.4) | 19 (31.1) | 17 (44.7) | .17 |
| Diabetes mellitus | 16 (16.2) | 8 (13.1) | 8 (21.1) | .29 |
| Chronic lung diseasea | 5 (5.1) | 2 (3.3) | 3 (7.9) | .37 |
| Cardiac diseaseb | 7 (7.1) | 2 (3.3) | 5 (13.2) | .10 |
| Immunosuppressionc | 2 (2.0) | 1 (1.6) | 1 (2.6) | 1.00 |
| Pregnancy | 3 (3.0) | 3 (4.9) | 0 | .28 |
| Initial laboratory findings, median (IQR) | ||||
| PaO2/FiO2, mm Hg | 274.6 (187.4–383) | 288.3 (228.4–398.4) | 250.3 (149.8–370.4) | .03 |
| Leukocyte count, ×109 cells/L | 5.9 (4.1–10.0) | 5.2 (3.6–8.4) | 7.2 (4.8–10.8) | .07 |
| Lymphocyte count, ×109 cells/L | 0.8 (0.5–1.2) | 0.9 (0.6–1.3) | 0.5 (0.3–0.9) | .001 |
| ALB, g/L | 38 (33.9–43.4) | 40.6 (35.3–43.6) | 35.8 (32.4–40.8) | .03 |
| CRP, mg/L | 23.9 (9.1–51.1) | 16.1 (7.8–33.1) | 45 (12.9–81.8) | .01 |
| PCT, ng/mL | 0.06 (0.03–0.09) | 0.06 (0.03–0.08) | 0.07 (0.03–0.25) | .01 |
| CD4+ T cells/μL | 149 (79–324) | 199 (108–476.3) | 94 (67–210) | .01 |
| CD8+ T cells/μL | 133 (77–278) | 152.5 (99.5–374.5) | 79 (49–159) | .02 |
| B cells/μL | 112 (66–178) | 146.5 (79.3–188.8) | 70 (32–123) | .01 |
| NK cells/μL | 106 (65–187) | 133 (69.3–207.5) | 97 (53–158) | .06 |
Data are presented as No. (%) unless otherwise indicated. Values in boldface indicate statistical signifcance (P < .05).
Abbreviations: ALB, albumin; BMI, body mass index; CRP, C-reactive protein; IQR, interquartile range; NK, natural killer; PaO2/FiO2, ratio of partial pressure of oxygen in arterial blood/fraction of inspiration O2; PCT, procalcitonin.
aChronic lung disease includes chronic obstructive pulmonary disease and interstitial lung disease.
bCardiac disease includes congestive heart disease and coronary atherosclerotic heart disease.
cImmunosuppression is defined as the receipt of anti-rejection drugs, or AIDS.
Laboratory Abnormalities
Among laboratory indicators tested at admission, inflammatory indexes such as CRP (P = .01) and PCT (P = .01) of the persistently virus-positive patients were significantly higher than those of virus-negative patients. The oxygenation index (P = .03), lymphocyte counts (P = .001), albumin (P = .03), CD4+ T cells (P = .01), CD8+ T cells (P = .02), and B cells (P = .01) were significantly lower in the virus-positive group than in the virus-negative group (Table 1). Other laboratory indicators, such as leukocyte count, hemoglobin, platelet count, coagulation profile, creatine, liver function, and myocardial enzyme showed no difference between the 2 groups (Supplementary Table 1). From the results, persistent virus-positive patients showed lower immune cell counts and higher inflammation levels.
Prolonged Duration of SARS-CoV-2 Shedding
Only 1 patient (1.6%) tested SARS-CoV-2 negative within 5 days, 9 patients (14.7%) tested negative within 10 days, and 49 (80.3%) tested negative within 20 days from illness onset. In addition, a small subset of 12 patients with SARS-CoV-2 had detectable levels of the virus up to 30 days from symptom onset. The median time of persistent viral shedding was 16 (IQR, 13–21) days. The virus-positive group showed a median viral shedding time of 19 days, which was significantly longer than that in the virus-negative group (median, 15 [IQR, 12–19] days; P = .002; Table 2). No apparent difference in time from illness onset to COVID-19 diagnosis was found between the 2 groups. SARS-CoV-2 RNA was positively detected in the stool specimens of 21 patients (21.2%), 9 of whom were virus negative (14.8%) and 12 of whom were virus positive (31.6%; P < .01). The results of fecal SARS-CoV-2 RNA testing turned negative in only 4 patients (19.0%) before 17 February and remained positive in 11 patients (18.0%) after testing for sputum viral RNA turned negative.
Table 2.
Treatment and Outcome of 99 Hospitalized Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection
| Variable | Total (N = 99) | Virus Negative (n = 61) | Virus Positive (n = 38) | P Value |
|---|---|---|---|---|
| Virological result, d | ||||
| Days of viral persistent positive | 16 (13–21) | 15 (12–19) | 19 (15–24) | .002 |
| Days from illness onset to diagnosis | 5 (2–7) | 5 (2.5–7.5) | 4 (1–6) | .05 |
| Fecal viral positive, No. (%) | 21 (21.2) | 9 (14.8) | 12 (31.6) | .000 |
| Treatment | ||||
| Antiviral treatment, No. (%) | 97 (97.9) | 61 (100) | 36 (94.7) | .15 |
| Days from illness onset to antiviral treatment start | 6 (4–8.5) | 6 (4–9.5) | 5.5 (3–7.8) | .15 |
| Two-antiviral combination therapy, No. (%) | 81 (81.8) | 50 (82) | 31 (81.6) | .96 |
| Corticosteroid treatment, No. (%) | 77 (77.8) | 47 (77) | 30 (78.9) | .82 |
| Days from illness onset to corticosteroid start | 8 (6–10) | 8 (6–10) | 7.5 (5–10) | .35 |
| Initial dosage of corticosteroid, mg/d | 60 (40–80) | 40 (40–80) | 60 (40–80) | .47 |
| Antibiotic treatment, No. (%) | 49 (49.5) | 27 (44.3) | 22 (57.9) | .19 |
| Days from illness onset to antibiotic start | 9 (6–13) | 9 (6–14) | 9.5 (5.5–11) | .29 |
| Intravenous immunoglobulin, No. (%) | 43 (43.4) | 19 (31.1) | 24 (63.2) | .002 |
| Days from illness onset to immunoglobulin use | 8 (6–11) | 8 (5–9) | 8 (6–12) | .44 |
| Severity score and outcome | ||||
| APACHE II | 6 (3–11) | 5 (2–8) | 9.5 (5–15) | .000 |
| ICU admission, No. (%) | 30 (30.3) | 10 (16.4) | 20 (52.6) | .000 |
| ICU length of stay, d | 7.5 (4–11) | 4 (3–5.8) | 8.5 (6.3–11) | .000 |
| Mechanical ventilation, No. (%) | 12 (12.1) | 2 (3.3) | 10 (26.3) | .001 |
| ECMO, No. (%) | 6 (6.1) | 0 | 6 (15.8) | .000 |
Data are presented as median (interquartile range) unless otherwise indicated. Values in boldface indicate statistical signifcance (P < .05).
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit.
Treatment Disparity Between 2 Groups
A majority of the patients (97.9%) received antiviral treatment, and 81 patients (81.8%) received 2 combined antiviral treatments with arbidol (200 mg 3 times daily) and lopinavir and ritonavir (LPV/RTV, 400 mg twice daily and 100 mg twice daily, respectively), whereas 16 patients (16.2%) received single antiviral treatment with arbidol or LPV/RTV (Table 2). Darunavir (800 mg once daily) was prescribed to replace LPV/RTV if patients experienced significant drug side effects. The duration from illness onset to antiviral treatment start was 6 (IQR, 4–8.5) days. There was no significant difference in antiviral treatment start time between the virus-positive and virus-negative groups. A total of 77 patients (77.8%) received corticosteroid treatment with an initial dosage of 60 (IQR 40–80) mg/day, and the time from illness onset to corticosteroid treatment was 8 (IQR 6–10) days. There was no significant difference in the application, initial dosage, or start time of corticosteroid treatment between the 2 groups (Table 2). Forty-nine patients (49.5%) were treated with antibiotics, and the median time from illness onset to antibiotic use was 9 (IQR 6–13) days. There was no difference in antibiotic use proportion or antibiotic start time between the 2 groups (P > .05). In addition, 43 patients (43.4%) received intravenous immunoglobulin treatment. The proportion of patients receiving immunoglobulin therapy in the virus-positive group was much higher than in the virus-negative group (63.2% vs 31.1%; P = .002).
Clinical Outcome
The patients with persistent SARS-CoV-2 RNA positivity showed greater disease severity (Table 2). Among 99 COVID-19 patients, a total of 30 (30.3%) were transferred to the intensive care unit (ICU), and the patients in the virus-positive group had a higher rate of ICU admission than the virus-negative group (52.6% vs 16.4%; P < .01). The median length of ICU stay for the 30 patients was 7.5 (IQR, 4–11) days, and in the virus-positive group, the length of ICU stay was 8.5 (IQR, 6.3–11) days, which was longer than that in the virus-negative group (4 [IQR 3–5.8] days; P < .01). The Acute Physiology and Chronic Health Evaluation II (APACHE II) score was used to evaluate the severity of hospitalized patients as well as to predict mortality [10]. The APACHE II score was 9.5 (IQR, 5–15) in virus-positive group, which was much higher than that of virus-negative patients, with a score of 5 (IQR, 2–8). Mechanical ventilation was performed in a minority of patients (12.1%). Compared with the virus-negative group, the virus-positive group had a higher rate of mechanical ventilation (26.3% vs 3.3%; P < .01). Moreover, all patients receiving extracorporeal membrane oxygenation treatment were from the virus-positive group (15.8% vs 0%; P < .01), indicating more severe lung injury and oxygenation.
Risk Factors for Prolonged Viral Shedding
We conducted a multivariable time-dependent Cox proportional hazards model to identify risk factors associated with the duration of SARS-CoV-2 RNA shedding (Table 3). Male sex (hazard ratio [HR], 0.58 [95% confidence interval {CI}, .35–.98]), immunoglobulin use (HR, 0.42 [95% CI, .24–.76]), APACHE II score (HR, 0.89 [95% CI, .84–.96]), and lymphocyte count (HR, 1.81 [95% CI, 1.05–3.10]) were independent factors associated with the duration of SARS-CoV-2 RNA shedding, whereas current smoking, hypertension, diabetes, days from illness onset to antiviral therapy, antiviral combination therapy, corticosteroid treatment, and days from illness onset to corticosteroid treatment and antibiotic treatment were not independent factors (Table 3). Interestingly, the use of immunoglobulin may prolong the duration of viral shedding. We found that the disease tended to be more severe in patients prescribed immunoglobulins, and these patients were older with higher APACHE II scores and worse immune function than those who did not use immunoglobulins (Supplementary Table 2).
Table 3.
Multivariable Analyses of Factors Associated With Duration of Severe Acute Respiratory Syndrome Coronavirus 2 RNA Detection in 99 Hospitalized Patients
| Variable | Unadjusted HR (95% CI) | P Value | Adjusted HR (95% CI) | P Valuea |
|---|---|---|---|---|
| Age | 0.99 (.97–1.00) | .067 | 0.99 (.97–1.00) | .098 |
| Male sex | 0.56 (.34–.94) | .029 | 0.58 (.35–.98) | .042 |
| Current smoking | 0.98 (.46–2.07) | .982 | 1.48 (.65–3.35) | .345 |
| Hypertension | 0.58 (.33–1.02) | .058 | 0.63 (.36–1.10) | .104 |
| Diabetes | 0.87 (.41–1.82) | .865 | 1.18 (.53–2.62) | .680 |
| Days from illness onset to antiviral treatment start | 0.96 (.90–1.02) | .221 | 0.95 (.89–1.01) | .105 |
| Two-antiviral combination therapy | 0.72 (.38–1.39) | .331 | 0.90 (.45–1.81) | .899 |
| Corticosteroid treatment | 0.85 (.46–1.57) | .599 | 1.00 (.53–1.89) | .990 |
| Days from illness onset to corticosteroid start | 0.94 (.87–1.02) | .127 | 0.94 (.86–1.02) | .121 |
| Immunoglobulin use | 0.38 (.22–.66) | .001 | 0.42 (.24–.76) | .004 |
| Days from illness onset to immunoglobulin start | 0.91 (.81–1.03) | .136 | 0.93 (.82–1.05) | .231 |
| Antibiotic treatment | 0.61 (.37–1.03) | .614 | 0.70 (.42–1.19) | .187 |
| APACHE II score | 0.89 (.85–.95) | .000 | 0.89 (.84–.96) | .002 |
| Lymphocyte count | 1.86 (1.16–2.99) | .010 | 1.81 (1.05–3.10) | .033 |
Values in boldface indicate statistical signifcance (P < .05).
aResults from the time-dependent Cox proportional hazard model. An HR of <1 indicates that the variable increases the duration of severe acute respiratory syndrome coronavirus 2 shedding. HRs in multivariable analyses were adjusted for age and sex.
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; CI, confidence interval; HR, hazard ratio.
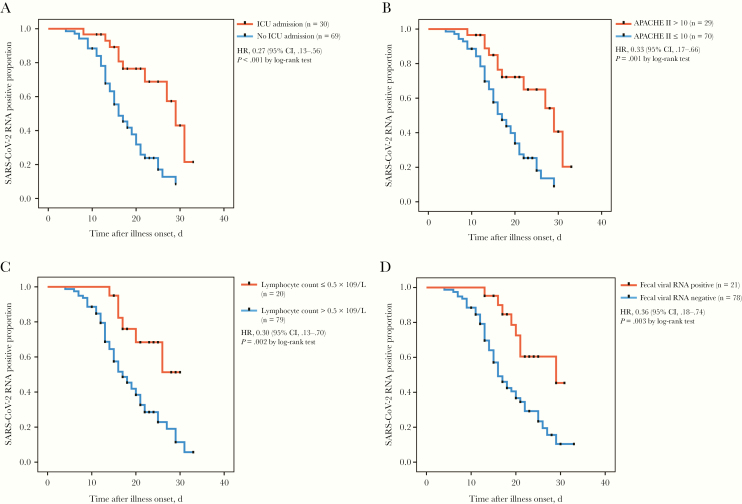
Using Kaplan–Meier survival analysis, we found that SARS-CoV-2 RNA clearance was associated with disease severity, which was significantly delayed in patients who were transferred to ICU and whose APACHE II score was >10 compared with that of patients in the general ward and whose APACHE II score was ≤10 (log-rank test, P < .001; Figure 1A and 1B). Compared with patients with a high lymphocyte count (>0.5 × 109/L), patients with low immunity (lymphocyte count ≤0.5 × 109/L) showed a significant delay in viral clearance (log-rank test, P = .002; Figure 1C). It took longer for fecal viral RNA-positive patients to clear SARS-CoV-2 RNA than fecal viral RNA-negative patients (log-rank test, P = .003; Figure 1D).
Figure 1.
A, Cumulative proportion of patients with detectable severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA among patients who were transferred to intensive care and those who were in the general ward, by day after illness onset. B, Cumulative proportion of patients with detectable SARS-CoV-2 RNA among those with Acute Physiology and Chronic Health Evaluation II score >10 and or ≤10, by day after illness onset. C, Cumulative proportion of patients with detectable SARS-CoV-2 RNA among those whose lymphocyte count was ≤0.5×109/L and those whose lymphocyte count was >0.5 ×109/L, by day after illness onset. D, Cumulative proportion of patients with detectable SARS-CoV-2 RNA among those with positive fecal viral RNA and those with negative fecal viral RNA, by day after illness onset. Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; CI, confidence interval; HR, hazard ratio; ICU, intensive care unit; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
DISCUSSION
Despite the ongoing spread of COVID-19, knowledge of the factors affecting prolonged viral shedding is still limited. Thus, we conducted a retrospective, single-center study of 99 hospitalized patients with confirmed SARS-CoV-2 infection identified between 19 January and 17 February 2020 in Zhejiang Province, China. We identified that male sex, immunoglobulin use, APACHE II score, and lymphopenia were independent risk factors associated with the duration of SARS-CoV-2 RNA shedding, whereas antiviral combination therapy and corticosteroid treatment were not independent factors. In addition, patients with a positive fecal viral RNA test needed more time to clear SARS-CoV-2.
Consistent with previous findings, the median age of hospitalized patients with SARS-CoV-2 infection was 54 years, and 22.2% patients were aged >65 years [11]. Patients with persistent SARS-CoV-2 RNA positivity were older. Although age >65 years was considered as a risk factor associated with disease severity [2]; our study did not find age to be a risk factor for viral clearance. The key difference in age between the 2 groups may be related to weak immune response as well as coexisting illnesses. A majority of COVID-19 patients had hypertension and diabetes, and a small proportion had chronic lung disease, cardiac disease, immunosuppression, or pregnancy. COVID-19 patients with underlying comorbidities, including hypertension, diabetes, and cardiovascular disease, were more likely to be transferred to ICU [12]. Underlying diseases has been shown to be related to prolonged viral shedding in SARS patients [6]. However, we found no significant differences between virus-positive patients and virus-negative patients with regard to comorbidity. This result suggests that underlying diseases such as hypertension and diabetes may be related to the patient’s disease prognosis but have little to do with the duration of viral shedding.
Male sex was found to be an independent risk factor for prolonged SARS-CoV-2 viral shedding. In SARS-CoV infection, males experience higher mortality than females [13]. Imbalanced levels of angiotensin-converting enzyme 2 (ACE2) between males and females may play an important role in the sex-based differences in the response to the disease [14]. ACE2 has been identified as the host receptor of SARS-CoV, which regulates both cross-species and human-to-human transmissions in SARS-CoV infection [15]. SARS-CoV-2 also uses the ACE2 receptor to facilitate viral entry into target cells [16].
Consistent with other reports, the most common laboratory abnormalities among the COVID-19 patients were depressed total lymphocyte and lymphocyte subset counts, as well as an elevated CRP level [2, 3, 17]. Compared with the virus-negative patients, patients with persistent SARS-CoV-2 positivity had lower lymphocyte count and T-cell subset count but higher CRP and PCT level. This suggested more severe cellular immune deficiency and inflammatory activation in SARS-CoV-2 virus–positive patients. Decreases in T-cell counts were strongly associated with the severity and progression of SARS and MERS in patients [18, 19]. The underlying reasons for lymphopenia may be the direct infection of lymphocytes by SARS-CoV-2, lymphocyte sequestration in the lung, or cytokine-mediated lymphocyte trafficking [20, 21], all of which may lead to extending the virus clearance time.
Although various drugs have been tested for COVID-19, there is still an urgent need for a clinically proven, effective antiviral treatment for SARS-CoV-2 infection. In this study, antiviral therapy mainly included LPV/RTV and arbidol. Nevertheless, we observed that there was no apparent effect on viral shedding from the use of these antiviral drugs or their combined use. Previous studies on the efficacy of LPV/RTV in SARS and MERS showed disparate results. Compared with those using ribavirin alone, improved outcomes for SARS and decreased viral loads were reported for patients using a combination of LPV/RTV and ribavirin [22], whereas no obvious antiviral effect against MERS-CoV with lopinavir was observed in vitro [23]. Another study revealed that LPV/RTV could improve pulmonary function without inhibiting virus replication in MERS-CoV infection [24]. Current evidence regarding the effect of arbidol against SARS-CoV-2 was rare. In vitro experiments have indicated that arbidol showed some antiviral activity against SARS-CoV [25]. It was previously demonstrated that remdesivir had excellent antiviral activity against MERS-CoV in vitro and in vivo [24]. Moreover, remdesivir exhibited a beneficial effect in the first case in the United States [26]. This novel nucleotide analogue had not been put into use in our center yet, but its therapeutic effect in illness improvement is worth attention. Overall, further studies are warranted to uncover the exact efficacy of these different antiviral agents on viral shedding.
Corticosteroids have been used frequently for the treatment of patients with severe COVID-19 by reducing inflammatory-induced lung injury [3]. Several studies have reported that the use of corticosteroids is associated with delayed viral RNA clearance in MERS or SARS patients and even with higher mortality in influenza pneumonia [27–29]. In this study, corticosteroid usage did not independently increase the risks for viral shedding regardless of the initial dosage or an earlier implementation. However, the percentage of corticosteroid use in our center reached up to 77%, and the dosages prescribed were relatively lower for these COVID-19 patients than for MERS and SARS patients in previous studies. It was reported that high dosages of corticosteroids (>150 mg/day) showed a similar influence on H7N9 infection with prolonged viral shedding time, whereas proper use of corticosteroids was associated with clinical improvement in SARS [30, 31]. Thus, more clinical data are urgently needed to indicate the effects of corticosteroids and the optimal scheme of their usage. In addition, the beneficial effect of immunoglobulin has also been observed in viral infection [32]. Our study found that immunoglobulin application was associated with delayed viral clearance. In fact, patients using immunoglobulin were more likely to be in the more severe condition. Thus, more studies should be done to confirm the association between immunoglobulin treatment and viral shedding.
We identified the predictive value of the APACHE II score in SARS-CoV-2 infection. Moreover, patients transferred to the ICU showed delayed SARS-CoV-2 RNA clearance. The result indicated that SARS-CoV-2 viral shedding was associated with disease severity. Similarly, MERS-CoV subgenomic messenger was detected more frequently in the severe group than in the mild group [5]. Severe patients had more prolonged MERS-CoV shedding, up to 18–27 days following the onset of symptoms [33]. SARS patients with APACHE II scores ≥20 were associated with an increased risk of transmission of SARS-CoV [34]. This can be explained by the higher APACHE II score, more severe immunosuppression, and higher viral load among patients in the ICU.
Fecal-oral transmission was considered as an additional route for SARS-CoV-2 spread. In this study, we found a high percentage of COVID-19 patients with positive fecal virus results, who needed more time to clear SARS-CoV-2 than those with negative fecal virus results. Fecal viral RNA can remain positive even after respiratory specimens test results return negative. Consistent with our results, stool specimens tested positive in up to 53% of COVID-19 patients, and the positive staining of ACE2 and SARS-CoV-2 nucleocapsid protein was observed in gastrointestinal epithelium as well as isolated infectious SARS-CoV-2 from feces [4]. The duration of SARS fecal viral excretion could last for >100 days [6]. Positive fecal viral RNA was highly suggestive of virus intestinal infection; thus, a longer isolated observation time should be considered in these COVID-19 patients.
Our study has some notable limitations. First, we only observed the hospitalization of COVID-19 patients for 28 days, and many patients remained in hospital with SARS-CoV-2 RNA positivity. The duration of viral shedding may be longer than this cutoff timepoint. Second, some patients were transferred to our hospital after the disease had progressed and become worse; hence, our cohort might represent a more severe range of COVID-19.
In conclusion, we found that male sex, immunoglobulin use, APACHE II score, and lymphopenia were independent risk factors associated with the duration of SARS-CoV-2 RNA shedding, whereas antiviral combination therapy and corticosteroid treatment were not. A prolonged SARS-CoV-2 RNA clearance time was associated with disease severity and fecal viral RNA positivity. Our findings suggest that more suitable antiviral agents and low-to-moderate corticosteroids rather than high dosages should be considered in the treatment of severe COVID-19; seeking solutions to enhance immune function and to reduce the inflammatory response was also of great importance for viral clearance. In addition, more data based on randomized controlled multicenter studies are needed to rigorously assess the clinical relevance of our proposed indicators.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. This study was funded by Science and Technology Department of Zhejiang Province (grant number 2020c03123-1); the Zhejiang Provincial Natural Science Foundation of China (grant number LQ20H030010); and the National Key Research and Development Program of China (grant number 2018YFC2000500). We thank all patients involved in the study and all the front-line medical staff in the First Affiliated Hospital, School of Medicine, Zhejiang University.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan W- J, Ni Z- Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 2020; 158:1831–33.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park WB, Poon LLM, Choi S-J, et al. Replicative virus shedding in the respiratory tract of patients with Middle East respiratory syndrome coronavirus infection. Int J Infect Dis 2018; 72:8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu W, Tang F, Fontanet A, et al. Long-term SARS coronavirus excretion from patient cohort, China. Emerg Infect Dis 2004; 10:1841–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Corman VM, Albarrak AM, Omrani AS, et al. Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clin Infect Dis 2016; 62:477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McGeer A, Green KA, Plevneshi A, et al. Toronto Invasive Bacterial Diseases Network Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clin Infect Dis 2007; 45:1568–75. [DOI] [PubMed] [Google Scholar]
- 9. World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. Geneva, Switzerland: WHO, 2020. [Google Scholar]
- 10. Zhou XY, Ben SQ, Chen HL, Ni SS. A comparison of APACHE II and CPIS scores for the prediction of 30-day mortality in patients with ventilator-associated pneumonia. Int J Infect Dis 2015; 30:144–7. [DOI] [PubMed] [Google Scholar]
- 11. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020; 382:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leong HN, Earnest A, Lim HH, et al. SARS in Singapore—predictors of disease severity. Ann Acad Med Singapore 2006; 35:326–31. [PubMed] [Google Scholar]
- 14. Clotet-Freixas S, Soler MJ, Palau V, et al. Sex dimorphism in ANGII-mediated crosstalk between ACE2 and ACE in diabetic nephropathy. Lab Invest 2018; 98:1237–49. [DOI] [PubMed] [Google Scholar]
- 15. Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J Virol 2020; 94:e00127–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 2020; 46:586–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wan S, Yi Q, Fan S, et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). medRxiv [Preprint]. Posted 12 February 2020. Available at: https://www.medrxiv.org/content/10.1101/2020.02.10.20021832v1. Accessed 10 March 2020. [Google Scholar]
- 18. Li T, Qiu Z, Zhang L, et al. Significant changes of peripheral T lymphocyte subsets in patients with severe acute respiratory syndrome. J Infect Dis 2004; 189:648–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ko JH, Park GE, Lee JY, et al. Predictive factors for pneumonia development and progression to respiratory failure in MERS-CoV infected patients. J Infect 2016; 73:468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lang ZW, Zhang LJ, Zhang SJ, et al. A clinicopathological study of three cases of severe acute respiratory syndrome (SARS). Pathology 2003; 35:526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Collins AR. In vitro detection of apoptosis in monocytes/macrophages infected with human coronavirus. Clin Diagn Lab Immunol 2002; 9:1392–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chu CM, Cheng VC, Hung IF, et al. HKU/UCH SARS Study Group Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax 2004; 59:252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chan JF, Chan KH, Kao RY, et al. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J Infect 2013; 67:606–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun 2020; 11:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khamitov RA, Loginova SI, Shchukina VN, Borisevich SV, Maksimov VA, Shuster AM. Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures [in Russian]. Vopr Virusol 2008; 53:9–13. [PubMed] [Google Scholar]
- 26. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020; 382:929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arabi YM, Mandourah Y, Al-Hameed F, et al. Saudi Critical Care Trial Group Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med 2018; 197:757–67. [DOI] [PubMed] [Google Scholar]
- 28. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated coronavirus RNA concentrations in adult patients. J Clin Virol 2004; 31:304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ni YN, Chen G, Sun J, Liang BM, Liang ZA. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care 2019; 23:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen RC, Tang XP, Tan SY, et al. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest 2006; 129:1441–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sung JJ, Wu A, Joynt GM, et al. Severe acute respiratory syndrome: report of treatment and outcome after a major outbreak. Thorax 2004; 59:414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mofenson LM, Moye J Jr, Bethel J, Hirschhorn R, Jordan C, Nugent R. Prophylactic intravenous immunoglobulin in HIV-infected children with CD4+ counts of 0.20 x 10(9)/L or more. Effect on viral, opportunistic, and bacterial infections. The National Institute of Child Health and Human Development Intravenous Immunoglobulin Clinical Trial Study Group. JAMA 1992; 268:483–8. [DOI] [PubMed] [Google Scholar]
- 33. Min CK, Cheon S, Ha NY, et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep 2016; 6:25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Raboud J, Shigayeva A, McGeer A, et al. Risk factors for SARS transmission from patients requiring intubation: a multicentre investigation in Toronto, Canada. PLoS One 2010; 5:e10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.