Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus has spread across the globe rapidly causing an unprecedented pandemic. Because of the novelty of the disease, the possible impact on the endocrine system is not clear. To compile a mini-review describing possible endocrine consequences of SARS-CoV-2 infection, we performed a literature survey using the key words Covid-19, Coronavirus, SARS CoV-1, SARS Cov-2, Endocrine, and related terms in medical databases including PubMed, Google Scholar, and MedARXiv from the year 2000. Additional references were identified through manual screening of bibliographies and via citations in the selected articles. The literature review is current until April 28, 2020. In light of the literature, we discuss SARS-CoV-2 and explore the endocrine consequences based on the experience with structurally-similar SARS-CoV-1. Studies from the SARS -CoV-1 epidemic have reported variable changes in the endocrine organs. SARS-CoV-2 attaches to the ACE2 system in the pancreas causing perturbation of insulin production resulting in hyperglycemic emergencies. In patients with preexisting endocrine disorders who develop COVID-19, several factors warrant management decisions. Hydrocortisone dose adjustments are required in patients with adrenal insufficiency. Identification and management of critical illness-related corticosteroid insufficiency is crucial. Patients with Cushing syndrome may have poorer outcomes because of the associated immunodeficiency and coagulopathy. Vitamin D deficiency appears to be associated with increased susceptibility or severity to SARS-CoV-2 infection, and replacement may improve outcomes. Robust strategies required for the optimal management of endocrinopathies in COVID-19 are discussed extensively in this mini-review.
Keywords: SARS-CoV2, COVID-19, Endocrine
Coronavirus disease 2019 (COVID-19), which was first reported in Wuhan, Hubei province, China, toward the end of 2019, swiftly spread around the globe and became a major pandemic [1]. It is caused by the virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the seventh coronavirus that is known to cause disease in humans [2]. Within 6 months, the number of deaths globally have exceeded 400 000, resulting in an unprecedented public health emergency in our time [3].
Because SARS-CoV-2 is a novel virus, limited data are available on the effect of the virus on the endocrine system, including the pancreas. Nevertheless, as the new SARS-CoV-2 is structurally similar to the SARS-CoV-1, studying the pathogenesis and clinical impact of the known disease should be helpful in understanding the possible effects of the novel disease [4].
1. Pathogenesis
SARS-CoV-1 and SARS-CoV-2 belong to the β-genus of coronavirus family [5]. The genetic sequence of SARS-CoV-2 has around 80% identity to SARS-Co V-1 [6]. In SARS-CoV-1 the defined receptor biding domain of the membrane Spike (S) protein of the virus uses host angiotensin-converting enzyme 2 (ACE2) as the receptor for fusion of viral and host membranes [7–9]. It appears that SARS-CoV-2 also uses the same mechanism for host attachment [5, 10, 11].
The inhaled virus binds to the epithelial cells of the nasal cavity [12]. Ciliated cells in the conducting airway are the primary cells where the virus binds. In this initial phase, the virus can be detected by nasal swabs even though the patient is asymptomatic [12]. The lifecycle of the virus begins when the S protein binds to ACE2. The S protein is a trimetric spike glycoprotein located on the envelope of the virus and is composed of an N-terminal peptide domain, C-terminal Collectrin-like domain, and a 40-residue intracellular segment [13]. Although ACE2 has structural similarity to angiotensin-converting enzyme, it is not inhibited by angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, but is upregulated with their use [14, 15]. ACE2 expression is highest in kidney, endothelium, lungs, and the heart [13, 16]. The main substrate for ACE2 is angiotensin II, and ACE2 acts as a negative regulator of the renin-angiotensin-aldosterone system (RAAS) by converting the active angiotensin and angiotensin II to the inactive angiotensin 1-7 [17, 18].
The S protein is a trimeric spike glycoprotein located on the envelope of the virus [13]. Initially, the S protein is in a metastable prefusion state. The S1 subunit triggers viral envelope fusion with the cell membrane via the endosomal pathway. This results in shedding of S1 from the prefusion conformation and allows S2 to attain a stable postfusion state [19–21]. The cleavage of S protein of SARS-CoV-1 is triggered by transmembrane protease serine-2 (TMPRSS2) and cathepsin. However, the exact molecules facilitating endocytosis of SARS-CoV-2 are yet to be fully elucidated [22]. Furthermore, clathrin-dependent and clathrin-independent endocytosis may also be involved in mediating the viral entry [23, 24]. Following cellular entry, the virus then releases the genome RNA into the host cell which get translated into viral replicase polyproteins pp1a and 1ab [25]. These proteins are further cleaved into small particles by viral proteinases. The polymerase produces more subgenomic mRNAs in a serial manner that are ultimately translated into the relevant viral proteins that are assembled in the endoplasmic reticulum and Golgi apparatus, along with the genome RNA into virions. The virions are then released via vesicles out of the cell (Fig. 1) [26, 27]. Evidence regarding the affinity of SARS-CoV-2 to ACE2 binding is inconclusive. One study suggests that SARS-CoV-2 S proteins bind to ACE2 with 10 to 20 times higher affinity compared with SARS-CoV-1, whereas another study reported that SARS-CoV-2 receptor biding domain binds to ACE2 with a similar affinity to the SARS-CoV-1 [5, 28]. Although human autopsy studies have shown that SARS-CoV-1 RNA is seen only in cells expressing ACE2, there is some evidence that the RNA material is also seen in cells lacking ACE2, indicating that there may be factors other than ACE2 playing a role in entry of these coronaviruses into the host cell [16, 29]. Nevertheless, there is sufficient evidence in favor of ACE2 as the mediator of viral entry into alveolar cells, and local harmful activation of the RAAS, such that treatment with recombinant ACE2 is being considered as a serious therapeutic option.
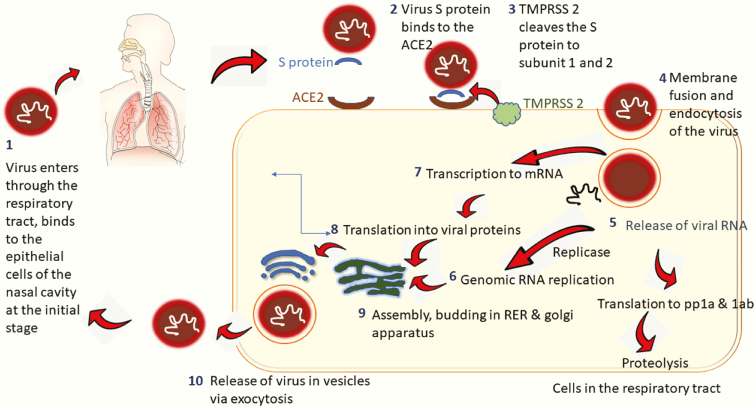
Figure 1.
Viral entry and cellular pathogenesis. The SARS-CoV-2 virus enters the respiratory tract via the epithelial cells in the nasal cavity (1). The virus binds via its membrane spike protein S, to the cell membrane protein ACE2 in lungs (2). TMPRSS2, another cell membrane protein, triggers cleavage of the S protein into 2 subunits (3). The S1 subunit promotes fusion of the viral envelope with the host cell membrane culminating endocytosis of the virus (4). The virus then releases its genomic RNA into the host cell (5). The viral RNA is translated into polyproteins pp1a and 1ab, both of which in turn undergo proteolysis by vital proteinases into small particles. In parallel, more genomic RNA are produced via the enzyme replicase (6). The genomic RNA gets transcribed into mRNA (7) and results in viral protein synthesis via translation (8). The replicated genomic RNA and the synthesized viral proteins are incorporated into virions in the RER and Golgi apparatus (9). The virions are ultimately released from the host cell as vesicles via exocytosis (10). pp1a and 1ab, viral replicase polyproteins; RER, rough endoplasmic reticulum; S, Spike protein; TMPRSS2, transmembrane protease serine 2.
Following the initial symptomatic period without a significant immune response, the second phase commences with an initial innate immune response [12]. This is seen in ~80% of patients, in whom the clinical course is relatively mild. Following entry of the virus into the host cells, the viral antigens are presented by antigen-presenting cells recognized by cytotoxic T lymphocytes. There is evidence that certain HLA polymorphisms are related to the susceptibility and protection from SARS-CoV-1 infection [30–32]. Both cellular and humoral immunity are subsequently activated following antigen presentation. A recent report revealed that the number of CD4+ and CD8+ T lymphocytes are significantly decreased in SARS-CoV-2-infected patients [33, 34].
The potentially life-threatening complication of coronavirus is acute respiratory distress syndrome (ARDS), which is secondary to a “cytokine storm” induced by the hyperactivation of the transcription factor NF-kappa B (NF-κB) with the release of massive amounts of pro-inflammatory cytokines and chemokines. NF-κB is activated directly by SARS-CoV-2 via pattern recognition receptors [35]. Down-regulation of ACE2 resulting from the binding of SARS-CoV-2, and the resultant increase in angiotensin II, activates STAT 3. Activation of STAT 3 leads to hyperactivation of the NF-κB pathway via the IL-6 amplifier and the resultant increase in proinflammatory cytokines and chemokines. Very high levels of cytokines including IL1-β, IL1RA, IL-7, IL-8, IL-9, IL-10, fibroblast growth factor 2 (FGF2), granulocyte colony stimulating factor, granulocyte macrophage colony stimulating factor, interferon-γ (IFN-γ), TNF-α, monocyte chemoattractant protein-1 (MCP1), macrophage inflammatory protein α, and vascular endothelial growth factor-A have been reported in those with severe COVID-19 [36, 37].
In addition to lung involvement, it is suggested that viruses entering the body via the nasal passage may gain access to the central nervous system via the olfactory bulb through synapses as in SARS-CoV-1 and Middle East respiratory syndrome-coronavirus (MERS-CoV) [38, 39], and this may also be responsible for the early symptoms of loss of taste and smell. Involvement of the brain has been reported in patients with severe COVID-19 [40]. There is also evidence that SARS-CoV-2 causes significant cardiac and renal disease, possibly from ACE2/renin-angiotensin system-related impact.
2. Pituitary
SARS-CoV-1 has been known to affect the hypothalamo-pituitary-adrenal axis (HPA) causing transient hypocortisolism. Less commonly, the hypothalamo-pituitary-thyroid axis is affected leading to secondary hypothyroidism [41]. Both reversible hypophysitis and a direct hypothalamic effect have been reported as possible mechanisms [41]. In SARS-CoV-1 survivors, hypocortisolism has been reported to develop gradually as a late complication over a number of weeks from the onset of infection [41].
Postviral syndromes manifest as low energy levels, low mood, and dizziness; these have been shown to be associated with postviral hypocortisolism and are dramatically improved with cortisol replacement [41]. Furthermore, insertion/deletion polymorphisms in the ACE gene coding for ACE receptors are associated with an increased risk of idiopathic chronic fatigue and chronic fatigue syndrome [42]. These effects have been suggested to be mediated by ACE receptors interacting with neurotransmitter pathways [41]. Because ACE2 is expressed in the hypothalamus and could be disrupted by the SARS-CoV-2 infection, it may play a role in postviral fatigue and associated hypocortisolism [43, 44].
It has been demonstrated that SARS-CoV enters the brain via an ACE2 receptor located in the olfactory bulb [39]. Furthermore, it is known that SARS-CoV-2 causes anosmia and ageusia, which could be related to a local or central pathology such as damage to the hypothalamus leading to hormonal deficiencies [45].
Evidence during the SARS epidemic in 2003 suggested that the amino acid sequence of SARS virus showed molecular mimicry with ACTH; this would lead to host antibodies against viral antigens binding to ACTH receptors, limiting the corticosteroid stress response [46]. Patients who developed hypocortisolism were found to recover their HPA axis within 1 year [47]. Thus, theoretically, supraphysiological corticosteroids could be beneficial, but the evidence is inconclusive. Molecular mimicry of SARS-CoV-2 with ACTH has not been reported yet.
Hyperprolactinemia is known to occur in response to any form of stress, including infections [48]. High prolactin levels have been shown in patients with severe sepsis and in infants with severe respiratory infections [49, 50]. Prolactin also has shown immunomodulatory and anti-inflammatory effects in experimental studies [51]. However, no data are available on susceptibility to infection among patients with hyperprolactinemia or any risk conferred by prolactin-lowering therapy. Table 1 summarizes the possible effects of SARS-CoV-2 on the endocrine system.
Table 1.
Possible Effects of SARS-CoV-2 on the Endocrine System
| Pathology | Possible Mechanism | Effect on Hormonal Axis | Clinical Features | Management Issues and Solutions |
|---|---|---|---|---|
| Pituitary | ||||
| Central hypocortisolism and hypothyroidism | Hypophysitis resulting from infiltration by virus [41] Hypothalamic involvement [41] Destruction of ACE2 in hypothalamus [43, 44] Molecular mimicry of SARS-CoV-1 to ACTH and subsequent host defense mechanisms [46] |
Impaired ACTH/cortisol production Low thyroid hormones sometimes with low TSH |
Postviral syndromes [41] | Cosyntropin/Synacthen test TSH and free T4 If deficient, hormone replacement in physiological doses [41] |
| Hyperprolactinemia | Dopaminergic stress response [48] | Transient hyperprolactinemia | Asymptomatic | Prolactin levels may be high during acute illness. Caution on interactions of DRA with CYP450 inducing antivirals and amine based pressors/inotropes [55, 56] |
| Electrolyte imbalances | ||||
| Hypernatremia | High fever, tachypnea, gastrointestinal losses, inability to take adequate fluids [52] | Hypernatremia | Impaired level of consciousness | Monitor electrolytes Replacement Convert desmopressin to parenteral form [52] |
| Hypokalemia | Gastrointestinal losses [52] Upregulation of the RAAS by degradation of ACE2 [53, 54] |
Hypokalemia | Clinical features of hypokalemia | Monitor electrolytes Replacement |
| Adrenal | ||||
| Hypoadrenalism | Adrenal necrosis and vasculitis from direct cytopathic effect or inflammatory response [29, 59] | Hypocortisolism | Postural hypotension Persistently low blood pressure Hyperkalemia and hyponatremia |
Serum 9 am cortisol Cosyntropin test Hydrocortisone therapy |
| Thyroid | ||||
| Hypothyroidism | Destruction of follicular and parafollicular cells of thyroid [83] | Primary hypothyroidism | Hypothyroid features | High TSH and low free T4 Thyroxine replacement |
| Decreased activity of type 1 deiodinase activity, increased activity of type 3 deiodinase activity, and down-regulation of hypothalamic pituitary axis [82] | Sick euthyroidism | Clinically not significant | Difficulty in differentiating during acute illness, test TSH and free T4 following recovery | |
| Hypophysitis/ hypothalamic involvement [41] | Central hypothyroidism | Hypothyroid features | Low TSH and free T4 Thyroxine replacement |
|
| Pancreas | ||||
| Hypo-/hyperglycemia | Direct viral injury on ACE2 expressing islet cells [89] Hyperglycemia glycosylates ACE2 and viral S protein, facilitating viral entry [92] Pancreatitis [90]: direct viral injury, response to systemic inflammation, immune-mediated injury |
Possible hypoinsulinemia Stress response up-regulates cortisol, growth hormone, and adrenergic activity with hyperglycemic effects |
Hyperglycemia Mild pancreatitis: minimal or no symptoms |
Hyperglycemia predicts poor prognosis Requires frequent monitoring and titration of treatment Potential anti-COVID therapies may cause hypo- and hyperglycemia [100] |
| Parathyroid | ||||
| No direct effect | Not identified | Not identified | None | |
| Gonads | ||||
| Hypogonadism | Entry of virus into spermatogonia and somatic cells using ACE2 receptors [123, 124] Destruction of seminiferous tubules and reduced spermatozoa from immune-mediated orchitis [126] |
Impaired spermatogenesis and androgen synthesis | Male hypogonadism and subfertility | Follow-up after recovery from acute infection |
A. Management Considerations in Patients with Preexisting (or Known) Pituitary Conditions
Electrolyte and water imbalances can occur in COVID-19 resulting from insensible loss caused by high fever and tachypnea, gastrointestinal loss such as vomiting and diarrhea, as well as the inability to take adequate fluids because of an impaired level of consciousness [52]. Hypokalemia had been documented that has been attributed to upregulation of the RAAS by degradation of ACE2 by SARS-CoV-2 with increased renal loss of potassium [53, 54]. Clinicians must be vigilant regarding possible electrolyte imbalances and should titrate any corticosteroid or desmopressin doses accordingly. If intranasal desmopressin cannot be administered because of impaired consciousness, it can be converted to a parenteral form (IV/IM) to allow more stringent titration [52]. Table 2 summarizes the management of patients with preexisting endocrine conditions who are affected with COVID-19.
Table 2.
Management of Patients with Preexisting Endocrine Conditions who are Affected with COVID-19
| Endocrine Condition | Management | ||
|---|---|---|---|
| Diabetes insipidus | Titrate the dose of desmopressin according to serum sodium, and osmolality. Convert to parenteral form (IV/IM) if intranasal route is not feasible [52] Desmopressin dose equivalents [138] |
||
| Tablets | Spray | Injections | |
| 100 µg | 2.5 µg | NA | |
| 200 µg | 5.0 µg | <0.5 µg | |
| 400 µg | 10.0 µg | <1.0 µg | |
| Hyperprolactinemia | Bromocriptine: may need dose adjustment because of interactions between lopinavir/ritonavir, which increase bromocriptine levels [55] Cabergoline: dose adjustment is not required |
||
| GH deficiency | Continue on the same dose of growth hormone in those with established GH deficiency [58] | ||
| Hypoadrenalism | Double the morning dose of hydrocortisone and continue 20 mg 4 times daily, or give doubled usual hydrocortisone dose (“sick day rule”), depending on the infection severity and patient characteristics [64] During an adrenal crisis, IM or IV hydrocortisone 100 mg stat followed by 200 mg over 24-hour infusion [66] Patients with primary hypoadrenalism do not require increasing fludrocortisone dose [65] Critical illness-related corticosteroid insufficiency (CIRCI): IV hydrocortisone 400 mg daily for 3 days or longer depending on the requirement [70] |
||
| Cushing’s syndrome | Continue medical management in those with active disease IV etomidate at a rate of 0.04 to 0.05 mg/kg/h for those with severe illness targeting a cortisol level 500-800 nmol/L [78, 79] Anticoagulation to be considered in acute illness [75] |
||
| Pheochromocytoma/paraganglioma | Treatment with initial alpha-adrenoceptor blockers followed by beta-adrenoceptor blockers depending on the blood pressure and heart rate [81] | ||
| Hypothyroidism | No thyroxine dose adjustments are required | ||
| Hyperthyroidism | Dose adjustment of antithyroid medications as usual according to the thyroid function tests. If blood tests cannot be performed, dose adjustments may be made based on thorough history and examination. Short-term block and replacement therapy as an alternative [87] |
||
| Hypoparathyroidism | Ensure a continuous supply of calcium supplements Maintain normocalcemia as hypocalcemia increases the risk of QT prolongation with chloroquine/hydroxychloroquine and azithromycin [112] |
||
| Vitamin D deficiency | Vitamin D supplements to achieve a target level of > 50 nmol/L (20 ng/mL) [130] During winter seasons, 2000-5000 IU daily up to 10 000 IU depending on the requirement [131, 132] |
||
| Hypogonadism |
Testosterone
Temporary discontinuation may be possible if medication is not available or changing to an alternative is possible (e.g., intramuscular injections to testosterone gel) Estrogen Conversion to transdermal formulations when applicable as the thrombosis risk is lower with transdermal compared to oral estrogen |
Based on the currently available evidence, patients with preexisting hyperprolactinemia may continue dopamine receptor agonist (DRA) therapy during the pandemic and during acute mild to moderate COVID-19. In patients with hyperprolactinemia, prolactin levels should not be measured to assess disease control during acute illness. It is noteworthy that patients on DRAs who develop COVID-19 are at risk of drug interactions. Antiretroviral agents lopinavir/ritonavir, which are currently being used for the treatment COVID-19 in some parts of the world, can inhibit CYP3A4 enzymes and may increase plasma bromocriptine levels [55]. However, the clinical significance of higher plasma bromocriptine levels is uncertain. Cabergoline is less affected by such enzyme modulations because only a small proportion of this is metabolized through the cytochrome P450 system [56]. In critically ill COVID-19 patients who require pressor support because of septic shock, use of amine derivatives (norepinephrine, epinephrine, dobutamine, dopamine) with DRAs may cause additive vasospasm and a rapid blood pressure rise resulting from pharmacological synergism [55]. Therefore, close monitoring is required and temporary discontinuation of DRAs in severe COVID-19 should be considered. Chloroquine is not known to adversely interact with DRAs. In fact, it has been shown to induce cell apoptosis in pituitary tumors when used in combination with cabergoline in animal studies [57]. However, these beneficial effects are unlikely to be observed during brief exposure.
Patients who have commenced GH therapy can be continued on the same dosage [58]. However, it is recommended that GH therapy be withheld in a GH-deficient patient who develops COVID-19 [58].
3. Adrenal
Autopsy studies during the SARS epidemic revealed adrenal necrosis, vasculitis of small veins of adrenal medulla, and adrenal infiltration with monocytes and lymphocytes that was caused by SARS-CoV-1 [29, 59]. Viral antigens and the genomic sequence of SARS-CoV-1 were demonstrated in the adrenal glands [29]. To date, this has not been reported with SARS-CoV-2; however, this requires attention and further studies.
4. Management Considerations
A. COVID-19 and Preexisting Adrenal Insufficiency
Patients with preexisting primary or secondary adrenal insufficiency are at high risk of complications during COVID-19 pandemic for multiple reasons. These patients are at increased risk of mortality as a result of infections triggering an adrenal crisis. Further, they are at high risk of infections, particularly involving the lower respiratory tract, partly from impairment of natural killer cell function [60]. Because upper respiratory tract infections are found to be the most common precipitating event for adrenal crises, stringent infection prevention measures are recommended [61].
These patients would be on steroid replacement in physiological doses, usually with hydrocortisone which needs continuation without disruption. Patients should have a “steroid emergency card” and bracelet with details of hydrocortisone dosing during an infection. Standard guidelines suggest doubling the usual steroid dose in accordance with “sick day rules,” in the event of mild to moderate infection [62]. However, based on the experiences during the COVID-19 pandemic, which is associated with continuous high levels of acute inflammation, it is suggested that this regimen might be inadequate because it can result in periods of glucocorticoid deficiency in these patients with ongoing continuous inflammation [63, 64]. Therefore, an immediate dose of doubled hydrocortisone morning dose, followed by replacement dose of 20 mg 4 times daily is proposed as a better approach in the current context because it provides a more evenly spaced and continuous glucocorticoid cover [64]. This should be tapered off to double the patients’ usual dose once they have no fever and show clinical improvement, followed by tapering down to the routine dose once they are completely asymptomatic [64]. However, an individualized approach is recommended, based on the patient characteristics and severity of the infection because it is evident that all patients with COVID-19 do not behave the same way. COVID-19-PCR positive, asymptomatic patients may not need to increase their routine dose. Thus, we recommend that it is imperative to have a discussion with the treating endocrinologist before making changes to the steroid replacement dose of a COVID-19 patient with adrenal insufficiency.
If hospitalized with pneumonia, patients should be closely monitored for features of acute adrenal insufficiency such as syncope, vomiting, hypotension, and electrolyte imbalances including hyperkalemia, hyponatremia, and hypoglycemia [65]. Severe pneumonia or any features of acute adrenal insufficiency would warrant IV or IM hydrocortisone treatment with 100 mg given immediately, followed by 200 mg every 24 hours as a continuous IV infusion or 6-hourly in divided doses, accompanied with appropriate fluid resuscitation [66]. Patients with primary adrenal insufficiency would not require additional fludrocortisone in this setting as hydrocortisone doses above 50 mg per day are known to exert adequate actions on mineralocorticoid receptors [65].
During SARS and MERS epidemics, pharmacological treatment with glucocorticoids was found to result in adverse outcomes. This was the basis for World Health Organization guidelines recommend against their routine use for COVID-19 patients [67, 68]. However, steroids in physiological doses for adrenal insufficiency would be beneficial and improve the outcomes in these patients. The decision on hydrocortisone treatment and the appropriate dose can be guided by baseline cortisol level, and the target level expected in severely ill patients on intensive care units (see the following section).
B. COVID-19 and Critical Illness-Related Corticosteroid Insufficiency
Usually, any stressful condition including infections results in activation of the HPA axis with increased secretion of corticosteroids to optimize the stress response. The strongest activators of HPA axis are hypoxia, hypotension, and sepsis. In some patients with critical illness or sepsis, this response may be inadequate because of impaired cortisol secretion or cortisol resistance caused by abnormalities in glucocorticoid receptors, known as critical illness-related corticosteroid insufficiency (CIRCI) [69]. The occurrence of CIRCI during an infection is associated with increased inflammatory markers, abnormalities in indicators of coagulation, and a prolonged intensive care unit stay [70]. CIRCI should be considered in all critically ill patients with COVID-19 who have hypotension refractory to fluid and vasopressor therapy [70]. Although uncommon, these patients may also demonstrate hyperkalemia, hyponatremia, and hypoglycemia [69, 70].
Based on the available evidence, a random serum cortisol of less than 275 nmol/L (10 µg/dL) or a serum cortisol rise by less than 248 nmol/L (9 µg/dL) at 1 hour following 250 µg cosyntropin/Synacthen can be used to diagnose CIRCI [69, 70]. However, it is important to be aware that this stimulation test may have a low sensitivity in critically ill patients and may be difficult to conduct [69]. Furthermore, one needs to be aware that threshold levels of cortisol will vary according to assay. These assay-related variations can be even more prominent in critically ill patients because of the presence of heterophile antibodies and cortisol metabolites, and can impact the diagnosis of CIRCI [69, 71]. For these reasons, a glucocorticoid trial can be considered based on clinical parameters, regardless of the cortisol response, following a discussion between the treating intensivist and endocrinologist. Patients with CIRCI can be treated with IV hydrocortisone (up to 400 mg/day) for 3 days or longer depending on the clinical parameters, with slow tapering of doses once the patient’s condition is stable [70]. The decision regarding hydrocortisone treatment should be made cautiously, and it is also important to be mindful about the possible drug interactions as well. Ritonavir is known to inhibit the cytochrome P4503A enzyme and thereby increase the exposure to corticosteroids and prolong their half-lives [72]. It is noteworthy that following a cytokine storm, there may be immune “exhaustion,” and corticosteroids may be harmful [35].
C. COVID-19 and Cushing’s Syndrome
Patients with Cushing’s syndrome (CS) may experience delays in diagnostic evaluation and surgical management, as well as interruptions in the continuous supply of medication during the COVID-19 pandemic, with disastrous consequences. Thus, it is imperative for the teams managing these patients to identify and address these issues [73].
There are no data regarding the behavior of COVID-19 in patients with CS. However, the metabolic and other complications associated with CS are likely to result in a poor prognosis. Diabetes and hypertension characteristically seen in CS have been identified as well-known poor prognostic factors in COVID-19 independent of age, and this may contribute to mortality among CS patients [74]. These patients may be susceptible to severe pneumonia with secondary bacterial infections from changes in white blood cell count and function, reduced lymphocytes with CD4 to CD8 ratio, and reduced action of natural killer cells secondary to glucocorticoid excess [75].
Coagulopathy associated with elevated D-dimer has been observed in COVID-19; this is consistently associated with multiorgan failure and poor outcome [76, 77]. It is well-known that increased production of fibrinogen, factor VIII, and von Willebrand factor along with impaired fibrinolysis in CS also gives rise to a pro-thrombotic state. This risk could possibly be aggravated if infected with SARS-CoV-2 [78].
The entry of SARS-CoV-2 into cells via ACE2 on the cell membrane leads to degradation of ACE2, thereby up regulating the RAAS, resulting in hypokalemia [54]. Hypokalemia can be more severe in patients with CS as they have preexisting corticosteroid-induced potassium depletion.
As has been discussed, there are several mechanisms that can contribute to increased mortality in CS patients with COVID-19. Therefore, it is crucial to adhere to standard infection prevention measures. During an infection, blood pressure and blood glucose levels needs close monitoring and management according to standard guidelines. Close monitoring of electrolytes is important. Prophylactic, low-dose low-molecular-weight heparin should generally be administered given there are no major contraindications [76]. Medical management should be considered for patients with active CS until the infection settles and they are stable to undergo definitive therapy. Intravenous etomidate can be considered in patients with severe illness needing parenteral glucocorticoid-lowering therapy with careful dose adjustments to achieve a target cortisol level. If the patient has coexisting acute kidney injury, further stringent dose titration is warranted. It is crucial to keep in mind that the precursor 11-deoxycortisol may crossreact in many assays [79, 80].
D. COVID-19 and Pheochromocytoma and Paraganglioma
Because of the current COVID-19 pandemic, regular follow-up of already diagnosed patients can be delayed. These patients are not at an increased risk of contracting the infection and general disease prevention measures can be followed. If infected with SARS-CoV-2, plasma and urinary metanephrines can be elevated from the associated stress response and measurement during illness will give false-positive results [81]. During the infection, treatment via α-adrenoreceptor blockade, or β -adrenoreceptor blockade after adequate α -adrenoreceptor blockade, can be considered to prevent possible cardiovascular complications [82].
5. Thyroid
A report on thyroid function tests in 48 patients infected with SARS-CoV-1 showed reduced free T3 and free T4 in 94% and 46% of patients, respectively. Serum TSH levels were also reduced in these patients, raising the possibility of either central hypothyroidism or the “sick euthyroid” syndrome [83]. In another follow-up study that looked at endocrine disorders among 61 patients who had SARS, 2 patients were identified as having subclinical thyrotoxicosis, 3 had central hypothyroidism, and 1 had primary hypothyroidism with positive thyroid autoantibodies [41]. The degree of hormonal derangement and clinical features were not described. Therefore, previously undiagnosed primary hypothyroidism and recovering central hypothyroidism or sick euthyroidism are possibilities that should be considered.
Acute and chronic phases of critical illness have different effects on the thyroid axis [84]. Sick euthyroid syndrome or “nonthyroidal illness syndrome” can manifest in patients with COVID-19, especially during the acute and recovery phase of the illness, which will further complicate their management. This mechanism is complex. During an acute illness, changes in thyroid hormone binding, cellular uptake, and decreased activity of type I deiodinase enzyme leading to decreased T4 to T3 conversion, can occur [84]. Type I deiodinase enzyme activity is reduced as it is influenced by various substances including circulating cortisol, cytokines, endogenous free fatty acids, and various drugs used in management [84]. Increased T3 catabolism in peripheral tissues can occur because of increased activity of type 3 deiodinase [84]. The cumulative effect is low circulating T3 levels [84]. In addition to these changes, down-regulation of the hypothalamo-pituitary axis leads to low circulating TSH and T4 levels during the course of illness [84]. Differentiating this from central hypothyroidism might be difficult in the acute stage and may require reevaluation later. If nonthyroidal illness syndrome is suspected, therapy with thyroxine or liothyronine is not currently recommended because of lack of clinical benefit and safety concerns [84].
Although there is conflicting evidence, 2 independent studies have shown the adverse effects of SARS-CoV-1 on the thyroid in autopsy samples. In 1 study, samples of all 5 patients showed destruction of follicular epithelia and exfoliation of epithelial cells into the follicles. There was also evidence of destruction of parafollicular cells [85]. Another similar study showed deformation, enlargement, and dystrophy of follicular cells as well as a reduced amount of thyroglobulin in follicular epithelial cells in all 4 patients who died of SARS [86]. However, in another autopsy study involving 4 patients who died of SARS-CoV-1, neither viral RNA material nor antigens were seen in the thyroid [87].
A recent report from Italy on a young female who recovered from SARS-CoV-2 describes the first reported case of subacute thyroiditis chronologically related to this viral infection [88]. With the known association of subacute thyroiditis with preceding viral infections, it is possible that the thyroiditis is etiologically related to SARS-CoV-2 infection.
A. Management Considerations of Preexisting Thyroid Conditions
If infected with COVID-19, patients with central hypothyroidism and primary hypothyroidism should be advised to continue the same dose or higher doses of thyroxine, depending on the clinical context. Patients on antithyroid drugs would need to exercise extra caution because signs of neutropenia and COVID-19 could mimic each other. Urgent medical care and a full blood count should be arranged for these patients in case of any doubt. For patients on antithyroid drugs, regular consultation with their physician may often be postponed, but medications can be titrated according to thyroid function tests. If blood tests are not available, a thorough clinical assessment and history is recommended. Consultation is advisable in situations such as pregnancy, poor disease control, and patients with adverse effects to medications. Definitive therapy is best postponed until the epidemic is controlled unless used as an emergency measure in a poorly controlled patient. Block-and-replacement therapy is also suggested if monitoring of thyroid function is not feasible for a considerable period [89]. In patients with underlying thyroid disease during an acute illness, thyroid function may not depict the actual thyroid status, highlighting the importance of clinical evaluation, if dose adjustments are required.
There is no clear evidence on the outcome of COVID-19 in patients on immunosuppressants [90]. However, patients who are on glucocorticoids and mycophenolate mofetil for thyroid eye disease should especially adhere to social distancing and infection control measures because of the potential risk of complications.
6. Pancreas
ACE2 is expressed in pancreatic cells. An animal study and a postmortem study among SARS fatalities showed ACE2 to be expressed in islets rather than the exocrine pancreas; specifically, in β and δ cells as opposed to α cells [91, 92]. In contrast, a population-based study analyzing bulk RNA-sequence data showed that exocrine tissues (predominantly duct cells) express more ACE2 than islet cells [93]. It is not known whether the SARS-CoV infection alters the patterns of pancreatic cell ACE2 expression, dependent on ethnic or other variations. In fact, animal studies have shown diabetes to increase ACE2 expression in pancreatic as well as lung and other tissues [94].
A study during the SARS epidemic demonstrated ACE2 expression in islet cells and a high incidence of hyperglycemia among SARS patients. The authors speculated that SARS-CoV-1 may directly infect islet cells causing their dysfunction, resulting in hyperglycemia or new-onset diabetes [92]. Pancreatic injury (hydropic degeneration, fatty degeneration, and interstitial proliferation) has been shown in some postmortem studies [95] but not in others [96]. Similarly, SARS-CoV-1 viral material was found within pancreatic cells in some [87], but not other postmortem studies [97]. Thus far, no postmortem studies during the COVID-19 pandemic have reported changes in pancreatic tissues. No studies have described differences in insulin level or insulin resistance to establish a direct link between the coronavirus infection and hyperglycemia.
Viral infections are known to cause type 1 diabetes by triggering the production of cross-reactive antibodies as a result of molecular mimicry or by activating cross-reactive T cells [98]. Although this is well-known with enterovirus, influenza virus, cytomegalovirus, rotavirus, and coxsackie virus infections, it has never (as yet) been described with coronavirus infections.
A. Glucose Homeostasis
During the SARS epidemic, in a cohort of 39 nondiabetic nonglucocorticoid-treated patients, 20 developed new-onset fasting hyperglycemia (>7 mmol/L), starting from the third day in the hospital and reversing by 2 weeks in the majority, whereas 2 patients continued to have diabetes 3 years later [92]. This was not observed in the control groups with non-SARS pneumonia, and healthy siblings of SARS patients. Postprandial plasma glucose, insulin levels, and insulin resistance were not reported.
Data from the current COVID-19 pandemic are limited. One study among patients with diabetes and COVID-19 reported that preprandial and postprandial glucose levels were above target in 29.4% and 64.5%, respectively, whereas 10% suffered at least 1 hypoglycemic event [99]. Details of their antidiabetic therapies, outcomes, or comparison with a control group were not reported. Anecdotal reports from experts caring for COVID-19 patients note a higher incidence of hyperglycemia, new-onset diabetes, diabetic ketoacidosis, and euglycemic ketoacidosis [100].
Any infection or acute illness would trigger inflammatory and stress responses with increase in cytokines, cortisol, sympathetic activity, and growth hormone. These will induce a state of insulin resistance causing hyperglycemia. To date, robust scientific data remain too scarce to confirm that coronavirus-infected patients develop hyperglycemia at a greater severity or frequency, beyond what is expected in any acute severe illness.
Irrespective of the cause, hyperglycemia predicts a poor prognosis and warrants prompt recognition and correction. Based on the experimental observations, a paracrine loop hypothesis has been suggested to explain this risk. SARS-CoV infects alveolar cells and pancreatic islet cells, causes hyperglycemia resulting from islet cell dysfunction, and this increases glycosylation of ACE2 and viral spike proteins facilitating viral entry in to host cells and thus setting up a vicious cycle [101].
Alpha-cell injury may predispose to hypoglycemia. However, an excess risk of hypoglycemia was not described during the SARS epidemic. One reported event of fatal hypoglycemic coma affected a patient with diabetes; however, the antidiabetic therapies administered were not described [102]. The same author noted an increased incidence of liver injury among patients with diabetes and SARS, which may also contribute to hypoglycemia [102]. To date, no link between alpha-cell function or glucagon levels and coronavirus infections have been described.
B. Pancreatitis
Despite widespread expression of ACE2 in pancreatic tissues, pancreatitis has been uncommon with coronavirus infections. No events of pancreatitis have been described during the SARS-CoV-1 or MERS-CoV epidemics attributable to the viral infection. During the current COVID-19 pandemic, mild pancreatitis (diagnosed on the basis of elevated serum amylase and/or lipase) has been described in patients with severe COVID-19 [93]. It is uncertain whether the mild pancreatic injury was a result of direct viral invasion or the systemic inflammatory response. Nevertheless, it is important to recognize this because pancreatitis itself could worsen ARDS, the major life-threatening sequelae of COVID-19.
C. COVID-19 in People with Diabetes
Prognostic and management implications of possible pancreatic injury on patients with COVID-19 and on people with preexisting diabetes are reviewed in detail elsewhere [103–105].
In brief, people with diabetes should ensure a healthy lifestyle, regular glucose monitoring, and adherence to pharmacotherapy during this pandemic. Although diabetes does not increase the risk of infection, it increases the risk for complications and death [74, 106]. Obesity, hypertension, and cardiovascular diseases are common comorbidities of diabetes and are all linked with adverse outcomes with COVID-19 [107, 108]. Maintaining good glycemic control improves outcomes [109]. Specific therapies for SARS-CoV-2 may have therapeutic implications when used in people with diabetes, thus warranting their cautious use [103].
D. COVID-19 in Patients with Pancreatic Neuroendocrine Tumors
Whether patients with these rare disorders are more susceptible to COVID-19 and its complications, or whether COVID-19 could precipitate tumor-related complications (infarct or necrosis) in these patients is unknown. Patients with glucagonoma may be at increased risk of severe COVID-19 because of hyperglycemia and a thrombophilic state [110]. Patients on immunosuppressive therapies will also be vulnerable to COVID-19. Implications for management of these patients during the COVID-19 pandemic are reviewed elsewhere [111].
7. Parathyroid
Although there is no evidence that primary hyper- or hypoparathyroidism are risk factors for COVID-19, any infection may impose challenges in the management of these diseases. Patients with chronic renal impairment and parathyroid dysfunction may be at risk for COVID-19 because of the underlying renal disease.
Intracellular calcium signaling is essential for the replication of certain viruses and the cellular outcomes [112]. It is known that the cytoplasmic domain of the 3a protein of SARS-CoV-1 binds calcium in vitro, causing a change in its protein conformation [113]. However, the role of calcium signaling in the context of COVID-19 is yet to be elucidated and needs further research. No data exist on ACE2 expression, viral invasion or inflammation of the parathyroid glands, or alterations in parathyroid hormone or calcium homeostasis during coronavirus infections. Although hypocalcemia has been reported in patients with severe illnesses in the past, no published data are available regarding COVID-19 disease severity and serum calcium levels. Unpublished data suggest a possible association between hypocalcemia and COVID-19 disease severity and prognosis.
A. Management Considerations of Preexisting Hypoparathyroidism
The availability of calcium supplements must be ensured for patients with hypoparathyroidism to prevent life-threatening complications of hypocalcemia. Patients with hypoparathyroidism should have access to their endocrine service provider if they develop symptoms of hypocalcemia, and advice regarding management should be given based on the symptoms and signs, pending laboratory confirmation of hypocalcemia. For patients with hyperparathyroidism awaiting surgery, timing of parathyroid surgery must be considered based on the risk profile of the patients and availability of surgical facilities.
Because the treatment of COVID-19 may involve drugs that can cause QT prolongation, such as chloroquine/hydroxychloroquine and azithromycin, care should be taken to optimize calcium levels before starting treatment with these agents [114]. Similarly, hypomagnesemia should be corrected for optimal calcium and vitamin D metabolism and to prevent QT prolongation [115].
8. Vitamin D
Vitamin D plays an important role in the immune system and reduces the risk of viral infections in many ways. The beneficial role of vitamin D in preventing the common cold is attributed to its effects on physical barrier function and innate and adaptive immunity [116].
Dancer et al. showed that vitamin D deficiency is a common problem in patients with ARDS and leads to inflammation in alveolar epithelial cells. They also showed that repletion of vitamin D before esophagectomy was associated with reduced alveolar damage in in vivo measurements [117]. Ethnic variations in the vitamin D-binding protein relating to differences in the inflammatory profile and disease severity has been demonstrated in tuberculosis patients [118].
Increased serum levels of pro-inflammatory cytokines associated with pulmonary inflammation and lung injury have been reported in studies with SARS-CoV and MERS-CoV-1 [119, 120]. Although the exact pathogenesis is yet to be elucidated, increased levels of proinflammatory cytokines (IL1B, IFN-γ, IP10, and MCP1) have been found in patients with COVID-19. Furthermore, the levels of inflammatory cytokines (granulocyte colony stimulating factor, IP10, MCP1, MIP1A, and TNF-α) were found to be associated with disease severity [36]. Vitamin D treatment seems to inhibit the T-helper-1 response and reduces serum levels of pro-inflammatory cytokines TNF-α and IFN-γ [121]. SARS-CoV-2 infection also results in an increase in the levels of cytokines from Th2 cells (e.g., IL-4, IL-10), which suppress inflammation [36]. Vitamin D and 1,25(OH)2D induce the production of Th2 cytokines, which are anti-inflammatory [122]. Therefore, vitamin D may play a role in reducing inflammation in COVID 19 by increasing the anti-inflammatory cytokines and reducing the pro-inflammatory cytokines. In addition, it has been found that the SARS-CoV-2 spike glycoprotein interacts with the human DPP4/CD26, which is important for its virulence [123]. Vitamin D may play a role in modulating the virulence of SARS-CoV-2 as it has been demonstrated that treatment of vitamin D deficiency leads to reduced expression of DPP4/CD26 [123, 124].
The RAAS is thought to mediate lung injury in COVID-19; its inhibition is being investigated as a potential treatment [125]. Vitamin D has been found to be a negative endocrine regulator of the RAAS: 1,25 (OH)2D3 down-regulates the RAAS by suppressing renin expression [126].
These mechanisms (Fig. 2) thus suggest that treatment of vitamin D deficiency may be of benefit in the prevention and treatment of COVID-19 infection.
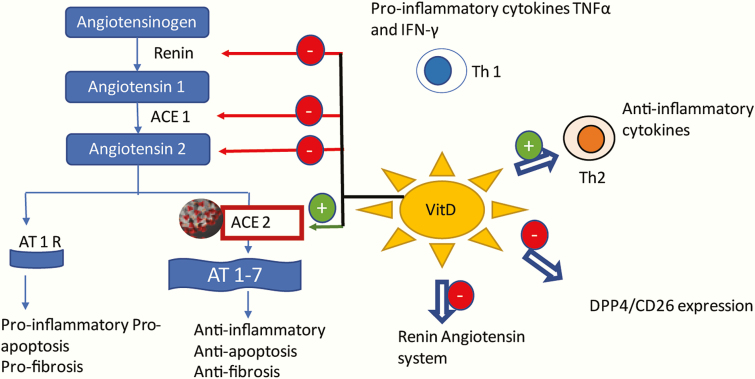
Figure 2.
Postulated mechanisms of vitamin D in prevention of COVID-19 infection. Vitamin D treatment inhibits the T-helper-1 cell (Th1) response, which reduces serum levels of pro-inflammatory cytokines and induces the production of anti-inflammatory Th2 cytokines. Vitamin D treatment downregulates the expression of DPP4/CD26, which may play a role in the virulence of the SARS-CoV-2. SARS-CoV-2 uses angiotensin-converting enzyme-2 (ACE2) for cellular entry. However, upregulation of ACE 2, protects against lipopolysaccharide induced acute lung injury. Vitamin D is found to be a negative endocrine regulator of RAAS. Vitamin D inhibited renin, ACE, and Ang II expression, and induced ACE2 levels. ACE2, converts angiotensin II to angiotensin 1-7. Upon binding AT1R, angiotensin II causes inflammation, fibrosis, and apoptosis. AT-(1-7) opposes the effects of angiotensin II by interacting with its own receptor. Red arrows indicates inhibitory action and green arrows, stimulatory action. ACE1, angiotensin-converting enzyme 1; ACE2, angiotensin-converting enzyme 2; AT1R, type 1 angiotensin 2 receptor; AT1-7, heptapeptide angiotensin (1-7); DPP4/CD26, dipeptidyl peptidase 4/cluster of differentiation 26; Th1, T helper 1 cells; Th2, T helper 2 cells.
Very low vitamin D levels are commonly found in ethnic minorities with increased skin melanin in the United Kingdom and the United States [127]. In a provisional analysis of COVID-19-related deaths in England and Wales, even after correcting for age and other sociodemographic characteristics and measures of self-reported health and disability, males and females of black ethnicity showed 1.9 times higher COVID-19-related deaths than those of white ethnicity. Further, males and females in the Bangladeshi and Pakistani ethnic groups were 1.8 and 1.6 times more likely to suffer a COVID-19-related death than males and females from the white ethnic group, respectively. Further research is needed to examine whether vitamin D status contributes to the higher COVID 19 mortality seen in these ethnicities in the United Kingdom [128].
Several assumptions can be made regarding the role of vitamin D deficiency in COVID-19 and disease severity based on the epidemiological and clinical data. Conditions associated with lower vitamin D levels such as chronic illnesses, smoking, and increased age and dark-skinned ethnicities have a higher case fatality with COVID-19. Furthermore, the onset of epidemic and higher case load in countries during the winter season also raises the possible association with low vitamin D status [129]. Nevertheless, these are simply correlations and do not necessarily imply causation, and other factors are clearly involved such as preferential involvement in patient-centered activities and socioeconomic status.
A. Vitamin D Replacement
Although increased susceptibility to infections has been found with vitamin D deficiency, evidence regarding the benefits of vitamin D supplementation in preventing infections or disease mortality has been inconsistent [130]. A meta-analysis that showed vitamin D supplementation reduced the risk of acute respiratory tract infections demonstrated benefits with daily or weekly vitamin D supplementation, but not with regimens containing large bolus doses. Protective effects were strongest in those with profound vitamin D deficiency at baseline [131].
Different thresholds of vitamin D levels have been recommended in studies for the protection of respiratory tract infections. A vitamin D threshold of > 50nmol/L (20 ng/mL) is thought to be adequate for the prevention of acute respiratory tract infections [132]. The degree of protection appears to be optimal when the serum vitamin D levels are in the range of 100 to 150 nmol/L (40-60 ng/mL) [129]. A daily dose of 2000 to 5000 IU of vitamin D was required to achieve this level during winter months because cutaneous vitamin D synthesis is minimal during this period [133]. Daily doses of vitamin D up to 10 000 IU/day are generally safe and not associated with any adverse effects [134]. However, the dose requirement may vary with dietary intake, genetics, baseline vitamin D levels, and environmental conditions.
Vitamin D treatment has been recommended on a background of COVID-19 by clinicians [135]. Considering the potential benefits of enhanced immunity, mitigation of the inflammatory response, epidemiological data on protection from severe infection and established safety, it is reasonable to consider vitamin D replacement in patients with demonstrable deficiency. Limited exposure to sunlight, and poor dietary conditions as a result of the lockdown in most countries, are important factors that would increase the need of vitamin D supplementation.
9. Gonads
Analysis of the tissue expression pattern of ACE2 in different human tissues has revealed a high level of expression in the human testis [136, 137]. According to a recent study, ACE2 is expressed in spermatogonia and somatic (Leydig and Sertoli) cells in the testis. It was shown that TMPRSS2 is concentrated in spermatogonia and spermatids. This is shown to be used for viral S protein priming [138]. This suggests that the testis is a high-risk organ vulnerable to SARS-CoV-2 infection. However, there are no similar studies on ACE2 expression in ovaries.
To date, there are no reports on the viral distribution or pathological effects of virus on human gonads from SARS-CoV-2 infection. A study conducted to define the organ distribution of SARS-CoV-1 in 2003 using autopsy samples of 2 male and 2 female patients who died of SARS in China revealed that virus RNA material or antigens were not present in the testis or ovary [87]. However, an autopsy-based study on 6 males who died of SARS-CoV-1 showed orchitis in all. There was marked germ cell destruction, reduced spermatozoon in the seminiferous tubule, thickened basement membrane, and leukocyte infiltration. In situ hybridization for SARS viral genomic material was negative in the samples. Therefore, the authors suggest possible immune-mediated damage as the cause of destruction rather than direct viral entry and damage [139]. There is no literature on the effect of these viruses on the human ovaries.
The available evidence suggests the vulnerability of the testes to viral entry because the abundance of ACE2 has not been demonstrated during SARS-CoV-1 epidemic. Even in the absence of viral material, immune-mediated orchitis was shown during the previous epidemic, the same consequence is possible in survivors of the current pandemic. Therefore, further studies to assess hypogonadism and spermatogenesis following recovery from acute illness are warranted.
A. Management Considerations of Preexistent Hypogonadism
For people with hypogonadism, continuing the same regimen of hormone replacement until a visit to the health care provider is advisable. There might be difficulty in accessing medications including testosterone injections. It may be reasonable to postpone replacement in case of lack of availability until nonurgent services resume because there are no major hazards of temporary discontinuation. However, substitution of the alternative dosage forms is an option. If testosterone gel preparation is substituted for injections, the gel can be commenced from the due date of the next testosterone injection [58]. In the case of female hormone replacement, it may be preferable to use transdermal formulations that are less likely to lead to a hypercoagulable state.
10. Conclusions
In the absence of literature relating to the effects of SARS-CoV-2 on endocrine organs, we have made speculations based on the evidence from SARS-CoV-1 infection during the years 2002 through 2003. Research on clinical effects during the current pandemic and follow-up of recovered patients is a priority, considering the potential for multitude of effects on endocrine organs. We have highlighted management strategies based on available data in the current pandemic and have also demonstrated the need for multifaceted research. The research needs include collection of carefully prepared clinical data, histological and autopsy studies, as well as basic science studies to understand the effect of SARS-Cov-2 on the endocrine system. Furthermore, studies on viral genomics to fill the gaps in the knowledge on effects of direct viral invasion and immune-mediated injury are needed. Clinicians should be encouraged to report their experience in managing patients with preexisting endocrine diseases to improve current practices, which are mostly empirical.
Glossary
Abbreviations
- ACE2
angiotensin-converting enzyme 2
- ARDS
acute respiratory distress syndrome
- CIRCI
critical illness-related corticosteroid insufficiency
- COVID-19
coronavirus-19
- CS
Cushing syndrome
- DRA
dopamine receptor agonist
- FGF2
fibroblast growth factor 2
- HPA
hypothalamo-pituitary-adrenal axis
- IFN-γ
interferon γ
- MCP1
monocyte chemoattractant protein 1
- MERS-CoV
Middle East respiratory syndrome-coronavirus
- RAAS
renin-angiotensin-aldosterone system
- S protein
Spike protein
- SARS-CoV-1
severe acute respiratory syndrome-coronavirus-1
- SARS-CoV-2
severe acute respiratory syndrome-coronavirus-2
- TMPRSS2
transmembrane protease serine 2
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: Data sharing is not applicable to this article because no datasets were generated or analyzed during the current study.
References
- 1. Mulatero P, Stowasser M, Loh KC, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89(3):1045-1050. [DOI] [PubMed] [Google Scholar]
- 2. Corman VM, Muth D, Niemeyer D, Drosten C. Hosts and sources of dndemic human coronaviruses. Adv Virus Res. 2018;100:163-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Worldometer. Coronavirus cases. https://www.worldometers.info/coronavirus/. doi: 10.1101/2020.01.23.20018549V2 [DOI] [Google Scholar]
- 4. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi: 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J Virol. 2020. doi: 10.1128/jvi.00127-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020. doi: 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270-273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Letko M, Munster V. Functional assessment of cell entry and receptor usage for lineage B β-coronaviruses, including 2019-nCoV. bioRxiv. 2020. doi: 10.1101/2020.01.22.915660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jin Y, Yang H, Ji W, et al. Virology, epidemiology, pathogenesis, and control of covid-19. Viruses. 2020. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mason RJ. Pathogenesis of COVID-19 from a cell biologic perspective. Eur Respir J. April 2020:2000607. doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87(5):E1-E9. [DOI] [PubMed] [Google Scholar]
- 14. Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605-2610. [DOI] [PubMed] [Google Scholar]
- 15. Furuhashi M, Moniwa N, Mita T, et al. Urinary angiotensin-converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens. 2015;28(1):15-21. [DOI] [PubMed] [Google Scholar]
- 16. Kuba K, Imai Y, Penninger JM. Angiotensin-converting enzyme 2 in lung diseases. Curr Opin Pharmacol. 2006;6(3):271-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iwai M, Horiuchi M. Devil and angel in the renin-angiotensin system: ACE-angiotensin II-AT1 receptor axis vs. ACE2-angiotensin-(1-7)-Mas receptor axis. Hypertens Res. 2009;32(7):533-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuster GM, Pfister O, Burkard T, et al. SARS-CoV2: should inhibitors of the renin–angiotensin system be withdrawn in patients with COVID-19? Eur Heart J. March 2020. doi: 10.1093/eurheartj/ehaa235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Wilde AH, Snijder EJ, Kikkert M, van Hemert MJ. Host factors in coronavirus replication. Curr Top Microbiol Immunol. 2018;419:1-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Belouzard S, Chu VC, Whittaker GR. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc Natl Acad Sci U S A. 2009;106(14):5871-5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10(2):102-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020. doi: 10.1186/s40779-020-00240-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang H, Yang P, Liu K, et al. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuba K, Imai Y, Ohto-Nakanishi T, Penninger JM. Trilogy of ACE2: a peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther. 2010;128(1):119-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perlman S, Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009;7(6):439-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science (80-). 2020. doi: 10.1126/science.aax0902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gu J, Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am J Pathol. 2007;170(4):1136-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Keicho N, Itoyama S, Kashiwase K, et al. Association of human leukocyte antigen class II alleles with severe acute respiratory syndrome in the Vietnamese population. Hum Immunol. 2009;70(7):527-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen YM, Liang SY, Shih YP, et al. Epidemiological and genetic correlates of severe acute respiratory syndrome coronavirus infection in the hospital with the highest nosocomial infection rate in Taiwan in 2003. J Clin Microbiol 2006;44(2):359-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang SF, Chen KH, Chen M, et al. Human-leukocyte antigen class I Cw 1502 and class II DR 0301 genotypes are associated with resistance to severe acute respiratory syndrome (SARS) infection. Viral Immunol. 2011;24(5):421-426. [DOI] [PubMed] [Google Scholar]
- 33. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9(1):727-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hirano T, Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020:3-5. doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. [published correction appears in Lancet January 30, 2020]. Lancet. 2020;395(10223):497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Steardo L, Steardo L Jr, Zorec R, Verkhratsky A. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol 2020;229(3):e13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82(15):7264-7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun 2020;87:18-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leow MK, Kwek DS, Ng AW, Ong KC, Kaw GJ, Lee LS. Hypocortisolism in survivors of severe acute respiratory syndrome (SARS). Clin Endocrinol (Oxf). 2005;63(2):197-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vladutiu GD, Natelson BH. Association of medically unexplained fatigue with ACE insertion/deletion polymorphism in Gulf War veterans. Muscle Nerve. 2004;30(1):38-43. [DOI] [PubMed] [Google Scholar]
- 43. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271-280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sriramula S, Xia H, Xu P, Lazartigues E. Brain-targeted angiotensin-converting enzyme 2 overexpression attenuates neurogenic hypertension by inhibiting cyclooxygenase-mediated inflammation. Hypertens (Dallas, Tex 1979). 2015;65(3):577-586. doi: 10.1161/hypertensionaha.114.04691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gane SB, Kelly C, Hopkins C. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology. 2020;58(3):299-301. [DOI] [PubMed] [Google Scholar]
- 46. Wheatland R. Molecular mimicry of ACTH in SARS - implications for corticosteroid treatment and prophylaxis. Med Hypotheses. 2004;63(5):855-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lofthouse M. Hypocortisolism in survivors of SARS. Nat Clin Pract Endocrinol Metab. 2005;1(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vilar L, Abucham J, Albuquerque JL, et al. Controversial issues in the management of hyperprolactinemia and prolactinomas - an overview by the Neuroendocrinology Department of the Brazilian Society of Endocrinology and Metabolism. Arch Endocrinol Metab. 2018;62(2):236-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vardas K, Apostolou K, Briassouli E, et al. Early response roles for prolactin cortisol and circulating and cellular levels of heat shock proteins 72 and 90α in severe sepsis and SIRS. Biomed Res Int. 2014;2014:803561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tasker RC, Roe MF, Bloxham DM, White DK, Ross-Russell RI, O’Donnell DR. The neuroendocrine stress response and severity of acute respiratory syncytial virus bronchiolitis in infancy. Intensive Care Med. 2004;30(12):2257-2262. [DOI] [PubMed] [Google Scholar]
- 51. Yu-Lee LY. Prolactin modulation of immune and inflammatory responses. Recent Prog Horm Res. 2002;57:435-455. [DOI] [PubMed] [Google Scholar]
- 52. Baldeweg SE, Ball S, Brooke A, et al. ; Society for Endocrinology Clinical Committee Society for Endocrinology Clinical Guidance: inpatient management of cranial diabetes insipidus. Endocr Connect. 2018;7(7):G8-G11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Filho AG, Ferreira AJ, Santos SH, et al. Selective increase of angiotensin(1-7) and its receptor in hearts of spontaneously hypertensive rats subjected to physical training. Exp Physiol. 2008;93(5):589-598. [DOI] [PubMed] [Google Scholar]
- 54. Dong C, Li X, Qifa S, Hu C, Su F, Dai J. Hypokalemia and clinical implications in patients with coronavirus disease 2019 (COVID-19). medRxiv. January 2020:2020.02.27.20028530. doi: 10.1101/2020.02.27.20028530 [DOI] [Google Scholar]
- 55. Cycloset, Parlodel (bromocriptine) dosing, indications, interactions, adverse effects, and more https://reference.medscape.com/drug/cycloset-parlodel-bromocriptine-343124. Accessed April 22, 2020.
- 56. Tulloch KJ, Dodin P, Tremblay-Racine F, Elwood C, Money D, Boucoiran I. Cabergoline: a review of its use in the inhibition of lactation for women living with HIV. J Int AIDS Soc. 2019;22(6):e25322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lin SJ, Wu ZR, Cao L, et al. Pituitary tumor suppression by combination of cabergoline and chloroquine. J Clin Endocrinol Metab. 2017;102(10):3692-3703. [DOI] [PubMed] [Google Scholar]
- 58. COVID-19 resources for managing endocrine conditions | Society for Endocrinology https://www.endocrinology.org/clinical-practice/covid-19-resources-for-managing-endocrine-conditions/. Accessed April 14, 2020.
- 59. Ding Y, Wang H, Shen H, et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200(3):282-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tresoldi AS, Sumilo D, Perrins M, et al. Increased infection risk in Addison’s disease and congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2020;105(2):418-429. doi: 10.1210/clinem/dgz006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. El-Maouche D, Hargreaves CJ, Sinaii N, Mallappa A, Veeraraghavan P, Merke DP. Longitudinal assessment of illnesses, stress dosing, and illness sequelae in patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2018;103(6):2336-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Arlt W; Society for Endocrinology Clinical Committee Society for Endocrinology Endocrine Emergency Guidance: emergency management of acute adrenal insufficiency (adrenal crisis) in adult patients. Endocr Connect. 2016;5(5):G1-G3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Prete A, Taylor AE, Bancos I, et al. Prevention of adrenal crisis: cortisol responses to major stress compared to stress dose hydrocortisone delivery. J Clin Endocrinol Metab. 2020;105(7). doi: 10.1210/clinem/dgaa133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Arlt W, Baldeweg SE, Pearce SHS, Simpson HL.. Clinical management guidance during the COVID-19 pandemic: adrenal insufficiency. Eur J Endocrinol. 2020:1-21. [Google Scholar]
- 65. Dineen R, Thompson CJ, Sherlock M. Adrenal crisis: prevention and management in adult patients. Ther Adv Endocrinol Metab. 2019;10:2042018819848218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101(2):364-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Auyeung TW, Lee JS, Lai WK, et al. The use of corticosteroid as treatment in SARS was associated with adverse outcomes: a retrospective cohort study. J Infect. 2005;51(2):98-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Clinical management of severe acute respiratory infection when COVID-19 is suspected https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed April 14, 2020.
- 69. Marik PE, Levitov A. The “koala stress syndrome” and adrenal responsiveness in the critically ill. Intensive Care Med. 2010;36(11):1805-1806. [DOI] [PubMed] [Google Scholar]
- 70. Annane D, Pastores SM, Rochwerg B, et al. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Crit Care Med. 2017;45(12):2078-2088. [DOI] [PubMed] [Google Scholar]
- 71. Cohen J, Ward G, Prins J, Jones M, Venkatesh B. Variability of cortisol assays can confound the diagnosis of adrenal insufficiency in the critically ill population. Intensive Care Med. 2006;32(11):1901-1905. [DOI] [PubMed] [Google Scholar]
- 72. Epperla N, McKiernan F. Iatrogenic Cushing syndrome and adrenal insufficiency during concomitant therapy with ritonavir and fluticasone. Springerplus. 2015;4:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pivonello R, Ferrigno R, Isidori AM, Biller BMK, Grossman AB, Colao A. Covid-19 disease and Cushing’s syndrome: recommendations for a special population with endogenous glucocorticoid excess. Lancet Diabet Endocrinol 2020;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Aranda G, Lopez C, Fernandez-Ruiz R, et al. Circulatory immune cells in Cushing syndrome: bystanders or active contributors to atherometabolic injury? A study of adhesion and activation of cell surface markers. Int J Endocrinol. 2017;2017:2912763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Isidori AM, Minnetti M, Sbardella E, Graziadio C, Grossman AB. Mechanisms in endocrinology: the spectrum of haemostatic abnormalities in glucocorticoid excess and defect. Eur J Endocrinol. 2015;173(3):R101-R113. [DOI] [PubMed] [Google Scholar]
- 78. van der Pas R, Leebeek FW, Hofland LJ, de Herder WW, Feelders RA. Hypercoagulability in Cushing’s syndrome: prevalence, pathogenesis and treatment. Clin Endocrinol (Oxf). 2013;78(4):481-488. [DOI] [PubMed] [Google Scholar]
- 79. Preda VA, Sen J, Karavitaki N, Grossman AB. Etomidate in the management of hypercortisolaemia in Cushing’s syndrome: a review. Eur J Endocrinol. 2012;167(2):137-143. [DOI] [PubMed] [Google Scholar]
- 80. Wong SWP, Yap YW, Narayanan RP, et al. Etomidate in the management of severe Cushing’s disease and MRSA bacteraemia in a district general hospital in the United Kingdom. Endocrinol Diabetes Metab Case Rep. 2019;2019. doi: 10.1530/EDM-19-0044. [DOI] [PubMed] [Google Scholar]
- 81. Yu R, Wei M. False positive test results for pheochromocytoma from 2000 to 2008. Exp Clin Endocrinol Diabetes. 2010;118(9):577-585. doi: 10.1055/s-0029-1237699 [DOI] [PubMed] [Google Scholar]
- 82. Lenders JW, Duh QY, Eisenhofer G, et al. ; Endocrine Society Pheochromocytoma and paraganglioma: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915-1942. [DOI] [PubMed] [Google Scholar]
- 83. Wang W, Ye Y, Yao H, et al. Evaluation and observation of serum thyroid hormone and parathyroid hormone in patients with severe acute respiratory syndrome. J Chin Antituberculous Assoc. 2003;25:232-234. [Google Scholar]
- 84. Jonklaas J, Bianco AC, Bauer AJ, et al. ; American Thyroid Association Task Force on Thyroid Hormone Replacement Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24(12):1670-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wei L, Sun S, Xu CH, et al. Pathology of the thyroid in severe acute respiratory syndrome. Hum Pathol. 2007;38(1):95-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sun S, Wei L, Zhang J, Xu Y, He FJ, Gu J. [Pathology and immunohistochemistry of thyroid in severe acute respiratory syndrome]. Zhonghua Yi Xue Za Zhi. 2005;85(10):667-670. [PubMed] [Google Scholar]
- 87. Ding Y, He L, Zhang Q, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203(2):622-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Brancatella A, Ricci D, Viola N, Sgrò D, Santini F, Latrofa F.. Subacute thyroiditis after SARS-CoV-2 infection. J Clin Endocrinol Metab. 105(7):dgaa276. doi: 10.1210/clinem/dgaa276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. COVID-19 resources for managing endocrine conditions. Society for Endocrinology https://www.endocrinology.org/clinical-practice/covid-19-resources-for-managing-endocrine-conditions/. Accessed April 18, 2020.
- 90. Romanelli A, Mascolo S. Immunosuppression drug-related and clinical manifestation of coronavirus disease 2019: a therapeutical hypothesis. Am J Transplant. 2020;20(7):1947-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fang HJ, Yang JK. Tissue-specific pattern of angiotensin-converting enzyme 2 expression in rat pancreas. J Int Med Res. 2010;38(2):558-569. [DOI] [PubMed] [Google Scholar]
- 92. Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(3):193-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Liu F, Long X, Zou W, et al. Highly ACE2 expression in pancreas may cause pancreas damage after SARS-CoV-2 infection. medRxiv. January 2020:2020.02.28.20029181. doi: 10.1101/2020.02.28.20029181 [DOI] [Google Scholar]
- 94. Roca-Ho H, Riera M, Palau V, Pascual J, Soler MJ. Characterization of ACE and ACE2 expression within different organs of the NOD mouse. Int J Mol Sci. 2017;18(3). doi: 10.3390/ijms18030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lang Z, Zhang L, Zhang S, et al. Pathological study on severe acute respiratory syndrome. Chin Med J (Engl). 2003;116(7):976-980. [PubMed] [Google Scholar]
- 96. Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202(3):415-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Shi X, Gong E, Gao D, et al. Severe acute respiratory syndrome associated coronavirus is detected in intestinal tissues of fatal cases. Am J Gastroenterol. 2005;100(1):169-176. [DOI] [PubMed] [Google Scholar]
- 98. Pusch E, Renz H, Skevaki C. Respiratory virus-induced heterologous immunity: part of the problem or part of the solution? Allergo J. 2018;27(3):28-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhou J, Tan J. Diabetes patients with COVID-19 need better blood glucose management in Wuhan, China. Metabolism. 2020;107:154216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. COVID-19 and Diabetes: Known Mechanisms and a “New Beast”? https://www.medscape.com/viewarticle/928629. Accessed April 18, 2020.
- 101. Brufsky A. Hyperglycemia, hydroxychloroquine, and the COVID-19 pandemic. J Med Virol. 2020;92(7):770-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. The Clinical Features of SARS Patients with Diabetes or Secondary Hyperglycemia. American Diabetes Association https://professional.diabetes.org/abstract/clinical-features-sars-patients-diabetes-or-secondary-hyperglycemia. Accessed April 18, 2020.
- 103. Katulanda P, Dissanayake HA, Ranathunga I, et al. et al. Prevention and management of COVID-19 among patients with diabetes: an appraisal of the literature [published online ahead of print, May 14, 2020]. Diabetologia.2020:1-13. doi: 10.1007/s00125-020-05164-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bornstein SR, Rubino F, Khunti K, et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8(6):546-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Drucker DJ. Coronavirus infections and type 2 diabetes—shared pathways with therapeutic implications. Endocr Rev. 2020;41(3). doi: 10.1210/endrev/bnaa011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fadini GP, Morieri ML, Longato E, Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Invest. 2020;43(6):867-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Chen C, Chen C, Yan JT, Zhou N, Zhao JP, Wang DW. [Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19]. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48(0):E008. [DOI] [PubMed] [Google Scholar]
- 108. Dietz W, Santos-Burgoa C. Obesity and its implications for COVID-19 mortality. Obesity (Silver Spring). 2020;28(6):1005. [DOI] [PubMed] [Google Scholar]
- 109. Zhu L, She ZG, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(6):1068-1077.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hofland J, Kaltsas G, de Herder WW. Advances in the diagnosis and management of well-differentiated neuroendocrine neoplasms. Endocr Rev. 2020;41(2):371-403. doi: 10.1210/endrev/bnz004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Casey RT, Valk GD, Schalin-Jantti C, Grossman AB, Thakker RV. Endocrinology in the time of COVID-19: Management of neuroendocrine neoplasms (NENs). Eur J Endocrino l.2020:1-24. doi: 10.1530/EJE-20-0424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhou Y, Frey TK, Yang JJ. Viral calciomics: interplays between Ca2+ and virus. Cell Calcium. 2009;46(1):1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Minakshi R, Padhan K, Rehman S, Hassan MI, Ahmad F. The SARS coronavirus 3a protein binds calcium in its cytoplasmic domain. Virus Res. 2014;191:180-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Nachimuthu S, Assar MD, Schussler JM. Drug-induced QT interval prolongation: mechanisms and clinical management. Ther Adv Drug Saf. 2012;3(5):241-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Uwitonze AM, Razzaque MS. Role of magnesium in vitamin D activation and function. J Am Osteopath Assoc. 2018;118(3):181-189. [DOI] [PubMed] [Google Scholar]
- 116. Rondanelli M, Miccono A, Lamburghini S, et al. Self-care for common colds: the pivotal role of vitamin D, vitamin C, ainc, and echinacea in three main immune interactive clusters (physical barriers, innate and adaptive immunity) involved during an episode of common colds - practical advice on dosages. Evidence-based Complement Altern Med. 2018;2018. doi: 10.1155/2018/5813095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Dancer RC, Parekh D, Lax S, et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax. 2015;70(7):617-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Coussens AK, Wilkinson RJ, Nikolayevskyy V, et al. Ethnic variation in inflammatory profile in tuberculosis. Plos Pathog. 2013;9(7):e1003468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wong CK, Lam CW, Wu AK, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136(1):95-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Mahallawi WH, Khabour OF, Zhang Q, Makhdoum HM, Suliman BA. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sharifi A, Vahedi H, Nedjat S, Rafiei H, Hosseinzadeh-Attar MJ. Effect of single-dose injection of vitamin D on immune cytokines in ulcerative colitis patients: a randomized placebo-controlled trial. Apmis. 2019;127(10):681-687. [DOI] [PubMed] [Google Scholar]
- 122. Cantorna MT, Snyder L, Lin YD, Yang L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients. 2015;7(4):3011-3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Vankadari N, Wilce JA. Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect. 2020;9(1):601-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Komolmit P, Charoensuk K, Thanapirom K, et al. Correction of vitamin D deficiency facilitated suppression of IP-10 and DPP IV levels in patients with chronic hepatitis C: a randomised double-blinded, placebo-control trial. Plos One. 2017;12(4):e0174608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Xu J, Yang J, Chen J, Luo Q, Zhang Q, Zhang H. Vitamin D alleviates lipopolysaccharide‑induced acute lung injury via regulation of the renin‑angiotensin system. Mol Med Rep. 2017;16(5):7432-7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Crowe FL, Jolly K, Macarthur C, et al. Trends in the incidence of testing for Vitamin D deficiency in primary care in the UK: a retrospective analysis of the Health Improvement Network (THIN), 2005–2015. BMJ Open. 2019;9(6):1-8. doi: 10.1136/bmjopen-2018-028355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Coronavirus (COVID-19) related deaths by ethnic group, England and Wales - Office for National Statistics https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/coronavirusrelateddeathsbyethnicgroupenglandandwales/2march2020to10april2020. Accessed May 24, 2020.
- 129. Grant WB, Lahore H, McDonnell SL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12040988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Gruber-Bzura BM. Vitamin D and influenza—prevention or therapy? Int J Mol Sci. 2018;19(8). doi: 10.3390/ijms19082419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. Bmj. 2017;356:i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Amrein K, Scherkl M, Hoffmann M, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020. doi: 10.1038/s41430-020-0558-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77(1):204-210. [DOI] [PubMed] [Google Scholar]
- 134. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Watkins J. Preventing a covid-19 pandemic. BMJ. 2020;368:m810. [DOI] [PubMed] [Google Scholar]
- 136. Chen Y, Guo Y, Pan Y, Zhao Z. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun. 2020;525. doi: 10.1016/j.bbrc.2020.02.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532(1–2):107-110. doi: 10.1016/s0014-5793(02)03640-2 [DOI] [PubMed] [Google Scholar]
- 138. Wang Z, Xu X. scRNA-seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS-CoV-2 infection in spermatogonia, Leydig and Sertoli cells. Cells. 2020;9(4). doi: 10.3390/cells9040920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Xu J, Qi L, Chi X, et al. Orchitis: a complication of severe acute respiratory syndrome (SARS). Biol Reprod. 2006;74(2):410-416. [DOI] [PMC free article] [PubMed] [Google Scholar]