Abstract
Background
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a novel pathogen causing the current worldwide coronavirus disease 2019 (COVID-19) pandemic. Due to insufficient diagnostic testing in the United States, there is a need for clinical decision-making algorithms to guide testing prioritization.
Methods
We recruited participants nationwide for a randomized clinical trial. We categorized participants into 3 groups: (1) those with confirmed SARS-CoV-2 infection, (2) those with probable SARS-CoV-2 infection (pending test or not tested but with a confirmed COVID-19 contact), and (3) those with possible SARS-CoV-2 infection (pending test or not tested and with a contact for whom testing was pending or not performed). We compared the frequency of self-reported symptoms in each group and categorized those reporting symptoms in early infection (0–2 days), midinfection (3–5 days), and late infection (>5 days).
Results
Among 1252 symptomatic persons screened, 316 had confirmed, 393 had probable, and 543 had possible SARS-CoV-2 infection. In early infection, those with confirmed and probable SARS-CoV-2 infection shared similar symptom profiles, with fever most likely in confirmed cases (P = .002). Confirmed cases did not show any statistically significant differences compared with unconfirmed cases in symptom frequency at any time point. The most commonly reported symptoms in those with confirmed infection were cough (82%), fever (67%), fatigue (62%), and headache (60%), with only 52% reporting both fever and cough.
Conclusions
Symptomatic persons with probable SARS-CoV-2 infection present similarly to those with confirmed SARS-CoV-2 infection. There was no pattern of symptom frequency over time.
Keywords: coronavirus, COVID-19, SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the pathogen responsible for the current global COVID-19 pandemic. Similar to its predecessor, SARS-CoV-1, this novel coronavirus can cause severe lower respiratory tract infection, often complicated by acute respiratory distress syndrome (ARDS). SARS-CoV-2, however, can also present with a wider variability in clinical syndromes and severity.
Guan et al. documented that cough (67%), fever (44%), and fatigue (38%) were the most common symptoms in 1099 patients admitted to hospitals throughout mainland China [1]. Another study out of China on 1012 non–critically ill patients noted fever (75%) and cough (52%) as the predominant symptoms, but also reported a higher rate of diarrhea (15%) compared with the predominant symptoms presented in a February 2020 Joint World Health Organization–China report of 55 924 laboratory-confirmed cases within China [2, 3]. Shortness of breath and cough (88%) were highly prevalent symptoms in 24 intensive care unit (ICU) patients in Washington, USA, while rhinorrhea was present in 17% of these patients [4]. In a recent Italian study of 202 confirmed infected patients, 64% reported alteration of sense of smell or taste [5–8]. A recent publication by the Centers for Disease Control and Prevention (CDC) COVID-19 Response Team examined 9282 health care personnel with confirmed SARS-CoV-2 infection and found that among those with complete data, 92% reported at least 1 symptom. Of those with symptoms, the most common were muscle aches (66%) and headache (65%), with absence of smell or taste grouped as “other symptoms” in 750 (16%) of the cases examined [9]. The full spectrum of the signs and symptoms of SARS-CoV-2 infection is still being elucidated.
Accurate identification of COVID-19 disease is critical to proper containment. Currently, the CDC recommends either a testing-based or symptom-based strategy when determining whether to institute transmission precautions in persons with confirmed or suspected SARS-CoV-2 infection, with similar strategies used to determine when to remove these isolation precautions [10]. In the setting of limited test availability, clinical decisions are often based on clinical suspicion and symptoms. Front-line health care workers need robust information on the clinical syndromes of COVID-19 in order to optimize the often scarce resources that are devoted to isolation and quarantine. To date, there is insufficient documentation in the literature on the symptoms of SARS-CoV-2-infected persons who do not require hospitalization. Here, we present the most common SARS-CoV-2 symptoms among symptomatic outpatients.
METHODS
Participants were recruited for a phase III randomized clinical trial comparing hydroxychloroquine vs placebo in the management of outpatient COVID-19 disease (ClinicalTrials.gov: NCT04308668). The parent trial was approved by the University of Minnesota institutional review board and conducted under a US Food and Drug Administration Investigational New Drug (IND) approval. Participants consented electronically by providing a digital signature after reading the consent document and passing a comprehension assessment. Recruitment began on March 17, 2020, and the data presented are through April 20, 2020. The parent trial is still actively recruiting participants.
The parent trial includes 2 distinct cohorts: (1) asymptomatic household contacts of a COVID-19 case or health care workers who have had a high-risk exposure event (postexposure prophylaxis) and (2) symptomatic, nonhospitalized household contacts of a COVID-19 case or health care workers who have had a high-risk exposure event (preemptive therapy to reduce progression to severe infection). The screening survey allows participants to indicate whether they are experiencing cough, fever, shortness of breath, headache, diarrhea, rhinorrhea, sore throat, fatigue, muscle aches, sinus congestion, or lack of smell, with persons reporting symptoms at the time of screening excluded from the postexposure arm of the study. Participants were recruited, via social media and traditional media exposure, from across the United States, and data were collected through self-reported online surveys. These surveys were emailed using the Research Electronic Data Capture (REDCap) system, and data were stored on the University of Minnesota secure REDCap database.
For this analysis, we reviewed all persons who screened positive for symptoms, regardless of whether they met criteria for enrollment. Symptomatic persons were subdivided into 3 groups: (1) confirmed SARS-CoV-2 infection, (2) probable infection, or (3) possible infection. Confirmed infection was determined by polymerase chain reaction (PCR) detection of SARS-CoV-2 virus per participant report. Probable infection was defined as those having a pending test or with no test but a positive-test-confirmed case contact. Possible infection was defined as those having a pending test or no test and whose contact also had a pending test or was not tested. This categorization was based on the initial screening survey, which all included participants completed.
Symptom duration and types of symptoms were collected by the electronic case report screening form. The timeline of symptom development was divided into tertiles based on the distribution of days since symptom development at time of screening reported by those with confirmed SARS-CoV-2 infection. Early infection was defined as having symptoms for 0 to 2 days, midinfection as 3 to 5 days, and late infection as 5 to 30 days.
Statistical analysis was completed using SPSS, version 25 (IBM, Armonk, NY, USA). Statistics were primarily descriptive. We compared the proportions of symptom prevalence between groups by the Fisher exact test. We chose a significance level of P < .01 to account for multiple comparisons.
RESULTS
Of the 1252 participants who completed a screening survey and were included in this analysis, there were 316 participants with confirmed infection, 393 with probable infection, and 543 with possible infection. All participants with confirmed infection in this analysis reported at least 1 symptom at the time of screening. The median age for the sample population (interquartile range [IQR]) was 45 (35–55) years, with no significant difference between the confirmed, probable, and possible groups. Health care workers comprised 37% of those included in this analysis.
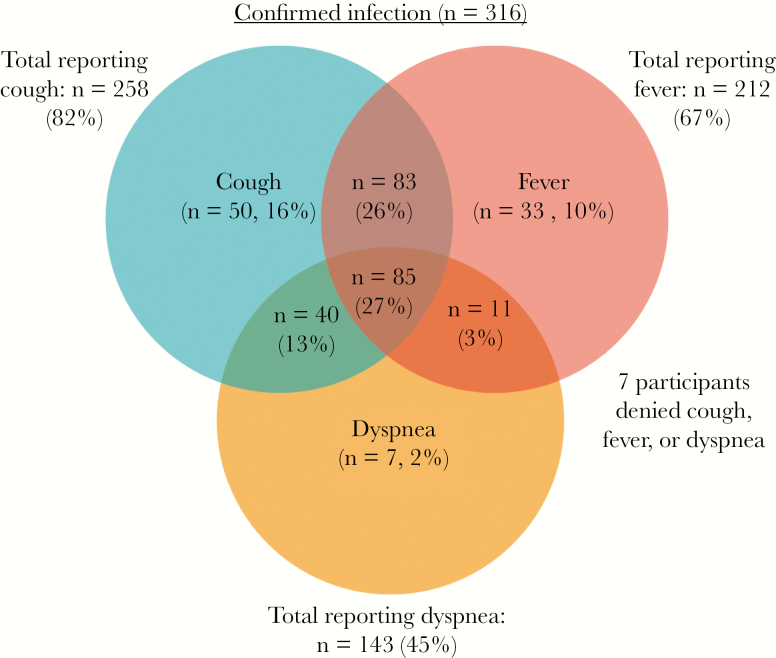
Among 316 nonhospitalized adults with confirmed SARS-CoV-2 infection, 258 (82%) reported cough, 212 (67%) reported fever, and 143 (45%) reported dyspnea, irrespective of the time from symptom development. Only 27% of participants with confirmed infection reported having all 3 symptoms of cough, fever, and dyspnea, while 53% of participants (168/316) had both fever and cough (Figure 1). When compared without regard for duration of symptoms, several symptoms demonstrated a significant difference between the confirmed infection group and unconfirmed group, including fever, headache, diarrhea, fatigue, myalgia, and anosmia (all P < .01), though the probable and possible infection groups appeared very similar (Table 1). Thus, when viewed without the context of symptom duration, it is difficult to separate probable and possible infections from one another. This may also suggest that those with confirmed infection had more severe (or multiple) symptoms, leading them to seek care and receive testing.
Figure 1.
Frequency of reported cough, fever, and dyspnea in 316 nonhospitalized adults with polymerase chain reaction–confirmed SARS-CoV-2 infection.
Table 1.
Comparison of Symptom Frequency and Percentage and Median Duration of Symptoms Among Those With Confirmed SARS-CoV-2 Infection, Probable Infection, or Possible Infection Across All Time Points
| Rates of Symptoms in All Cases | ||||
|---|---|---|---|---|
| Confirmed Infection | Probable Infection | Possible Infection | P | |
| Total, No. | 316 | 393 | 543 | |
| Duration of symptoms, d | 5 [3–11] | 2 [1–5] | 3 [1–7] | |
| Symptom severitya | 61 [50–72] | 50 [32–64] | 54 [37–66] | |
| Cough | 258 (82) | 326 (83) | 430 (79) | .32 |
| Fever | 212 (67) | 175 (45) | 245 (45) | <.001* |
| Dyspnea | 143 (45) | 139 (35) | 231 (43) | .02 |
| Headache | 191 (60) | 197 (50) | 261 (48) | .002* |
| Diarrhea | 121 (38) | 90 (23) | 140 (26) | <.001* |
| Rhinorrhea | 52 (16) | 80 (20) | 101 (19) | .42 |
| Sore throat | 125 (40) | 182 (46) | 270 (50) | .02 |
| Fatigue | 195 (62) | 183 (47) | 248 (46) | <.001* |
| Myalgia | 165 (52) | 170 (43) | 225 (41) | .007* |
| Sinus congestion | 83 (26) | 76 (19) | 106 (20) | .04 |
| Anosmia | 102 (32) | 68 (17) | 121 (22) | <.001* |
Values are No. (%) or median [interquartile range]. Significance was measured using the Fisher exact test.
aMeasured on a visual analog scale, 0–100 mm.
*Statistically significant difference between confirmed and probable infection groups.
To further explore the question of symptom temporality in SARS-CoV-2 infection, we examined reported rates of symptoms in participants with confirmed infection who completed the screening survey during early infection (n = 77), midinfection (n = 84), and late infection (n = 155) (Table 2). There was a borderline significant difference in the prevalence of fatigue across the 3 time points (P = .011). There was no significant difference in the prevalence of the remaining symptoms included in our screening survey across these time points.
Table 2.
Examination of Symptom Frequency and Percentage Across Early, Mid, and Late Infection for Those With Confirmed Positive SARS-CoV-2 Infection as a Means of Assessing Temporality of Symptoms
| Symptoms in Positive Cases, No. (%) | |||||
|---|---|---|---|---|---|
| Early Infection (n = 77) | Midinfection (n = 84) | Late Infection (n = 155) | P | All (n = 316), No. (%) | |
| Cough | 63 (82) | 62 (74) | 134 (86) | .06 | 259 (82) |
| Fever | 51 (66) | 58 (69) | 103 (66) | .93 | 212 (67) |
| Dyspnea | 31 (40) | 42 (50) | 71 (46) | .47 | 144 (46) |
| Headache | 46 (60) | 59 (70) | 86 (56) | .08 | 191 (60) |
| Diarrhea | 27 (35) | 27 (32) | 67 (43) | .20 | 121 (38) |
| Rhinorrhea | 13 (17) | 14 (17) | 25 (16) | <.99 | 52 (16) |
| Sore throat | 20 (26) | 31 (37) | 64 (42) | .81 | 115 (36) |
| Fatigue | 49 (64) | 62 (74) | 84 (54) | .01* | 195 (62) |
| Myalgia | 44 (57) | 50 (60) | 71 (46) | .08 | 165 (52) |
| Sinus congestion | 21 (27) | 25 (30) | 37 (24) | .59 | 83 (26) |
| Anosmia | 19 (25) | 27 (32) | 56 (36) | .21 | 102 (32) |
Significance was measured using the Fisher exact test.
*Statistically significant difference between mid and late infection time points.
The median duration of symptoms at the time of screening (IQR) was slightly longer in the confirmed group, at 5 (3–11) days, compared with 2 (1–5) days for probable infection and 3 (1–7) days for possible infection. In early infection, those with confirmed infection were more likely than those with unconfirmed infection to report fever, headache, fatigue, myalgia, and diarrhea (all P < .01). At later time points, there were fewer differences between these groups (Supplementary Tables 1–3).
DISCUSSION
Using symptoms to guide diagnostic decision-making in suspect COVID-19 infection may be problematic, particularly when symptoms have been present for several days. Cough, fever, and dyspnea have been previously described as the 3 most common symptoms of SARS-CoV-2 infection, promoted in guidelines as potential screening symptoms when deciding to test or not test a patient [11]. The data presented here demonstrate some pitfalls in using these symptoms, without further context, to guide diagnostic decisions. In our cohort, those with confirmed infection had similar reported symptoms in early, mid, and late infection, without a clear evolution of symptoms over time. Those with confirmed and probable infection had similar symptom profiles when viewed at similar time points, with only fever being consistently more common in those with confirmed infection. In contrast, those with possible infection had a markedly different symptom profile in early infection compared with those with confirmed infection (Supplementary Table 1). As time from initial symptoms lengthened, the differences in the symptom profiles of confirmed and possible infection decreased (Supplementary Tables 2 and 3). Additionally, when viewed without temporal context, it became difficult to separate probable and possible infections (Table 1).
These data underscore the protean ways in which COVID-19 can manifest and the need for wide-scale testing. Prioritizing testing of those with compatible symptoms of short duration and a confirmed COVID-19 case contact would be highest yield based on a similar presentation as those with confirmed infection. Compatible symptoms in the absence of a confirmed case contact or of long duration, however, are less helpful when trying to determine the likelihood of SARS-CoV-2 infection.
As with any study, there are limitations to our analysis. First, our data rely on patient-reported symptoms without further clarification of the quality of those symptoms, such as diarrhea without specification of frequency. Second, we are exclusively studying outpatient disease; thus our findings are not generalizable to those with more severe disease who require hospitalization. Third, the lack of prompt (or available) testing nationwide significantly affected the composition of confirmed, probable, and possible cases. Often, the person screening for our study or their contact was simply unable to have testing performed. Among the 460 symptomatic health care workers, only 220 (48%) received test results. Further, ill contacts of study subjects were frequently unable to undergo testing. Additionally, it is possible that more stringent criteria were imposed on study subjects when seeking testing, thus biasing the symptoms reported within the confirmed case group.
In summary, when making diagnostic decisions for those with potential SARS-CoV-2 infection who present with mild disease, clinicians should consider that COVID-19 can present in a variety of ways. In areas where SARS-CoV-2 testing remains limited, clinicians can use the information we present to guide clinical decision-making and improve allocation of limited resources.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
This work was supported by Jan and David Baszucki, Steve Kirsch, the Alliance of Minnesota Chinese Organizations, the Minnesota Chinese Chamber of Commerce, and the University of Minnesota. Personnel were supported through the Doris Duke Charitable Foundation through a grant supporting the Doris Duke International Clinical Research Fellows Program at the University of Minnesota. Katelyn Pastick and Elizabeth Okafor are Doris Duke International Clinical Research Fellows. Sarah Lofgren is supported by the National Institute of Mental Health (K23MH121220). Caleb Skipper is supported by the Fogarty International Center (D43TW009345). Drs. Radha Rajasingham and Matthew Pullen are supported by the National Institute of Allergy and Infectious Disease (K23AI138851, T32AI055433).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Guan WJ, Ni ZY, Hu Y, et al. . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382(18):1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang X, Fang J, Zhu Y, et al. . Clinical characteristics of non-critically ill patients with novel coronavirus infection (COVID-19) in a Fangcang Hospital. Clin Microbiol Infect. 2020. doi: 10.1016/j.cmi.2020.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19). 2020. Available at: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf. Accessed 1 July 2020. [Google Scholar]
- 4. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. . Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med 2020; 382:2012–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gane SB, Kelly C, Hopkins C. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology 2020; 58:299–301. [DOI] [PubMed] [Google Scholar]
- 6. Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020. doi: 10.1148/radiol.2020201187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Filatov A, Sharma P, Hindi F, Espinosa P. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus 2020; 12:e7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spinato G, Fabbris C, Polesel J, et al. . Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA 2020; 323:2089–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. CDC COVID-19 Response Team. Characteristics of health care personnel with COVID-19 - United States, February 12-April 9, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:477–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19) - discontinuing transmission-based precautions Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-hospitalized-patients.html. Accessed 1 June 2020.
- 11. Centers for Disease Control and Prevention. Evaluating and testing persons for coronavirus disease 2019 (COVID-19) 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html: National Center of Immunization and Respiratory Diseases. Accessed 11 April 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.