Summary
Background
Duration of persistence of SARS-CoV-2 in the upper respiratory tract of infected individuals has important clinical and epidemiological implications.
Aim
We aimed to establish the duration and risk factors for persistence of SARS-CoV-2 in the upper respiratory tract of asymptomatic infected individuals.
Methods
Data of repeat rRT-PCR (real-time reverse transcription-polymerase chain reaction) test done for SARS-CoV-2 infected individuals at our institute at Jodhpur, India were analysed from 19 March to 21 May 2020. Duration of virus persistence was estimated with parametric regression models based on weibull, log-normal, log-logistic, gamma and generalized gamma distributions. Factors associated with prolonged viral persistence were analysed with the best-fitting model.
Results
Fifty-one SARS-CoV-2 infected individuals with repeat rRT-PCR test were identified with 44 asymptomatics. The asymptomatic individuals had median virus persistence duration of 8.87 days (95% CI: 7.65–10.27) and 95 percentile duration of 20.70 days (95% CI: 16.08–28.20). The overall median virus persistence including both symptomatic and asymptomatic individuals was found to be 9.18 days (95% CI: 8.04–10.48). Around one-fourth asymptomatics (10/44) demonstrated SARS-CoV-2 persistence beyond 2 weeks. Age <60 years and local transmission were found to be significantly associated with longer virus persistence among asymptomatic individuals on univariate regression but not in multivariate analysis.
Conclusion
Recommended home isolation duration for SARS-CoV-2 infected individuals in India should be extended from 17 days to at least 3 weeks. Prolonged persistence of SARS-CoV-2 in a considerable proportion of asymptomatic individuals merits attention with regard to ensuring universal infection prevention precautions irrespective of symptomatic status.
Introduction
As on 21 June 2020, COVID-19 has resulted in 8.7 million cases and 0.46 million deaths.1 India has become the fourth most affected country worldwide with around 0.41 million confirmed COVID-19 cases.1 The persistence of SARS-CoV-2 in body fluids has important clinical and epidemiological implications. A negative rRT-PCR (real-time reverse transcription-polymerase chain reaction) result had been considered as a surrogate marker of non-infectiousness of the individual. Consequently, it had been required for discharge of individuals undergoing treatment or hospital isolation in India until 8 May 2020, when testing prior to discharge had been discontinued under the revised strategy.2,3 Also, the recommended duration of home isolation for both health workers and general public is being guided by expectation of viral negativity at the end of the isolation period.3
Materials and methods
Both oropharyngeal and nasopharyngeal swab specimens were taken from the individuals meeting suspect case definition for COVID-19 at our institute in Jodhpur, India.4 Nucleic acid extraction and rRT-PCR for SARS-CoV-2 was done as per protocol approved by National Institute of Virology, Indian Council of Medical Research.5 From among those found positive for SARS-CoV-2, duration of virus persistence was considered from the symptom onset date till the first negative rRT-PCR result. For asymptomatic individuals, the date of collection of first positive sample was taken instead of symptom onset. R software version 4.0.0 (with Survival and Flexsurv packages) was used for analysis of virus persistence duration with weibull, log-normal, log-logistic, gamma and generalized gamma models.6 The duration estimates were taken from the best-fitting model based on minimum Akaike information criterion (AIC) value. Standard maximum likelihood approach was used to obtain the best model fit to actual data.
The SARS-CoV-2 infected individuals from Jodhpur district, India were classified as having local transmission. Individuals who had been evacuated from Iran in March 2020 and had been quarantined were classified as having transmission from abroad. Univariate and multivariate parametric regression was conducted with the best-fitting distribution for asymptomatic individuals with age, gender and type of transmission as co-variates. Interval and right censoring of duration of virus persistence were accounted for in the regression analysis.
Results
We analysed data of 9760 rRT-PCR tests conducted at our institute from 19 March to 21 May 2020, out of which 425 positive test results were obtained. Fifty-one SARS-COV-2 infected individuals had undergone repeat rRT-PCR testing after first positive test, out of which 44 were asymptomatic and 7 were symptomatic.
Around 80% (35/44) of the asymptomatic individuals were males (Table 1). Nearly one-fourth asymptomatic individuals (10/44) had virus persistence longer than 14 days. Maximum duration of shedding among them was 25 days (Supplementary file S1). The 10 asymptomatic individuals demonstrating local transmission were significantly younger than the 34 individuals who had transmission from abroad (P = 0.020 on unpaired t-test). The log-logistic model was found to be the best-fitting for estimating the duration of virus persistence (Table 2).
Table 1.
Baseline characteristics of the 44 asymptomatic SARS-CoV-2 infected individuals studied for the duration of virus persistence in upper respiratory tract
| Characteristics | Local transmission | Transmission from abroad | Total |
|---|---|---|---|
| N = 10 | N = 34 | N = 44 | |
| Age in years, mean (SD) | 39.3 (23.4) | 61.5 (10.3) | 56.4 (17.1) |
| Gender, n (%) | |||
| Male | 8 (80.0) | 27 (79.4) | 35 (79.5) |
| Female | 2 (20.0) | 7 (20.6) | 9 (20.5) |
SD, standard deviation.
Table 2.
Fit of parametric survival models for estimation of duration of virus persistence in upper respiratory tract of asymptomatic SARS-CoV-2 infected individuals (n = 44)
| Type of distribution used in model | Minus 2 log likelihood value | No. of model parameters (k) | AIC value (−2 log likelihood +2k) |
|---|---|---|---|
| Weibull | 250.15 | 2 | 254.15 |
| Log-normal | 244.22 | 2 | 248.22 |
| Log-logistic | 243.39 | 2 | 247.39 |
| Gamma | 245.90 | 2 | 249.90 |
| Generalized gamma | 244.22 | 3 | 250.22 |
AIC, Akaike information criterion.
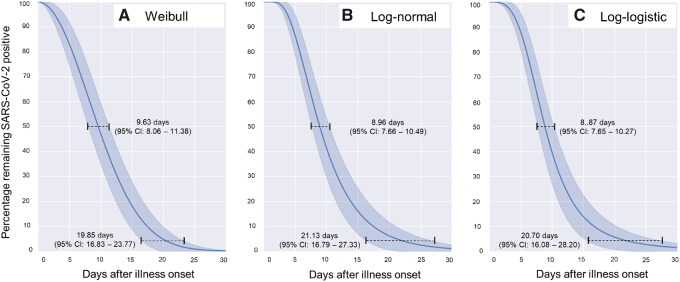
The median and 95 percentile durations of SARS-CoV-2 persistence in upper respiratory tract of asymptomatic individuals was found to be 8.87 days (95% CI: 7.65–10.27) and 20.70 days (95% CI: 16.08–28.20), respectively (Figure 1). While taking both the asymptomatic and symptomatic individuals together, the overall median and 95 percentile durations were found to be 9.18 days (95% CI: 8.04–10.48) and 20.92 days (95% CI: 16.61–27.55), respectively. The virus persistence duration of 10.98 days (95% CI: 8.38–14.44) for symptomatic individuals was not found to be significantly longer than that of asymptomatic individuals (P = 0.222).
Figure 1.
Duration of persistence of SARS-CoV-2 in the upper respiratory tract of asymptomatic individuals as fitted to (A) Weibull, (B) Log-normal and (C) Log-logistic distributions (n = 44)
Regression analysis for asymptomatic individuals was conducted with the best-fitting Log-logistic model. Upon univariate analysis, age <60 years and local transmission were found to be significantly associated with longer persistence of SARS-CoV-2 in the upper respiratory tract (Table 3). None of the co-variates were found to be significantly associated with prolonged virus persistence when age, gender and type of transmission were taken together in the multivariate model (Table 3).
Table 3.
Log-logistic regression for plausible risk factors for prolonged duration of virus persistence in the upper respiratory tract of asymptomatic SARS-CoV-2 infected individuals (n = 44)
| Co-variates | Exp (β) value (95 % CI) | P-value (Wald test) | AIC value for model |
|---|---|---|---|
| Univariate regression (one variable at a time) | |||
| Age ≥60 years vs. <60 years | 0.69 (0.52–0.92) | 0.011 | 243.01 |
| Male vs. female gender | 0.71 (0.48–1.06) | 0.097 | 246.80 |
| Local transmission vs. transmission from abroad | 1.48 (1.04–2.10) | 0.029 | 244.56 |
| Multivariate regression (all three variables together) | |||
| Age | 0.77 (0.57–1.03) | 0.076 | 242.06 |
| Gender | 0.72 (0.51–1.00) | 0.051 | |
| Type of transmission | 1.28 (0.90–1.81) | 0.171 | |
AIC, Akaike information criterion.
Discussion
Prior to the introduction of home isolation strategy on 10 May 2020,7 India had adopted the policy of universal health-facility-based isolation of all SARS-CoV-2 infected individuals irrespective of symptomatic status. This provided us with a unique opportunity to study the virus persistence among the asymptomatic individuals admitted at our institute.
Longer virus persistence has been demonstrated in severe illness as compared to mild COVID-19.8 However, we did not find a statistically significant difference in virus persistence based on symptomatic status. Our finding of median duration of virus persistence of 9.18 days among all infected individuals was comparable to the estimate of 10 days as per laboratory surveillance data from India3 and 10–12 days reported from China.9,10 It was, however, lower than the 15 days reported from South Korea.11 Considering only asymptomatic individuals, the median virus persistence of 8.87 days reported by us nearly matched that of 9 days reported from the Diamond Princess ship, indicating similar transmission dynamics.12 The present study adds to the evidence of prolonged persistence of SARS-CoV-2 in upper respiratory tract in a considerable proportion of infected individuals.8,9,11,13
Association of local transmission with longer virus persistence on univariate analysis could have been confounded by the locally infected individuals being significantly younger than those evacuated from abroad. Possible association of younger age and variation in transmission pattern with prolonged virus persistence merits further exploration.
Although virus persistence measured through positive rRT-PCR of swab samples does not necessarily mean that the individual is infective, greater viral load indicated by lower cyclic threshold values correlates with cell culture infectivity.13 Therefore, the finding of prolonged virus persistence even among asymptomatic individuals has important public health implications when large number of individuals with varying viral loads are considered in the community.
A study from the USA had recommended that people infected with SARS-CoV-2 should cease infection prevention precautions and should return to work only after 33 days of symptom onset or a negative test result.14 Furthermore, the finding of prolonged and intermittent viral shedding also helps explain why earlier many ‘recovered’ patients had re-tested positive after being discharged from hospital.15,16 These findings reset the expectation of viral shedding to a longer duration, especially for asymptomatic SARS-CoV-2 infected individuals.
Our estimates could be generalizable to settings wherein large proportion of infected individuals remain asymptomatic. We had the limitation that we could not separately estimate the duration of virus persistence in nasopharynx and oropharynx as both the swap tips were inserted together in the viral transport medium to increase the possibility of SARS-CoV-2 detection.
Conclusions
Based on our findings, a prolongation of current home isolation guidance from 17 days7 to at least 21 days may be recommended in India. During the course of the COVID-19 pandemic, the emerging evidence has supported the role of asymptomatic individuals in transmission and prolonged viral shedding in considerable proportion of individuals.17 This has prompted the WHO guidance to the general public on wearing masks in areas with suspected transmission, high population density or where physical distancing is not feasible.18 The evidence of prolonged virus persistence among asymptomatic individuals in the present study further emphasizes the need for universal infection prevention precautions irrespective of symptomatic status.
Supplementary material
Supplementary material is available at QJMED online.
Authors’ contributions
M.K.Garg conceived the idea of the study. S.S. extracted the data and S.S. and R.K. conducted the analysis. S.S. wrote the draft manuscript with further inputs from M.K.Garg, P.B. and M.K.Gupta. P.B. coordinated the data collection and V.L.N. coordinated the testing of samples. S.M. provided overall supervision of the testing, isolation, clinical care and research related to COVID-19 at AIIMS, Jodhpur, India. All authors approved the final manuscript.
Ethical approval
The study has been approved by the Institutional Ethics Committee of All India Institute of Medical Sciences (AIIMS), Jodhpur, India.
Supplementary Material
Acknowledgements
We acknowledge the help of personnel involved in testing and clinical care of SARS-CoV-2 infected individuals at All India Institute of Medical Sciences (AIIMS), Jodhpur, India.
Funding
Department of Health Research/ Indian Council of Medical Research, Government of India is acknowledged for funding the establishment and operation of Regional -Viral Diagnosis & Research Laboratory (VDRL) at AIIMS Jodhpur, which was uitlized for laboratory testing for COVID-19. The authors declare that no other funding was recieved from any source for the study and preparation of this article.
Conflict of interest. The authors declare that there are no conflicts interests for publication of this article. The views expressed in this article are those of the authors alone and do not necessarily represent the views of their organizations.
References
- 1.WHO. Situation Update no. 153 for COVID-19–21 June 2020 World Health Organization, Geneva, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200621-covid-19-sitrep-153.pdf?sfvrsn=c896464d_2 (22 June 2020, date last accessed).
- 2.MoHFW. Revised Discharge Policy for COVID-19 (dated 8 May 2020) Ministry of Health and Family Welfare, Government of India, New Delhi, 2020. https://www.mohfw.gov.in/pdf/ReviseddischargePolicyforCOVID19.pdf (16 June 2020, date last accessed).
- 3.MoHFW. Frequently Asked Questions (FAQs) on Revised Discharge Policy for COVID-19 (dated 8 May 2020) Ministry of Health and Family Welfare, Government of India, New Delhi, 2020. https://www.mohfw.gov.in/pdf/FAQsonRevisedDischargePolicy.pdf (16 June 2020, date last accessed).
- 4.ICMR. Revised Strategy of COVID-19 Testing in India—Version 3 (dated 20 March 2020) Indian Council of Medical Research, New Delhi, 2020. https://www.mohfw.gov.in/pdf/ICMRrevisedtestingstrategyforCOVID.pdf (16 June 2020, date last accessed).
- 5.ICMR. Standard Operating Procedure for Detection of 2019-nCoV in Suspected Human Cases by rRT-PCR Indian Council of Medical Research - National Institute of Virology, Pune, 2020. https://www.icmr.gov.in/pdf/covid/labs/2_SOP_for_Confirmatory_Assay_for_2019_nCoV.pdf (16 June 2020, date last accessed).
- 6.R Core Team. R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing, Vienna, Austria, 2020. https://www.R-project.org (18 June 2020, date last accessed)
- 7.MoHFW. Revised Guidelines for Home Isolation of Very Mild/Pre-symptomatic COVID-19 Cases (dated 10 May 2020) Ministry of Health and Family Welfare, Government of India, New Delhi, 2020. https://www.mohfw.gov.in/pdf/RevisedguidelinesforHomeIsolationofverymildpresymptomaticCOVID19cases10May2020.pdf (16 June 2020, date last accessed).
- 8. Sun J, Xiao J, Sun R, Tang X, Liang C, Lin H, et al. Prolonged persistence of SARS-CoV-2 RNA in body fluids. Emerg Infect Dis 2020; 26:doi: 10.3201/eid2608.201097 (8 May 2020, published online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020; 382:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lan L, Xu D, Ye G, Xia C, Wang S, Li Y, et al. Positive RT-PCR test results in patients recovered from COVID-19. JAMA 2020; 323:1502–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sakurai A, Sasaki T, Kato S, Hayashi M, Tsuzuki S-I, Ishihara T, et al. Natural history of asymptomatic SARS-CoV-2 infection. N Engl J Med 2020; doi:10.1056/NEJMc2013020 (12 June 2020, published online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bullard J, Dust K, Funk D, Strong JE, Alexander D, Garnett L, et al. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin Infect Dis 2020; doi:10.1093/cid/ciaa638 (22 May 2020, published online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gombar S, Chang M, Hogan CA, Zehnder J, Boyd S, Pinsky BA, et al. Persistent detection of SARS-CoV-2 RNA in patients and healthcare workers with COVID-19. J Clin Virol 2020; 129:104477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kang H, Wang Y, Tong Z, Liu X.. Re-test positive for SARS-CoV-2 RNA of “recovered” patients with COVID-19: persistence, sampling issues, or re-infection? J Med Virol 2020; doi : 10.1002/jmv.26114 (3 June 2020, published online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hoang VT, Dao TL, Gautret P.. Recurrence of positive SARS-CoV-2 in patients recovered from COVID-19. J Med Virol 2020; doi:10.1002/jmv.26056 (25 May 2020, published online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao HJ, Lu XX, Deng YB, Tang YJ, Lu JC.. COVID-19: asymptomatic carrier transmission is an underestimated problem. Epidemiol Infect 2020; 148: e116. doi: 10.1017/S0950268820001235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO. Advice on the Use of Masks in the Context of COVID-19—interim Guidance (5 June 2020) World Health Organization, Geneva, 2020. https://apps.who.int/iris/rest/bitstreams/1279750/retrieve (9 June 2020, date last accessed).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.