Rheumatology key message
Anakinra as a sole treatment may be useful in the immune-mediated hyperinflammatory phase of COVID-19.
Dear Editor, Coronavirus disease 2019 (COVID-19) is a global pandemic caused by the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2). COVID-19 is characterized by a wide range of clinical manifestations that may become severe by progressing towards acute respiratory distress syndrome and death [1]. After an initial viral phase with fever, coughing and mild upper respiratory tract symptoms, some COVID-19 patients may experience a hyperinflammatory phase manifested with biological changes of a cytokine storm syndrome (with increased levels of ferritin and CRP), which are similar to those observed after chimeric antigen receptor (CAR) T-cell therapy and in haemophagocytic lymphohistiocytosis (HLH), as well as in macrophage activation syndrome (MAS), a secondary HLH form, classically associated with autoimmune or inflammatory conditions [2]. Clinically, this hyperinflammatory syndrome is invariably associated with a rapid respiratory deterioration due to a bilateral inflammatory pneumonia [1, 3, 4].
Based on this immunological rationale, glucocorticoids [5] and tocilizumab (an anti-IL-6 agent) [4] have been used with benefit in a retrospective case series and a prospective open-label single-arm study, respectively. IL-1 may also play a determinant role in the cytokine storm syndrome of COVID-19 by activating the inflammasome after the interaction of the SARS-CoV-2 with specific Toll-like receptors, leading to a final uncontrolled production of active mature IL-1β, a mediator known to be involved in fever and inflammatory and fibrotic pulmonary changes [6]. Although intravenous IL-6 and IL-1 blockade strategies are being prospectively studied in clinical trials, no cases of COVID-19 managed with subcutaneous IL-1 agents have been communicated yet. Herein, we report the first case of severe COVID-19 pneumonia successfully treated with subcutaneous anakinra (a recombinant IL-1 receptor antagonist or IL-1Ra).
A non-smoker 47-year-old man with no remarkable medical history except for well-controlled and untreated asthma, and probable intolerance (because of previous neuropsychiatric effects) to glucocorticoids, was admitted with a seven-day history of fever and dry cough, together with additional shortness of breath during the last three days. Physical examination revealed a normal blood pressure, fever (38.3°C), tachypnoea (25 breaths per min) with baseline oxygen saturation of 92%. A polymerase chain reaction of nasopharyngeal swab confirmed SARS-CoV-2 infection. A chest radiography on admission showed bilateral, patchy, hazy opacities in the middle and lower lung fields (Fig. 1A). Laboratory tests revealed lymphopenia (630 cells/µl), increased levels of acute phase reactants, including CRP (140 mg/l; normal range 0–5 mg/l), ferritin (807 ng/ml; normal range 20–300 ng/ml), IL-6 (12.5 pg/ml; normal value <1.8 pg/ml), serum lactate dehydrogenase (360 U/l; normal range 14–280 U/l), and triglycerides (374 mg/dl; normal range 25–200 mg/dl). A normal platelet count (303 000/µl) and elevated d-dimer level (1773 ng/ml; normal value <500 ng/ml) were also detected. Serologies for HIV and hepatitis A, B and C viruses were negative. On day 1, treatment was started with azithromycin, hydroxychloroquine, enoxaparin 40 mg/day and oxygen, with high-flow nasal cannula. On day 4, the respiratory condition deteriorated despite the increase of high-flow oxygen therapy. A computed tomography pulmonary angiography revealed bilateral diffuse lung opacities involving all lobes with scattered areas of ground-glass opacity with no signs of pulmonary embolism (Fig. 1B). Subsequently, anakinra was initiated at 100 mg every 6 h subcutaneously. No glucocorticoids were administered because of the potential neuropsychiatric intolerance experienced in the past. A rapid and progressive improvement of the fever and respiratory manifestations was observed, with a sustained clinical stabilization and normalization of the inflammatory parameters after 10 days. At this moment, anakinra was reduced to 100 mg every 8 h until completing a total duration of treatment of 14 days. Finally, on day 19, the patient was discharged with no need for oxygen supplementation. The chest X-ray at this time showed clear improvement of the pulmonary infiltrates (Fig. 1C). No adverse reactions or laboratory abnormalities attributed to anakinra were observed. The patient signed an informed consent to publish his medical information.
Fig. 1.
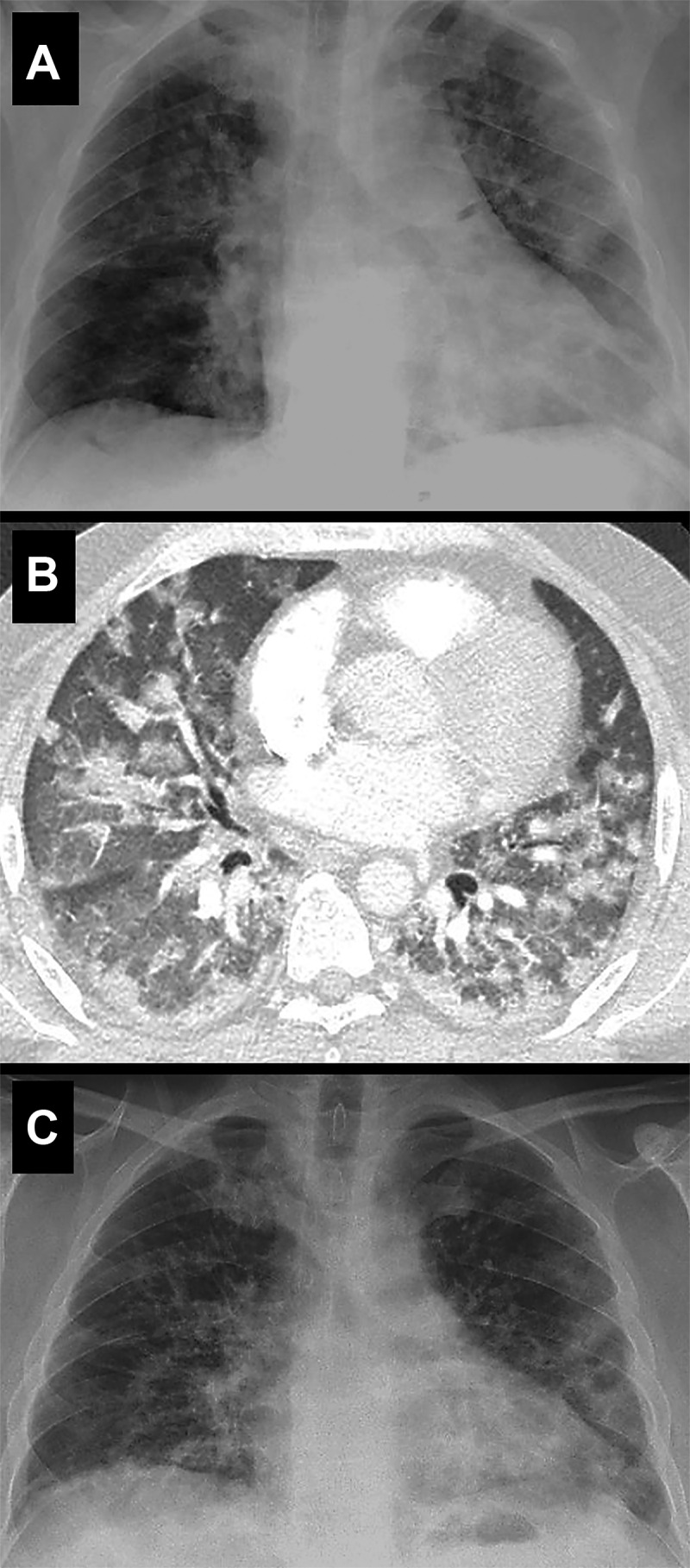
Pulmonary radiological findings
(A) A frontal chest X-ray (day 1) showed bilateral, patchy, hazy opacities in both lungs. (B) At the time of disease worsening (day 4), a computerized tomography revealed bilateral diffuse lung opacities involving all lobes with scattered areas of ground-glass opacity with a predominant central involvement. (C) A frontal chest X-ray obtained 12 days after starting anakinra (day 16) confirmed the imaging improvement with persistence but reduced bilateral patchy opacities in the lungs.
Immunomodulatory or immunosuppressive strategies targeting different components of the cytokine storm occurring in CAR-T cell therapy and HLH/MAS associated to autoimmune diseases have shown to control these systemic inflammatory situations [3, 7, 8]. Similar therapeutic approaches have been also tested in patients with COVID-19 by using glucocorticoids [5] and tocilizumab [4], with initial positive results in non-randomized controlled studies. With regard to anakinra, its subcutaneous administration has also been useful in patients with autoinflammatory diseases and MAS associated to autoimmune diseases [7, 8]. In patients with sepsis [9] and those with MAS refractory to subcutaneous anakinra [10], the administration of very high doses of anakinra (from 400 mg to >3000 mg per day) in intravenous perfusion during 3–21 days, respectively, has shown good results with no major safety concerns [9, 10].
Anakinra is labelled to be administered subcutaneously at a dose of 100 mg/day in patients with autoinflammatory diseases [7]. Its intravenous administration has been used in septic situations based on the peripheral vasoconstriction and hypoperfusion accompanying these processes, which may reduce subcutaneous drug absorption [9]. Because COVID-19 is not characterized by hypotension and compensatory peripheral vasoconstriction, the use of a subcutaneous way is thought to provide a good biodisponibility (usually >90%) and, consequently, a full effect. The increased dose of anakinra used in our patient (400 mg/day) is being similarly used in an ongoing open-label trial with intravenous anakinra in severe COVID-19 (NCT04324021).
In conclusion, to the best of our knowledge we report the first case of severe COVID-19-associated pneumonia successfully treated with subcutaneous anakinra alone, with no safety problems. Although the current management of moderate to severe COVID-19 during the immune/inflammatory phase with glucocorticoids and/or targeted cytokine therapies, mostly IL-6 and IL-1 blockers, is not supported by any robust scientific evidence yet, we look forward to unveiling the real usefulness of these drugs and the correct time of their administration once the results of different clinical trials are published anytime soon.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Huang C, Wang Y, Li X. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crayne CB, Albeituni S, Nichols KE, Cron RQ.. The immunology of macrophage activation syndrome. Front Immunol 2019;10:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mehta P, McAuley DF, Brown M. et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sciascia S, Aprà F, Baffa A. et al. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in severe patients with COVID-19. Clin Exp Rheumatol 2020; PMID: 32359035. [Epub ahead of print]. [PubMed] [Google Scholar]
- 5. Wu C, Chen X, Cai Y. et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;:e200994. Doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conti P, Ronconi G, Caraffa A. et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents 2020;34: 1;doi:10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 7. Soriano A, Soriano M, Espinosa G. et al. Current therapeutic options for the main monogenic autoinflammatory diseases and PFAPA syndrome: evidence-based approach and proposal of a practical guide. Front Immunol 2020;11:865.doi: 10.3389/fimmu.2020.00865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watanabe E, Sugawara H, Yamashita T. et al. Successful tocilizumab therapy for macrophage activation syndrome associated with adult-onset Still’s disease: a case-based review. Case Rep Med 2016;2016:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shakoory B, Carcillo JA, Chatham WW. et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med 2016;44:275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Monteagudo LA, Boothby A, Gertner E.. Continuous intravenous anakinra infusion to calm the cytokine storm in macrophage activation syndrome. ACR Open Rheumatol 2020;2:276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]


