Abstract
Context
While individuals with diabetes appear to be at similar risk for SARS-CoV-2 infection to those without diabetes, they are more likely to suffer severe consequences, including death. Diabetic ketoacidosis (DKA) is a common and potentially lethal acute complication of diabetes arising from a relative insulin deficiency, which occurs more often in those with type 1 diabetes and in the setting of moderate to severe illness. Early reports indicate that among patients with pre-existing diabetes, DKA may be a common complication of severe COVID-19 and a poor prognostic sign.
Case Description
This clinical perspective explores the key elements of caring for individuals with DKA during the COVID-19 pandemic through 2 cases. Topics addressed include diagnosis, triage, and the fundamental principles of treatment with a focus on the importance of characterizing DKA severity and medical complexity to determine the best approach.
Conclusions
As discussed, some tenets of DKA management may require flexibility in the setting of COVID-19 due to important public health goals, such as preventing transmission to highest risk individuals, reducing healthcare worker exposure to infected patients, and preserving personal protective equipment. Evidence for alternative treatment strategies is explored, with special attention placed on treatment options that may be more relevant during the pandemic, including use of subcutaneous insulin therapy. Finally, DKA is often a preventable condition. We include evidence-based strategies and guidance designed to empower clinicians and patients to avoid this serious complication when possible.
Keywords: diabetes, pancreatic and gastrointestinal hormones, diabetic ketoacidosis
Case Presentations
Case 1
A 53-year-old woman with hypertension, hyperlipidemia, and type 2 diabetes (T2D) presented to an Emergency Department (ED) in Boston, Massachusetts, with shortness of breath and fever. Symptoms began 12 days prior when she noted fatigue and malaise. Due to concern for work-related exposure to COVID-19, she was seen by her primary care physician and tested negative. In the subsequent week she developed sore throat, diarrhea, fever, and progressive dyspnea. Repeat SARS-CoV-2 testing was now positive. She was prescribed azithromycin and hydroxychloroquine, but due to worsening dyspnea, fever, and anorexia sought further evaluation. Upon arrival to the ED, she appeared ill, tachypneic, and unable to speak in full sentences. Oxygen saturation was 78% on ambient air. On examination, she had decreased breath sounds bilaterally, acanthosis nigricans, and central adiposity, body mass index 33 kg/m2. The remainder of the physical examination was normal. Biochemical evaluation was notable for normal renal and hepatic function, glucose 151 mg/dL (8.3 mmol/L), bicarbonate 20 mmol/L, lactic acid 1.2 mmol/L, pH 7.41, elevated inflammatory markers (ferritin 440 µg/L, erythrocyte sedimentation rate 93 mm/hour, C-reactive protein 95.4 mg/dL, interleukin-6 (IL-6) 24.4 pg/mL [ref. <1.64]) and was confirmed SARS-CoV-2 positive. She had bibasilar consolidation concerning for COVID pneumonia on imaging. Shortly after admission her oxygen requirements increased (15 L) and was she was transferred to the intensive care unit (ICU) for hypoxemic respiratory failure and was subsequently intubated. She had a longstanding history of T2D initially treated with oral agents and transitioned to insulin 1 year ago with most recent hemoglobin A1c (HbA1c) of 7.5%. She had no known diabetes-related complications and had never been hospitalized for glycemic control. Prior to admission, her diabetes medications included metformin 1000 mg twice daily, glimepiride 4 mg daily, empagliflozin 10 mg daily, exenatide 2 mg weekly, and glargine 22 units nightly. She took all medications on the day of ED presentation. Diabetes medications were discontinued on admission and glycemic control was at goal with minimal correctional insulin and poor oral intake. In the next 24 hours she developed metabolic acidosis with elevated anion gap, glucose 192 mg/dL (10.6 mmol/L), bicarbonate 15 mmol/L, venous blood pH 7.24, and lactic acid 1.3 mmol/L. β-Hydroxybutyrate was elevated at 6.1 mmol/L. She was started on intravenous insulin (IVI) with dextrose support for treatment of diabetic ketoacidosis (DKA).
Case 2
A 45-year-old man with no prior medical history presented to the ED with fatigue and symptomatic hyperglycemia (polyuria, polydipsia). He was last seen by his primary care physician several years ago. He denies taking any medications, supplements, or substance use. Following what he describes as a “stomach bug” approximately 1 week prior, he complained of lethargy and “extreme thirst like he can’t get enough water.” Upon acute care presentation he was hypertensive (149/95 mmHg), afebrile, and with oxygen saturation of 83% on ambient air, which improved with 6 L of supplemental oxygen. On examination body mass index was 28 kg/m2 and his breathing was effortful, but was otherwise normal. Biochemical evaluation was notable for glucose of 599 mg/dL (33.2 mmol/L), bicarbonate 15 mmol/L, estimated glomerular filtration rate 38 mL/minutes, and an elevated anion gap. Urinanalysis showed 2+ ketones and 3+ glucose; β-hydroxybutyrate was >9.0 mmol/L. Venous pH was 7.18, effective serum osmolality 305 mOsm/kg, lactic acid 2.4 mmol/L, and HbA1c 12.6%. Serum and urine toxicology screens were negative. A test for SARS-CoV-2 was positive. He denied any prior history of impaired glucose intolerance but reported a strong family history of T2D and no known autoimmune disease. He was admitted for COVID-19 and treatment of new-onset diabetes with DKA and acute kidney injury (AKI).
Background
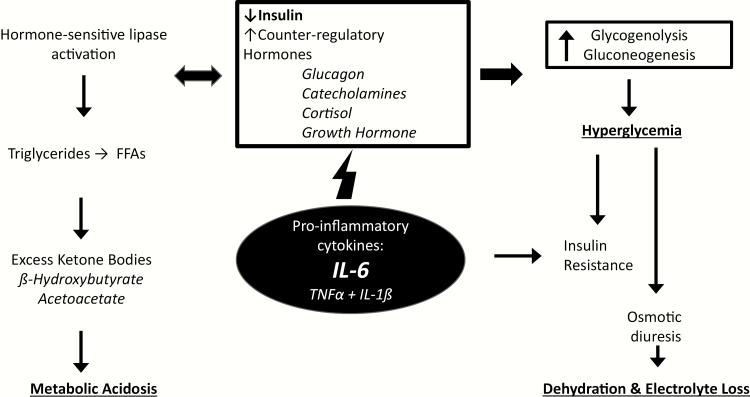
DKA is the most common hyperglycemic crisis, which also includes hyperosmolar hyperglycemia syndrome (HHS) and the overlap syndrome of hyperosmolar ketoacidosis (HK) (1). DKA occurs in the setting of relative or absolute insulin deficiency that leads to reduced glucose utilization and unchecked lipolysis, causing excessive ketone body formation and acidosis. DKA itself is an inflammatory state (2), but is often accompanied by an underlying severe illness (Fig. 1).
Figure 1.
Conceptual framework of the pathophysiology of diabetic ketoacidosis. In DKA, insulin insufficiency sets off a cascade of maladaptive physiologic responses including formation of excessive ketone bodies leading to metabolic acidosis, and hyperglycemia resulting in progressive hypovolemia and electrolyte loss. In the setting of inflammatory illness, cytokines and other factors drive this process. IL-6 levels have been shown to be elevated in both DKA and COVID-19, and may be an important prognostic factor. FFA, free fatty acids; TNFα, tumor necrosis factor α; IL-6, interleukin 6; IL-1β, interleukin 1β.
Initial studies from the 1970s and 1980s outlined the basic tenets of treatment, which are well described in hundreds of references and have remained largely unchanged in the past 20 years (3). While IVI has been the most studied approach to administering variable-dose insulin to the hospitalized patient with DKA, this poses a challenge in the setting of COVID-19 as it requires patient interactions every 1 to 2 hours. In this time when limiting these interactions is essential, particularly in areas where there are shortages of personal protective equipment (PPE), alternative approaches to frequent bedside care are being considered by hospital clinicians and leadership.
Along these lines, clinicians have reconsidered alternative methods for management of hyperglycemic crisis to achieve the triple goal of protecting patients with diabetes from SARS-CoV-2 exposure, reducing exposure to healthcare personnel during the care of COVID-19 patients, and limiting use of the ICU beds for non-COVID-19 care. Such methods include identifying patients who may be treated outside of the hospital or for a short period in the ED. In addition, the role of subcutaneous insulin for management of mild to moderate DKA has resurfaced as not only an evidenced-based alternative to IVI requiring less frequent glucose monitoring but also as a critical tool to employ during the pandemic to reduce the time required at the bedside managing insulin.
The purpose of this communication is to provide guidance for clinicians who care for patients at risk for DKA and patients who develop DKA in the setting of SARS-CoV-2 infection. The discussion also addresses the interest to protect both healthcare personnel and uninfected patients from unnecessary exposure (4). While most of the management strategies discussed will be based on randomized controlled clinical trials (RCT), many of these are small and not necessarily generalizable to the current COVID-19 clinical environment. Therefore, some modifications are suggested to published approaches in places where the authors have experience. It is emphasized that it is generally not recommended that hospitals make major changes to their current approach to managing DKA, as this may cause unintended consequences and result in the reverse outcome of requiring more ICU resources and time at the bedside.
Epidemiology of DKA: An Update
In both the United States and the United Kingdom, DKA hospitalizations have risen sharply in the last decade, despite a decrease in the early part of this century (5, 6). Fortunately, however, mortality from DKA has progressively declined over the last 3 decades and is most recently <0.5% in the United States. Traditionally, mortality has been reported to be highest at the extremes of age, although mortality rates in elderly people have also declined dramatically during the past 10 years (7). In type 1 diabetes (T1D), DKA is most often caused by missed insulin doses but death is rare with prompt treatment, whereas in type 2 diabetes (T2D) underlying severe illness is almost always the direct cause of both the DKA and ensuing death. Although DKA is more likely to occur in T1D, it is estimated that most DKA cases worldwide occur in T2D due to its higher prevalence. Probably as a result of increased diabetes screening and early recognition, DKA now occurs more frequently in persons with established diabetes rather than at the time of the initial diagnosis (7).
Since the year 2000, 2 new patient subtypes being hospitalized for DKA have been identified. The first was the “ketosis-prone diabetes” population. Described in the early 2000s after an increase in DKA cases among persons with obesity and T2D was reported (8), these patients had impaired insulin levels but lacked typical autoimmune markers of T1D, and their beta-cell function recovered quickly after treatment. The other group is what the American Diabetes Association (ADA) has described as “euglycemic DKA” (euDKA), which makes up about 10% of patients with DKA. As seen in Case 1, euDKA is characterized by metabolic acidosis and increased total body ketone concentration, but with glucose levels ≤250 mg/dL (9, 10). euDKA was first described in 1972, when Munro et al. reported 37 cases of patients with T1D who developed severe DKA with glucose <16.5 mmol/L (<300 mg/dL) (11), and has been described to occur most commonly in pregnant women. However, the introduction of the sodium–glucose cotransporter 2 (SGLT2) inhibitor medications in 2013 has identified another population at risk for euDKA. In December 2015, the Food and Drug Administration added a label to SGLT2 inhibitors warning that these medications might increase the risk for DKA (12). Since 2015, following the publication of several cardiovascular outcome trials demonstrating benefit and new formal indications for patients with cardiovascular disease, sales for these drugs have increased dramatically and expert guidance around this condition was published (13). There are several management considerations in the acute setting as discussed below and through Case 1.
On a positive note, DKA in general is still more common in younger individuals and it is thus reassuring that children and young adults appear to have lower rates of severe COVID-19 (14). Diabetes has not been identified as a risk factor in major reports of SARS-CoV-2 infections in children. Reports from pediatric endocrinologists in COVID-19 hotspots globally indicate that children, adolescents, and young adults (<25 years old) with diabetes have so far not shown a different disease pattern with the virus compared with children and younger people who do not have diabetes (15).
DKA and COVID
In the COVID-19 pandemic, it has been established that diabetes is a frequent comorbidity and is associated with increased severity of complications and mortality (16). DKA has been reported in COVID-19 (17, 18), as with other severe infections (19), in patients with T1D and patients with T2D. Following early studies from China reporting a wide prevalence of diabetes ranging between 7.4% and 19.5% 20-22), several studies reported higher prevalence among those requiring hospitalization and/or those who died. Data from Italy, for example, reported that 35.5% of patients who died had diabetes, a prevalence greater than 3 times that of the general population (23). In Chinese cohorts, ICU care was more often required for patients with diabetes (22.2% vs 5.9%, P = .009) (21), and acute respiratory distress syndrome (ARDS) was 2.34-fold higher (95% confidence interval [CI], 3.6-24.2%; P = .002) (24). As would be expected from the increased complications, mortality was also higher. One study from Wuhan of 191 patients showed an OR of 2.85 (95% CI, 1.25-6.05, P = .0062) in patients with diabetes compared with a nondiabetic population (25).
While a high severity of illness, organ failure, and mortality among adults with diabetes is being reported, what is less known is the metabolic complications specific to COVID-19. Viral infection and its association with diabetes is a familiar concept. For example, viruses have long been implicated in the development of T1D, such as cytomegalovirus, Epstein–Barr, mumps, rotavirus, rubella, and, in particular, enteroviruses and coxsackievirus (26). Hepatis C infection is a well-known risk factor for T2D that is also associated with beta-cell dysfunction. One small study compared the pancreata of hepatitis C virus (HCV)-positive and -negative donors and found the beta-cell of the HCV-positive donors had presence of the virus in the cytoplasm that was accompanied by morphological changes in mitochondria and reduced in vitro glucose-stimulated insulin secretion (27). In regard to coronaviruses, it has been shown that SARS-CoV-1 binds to the ACE2 receptor in the pancreatic islets, and it is postulated that this could cause damage and acute diabetes (28). While there have not been similar published findings related to the new SARS-CoV-2 virus, this theoretical pathophysiology could lead to insulinopenia and increased risk of DKA, especially for those with established T2D. However, at the time of publication, data on SARS-CoV-2 are limited and this mechanism remains speculative.
There are insufficient data to determine if DKA is more prevalent in COVID-19 and if SARS-CoV-2 poses an increased risk over other severe infectious diseases. To date, only 1 study has described the prevalence of acidosis and ketoacidosis in 658 hospitalized patients with confirmed COVID-19 (29). Of the cohort, 42 (6.4%) presented with positive urine or serum ketones, and, of these, 3 (7%) patients met the ADA criteria for DKA. Those with ketosis were about twice as likely to have diabetes at baseline, and the 3 individuals who developed DKA had underlying diabetes (1 with T1D, 2 with T2D). Also noted was that patients with ketosis itself with or without acidosis were younger (median age 47 vs 58 years, P = .003) and had had higher rates of ARDS (28.6% vs 13.5%, P = .007), acute liver injury (14.3% vs 5.4%, P = .042), digestive disorder (31.0% vs 12.0%, P < .001), required mechanical ventilation (21.4% vs 6.7%, P = .002), and had longer length of hospital stay (19.0 [12.8–39.0] days vs 16.0 [10.0–24.0] days, P < .001). Ketosis was also associated with higher mortality (21.4% vs 8.9%, P = .017). Larger cohorts are clearly needed to understand the true incidence and nature of DKA in COVID-19.
One likely important finding among COVID-19 patients is that severe disease is accompanied by high levels of inflammatory markers, which are also elevated in the setting of DKA independent of accompanying illness (Fig. 1) (2, 25). IL-6 in particular has been highlighted as likely playing a role in a maladaptive immune response to the SARS-CoV-2 virus and has been proposed as a possible treatment target (30). IL-6 has also been found to be elevated in DKA and is thought to be mostly a driver of ketosis rather than a result, but this is less clear (2). Whether the inflammatory cascades engaged in DKA and severe COVID-19 are synergistic in leading to worse clinical outcomes remains to be seen.
Considerations in the General Management of DKA
As noted, the underlying mechanism of DKA is insulin deficiency resulting in decreased glucose utilization by peripheral tissues, leading to increased counter-regulatory hormone levels, increased hepatic glucose production, and excess free fatty acid oxidation to ketoacids. The key elements of DKA treatment have been well established, and several professional societies, international organizations, and experts have maintained published guidance on management (10, 31). The main tenets of DKA management have not changed in decades and include the triad of fluid resuscitation, potassium repletion, and insulin replacement. Patients with hyperglycemic crisis present with profound dehydration and fluids are one of the most crucial aspects of treatment. With the goal of expanding intravascular volume and restoring perfusion, isotonic saline (0.9% NaCl) is often the preferred solution for fluid resuscitation. The subsequent choice of fluids depends on sodium and glucose concentrations. In the setting of acidosis and insulin deficiency, potassium shifts into the extracellular space. With insulin therapy and fluid administration potassium will shift intracellularly and close monitoring and potassium repletion is needed. Given lack of evidence demonstrating benefit in mortality or time to resolution of ketoacidosis (32), the routine use of bicarbonate therapy in DKA is not recommended and administration is generally reserved for patients with life-threatening acidosis (pH < 6.9).
Historically and at present, the most common hospital setting utilized to administer DKA care has been the ICU. In the uncomplicated patient, this is largely due to the frequent glucose monitoring required in individuals receiving IVI where insulin adjustments are required frequently. In the case of DKA, ketosis, acidosis, and elevated fatty acids all contribute to insulin resistance (31, 33), as does hyperglycemia itself (34), and as these resolve with simultaneous therapies (fluids and insulin), hypoglycemia risk is extremely high. It is well established that the major pitfalls of DKA treatment include first inadequate potassium supplementation and, second, failure to prevent hypoglycemia (35). A close third on this list of pitfalls is the recurrence of ketoacidosis in the case of ineffective transitioning of patients from IVI to subcutaneous insulin therapy. While there are numerous publications on how to avoid poor transitions, perhaps the most innovative and practical one tested the impact of administering long-acting subcutaneous insulin, for example, glargine, in the early phases of DKA treatment, and during IVI, in order to prevent rebound ketoacidosis and/or marked hyperglycemia (36).
As noted, DKA due to the use of SLGT2 inhibitors has led to DKA in the absence of marked hyperglycemia reported in hospitalized patients (13, 37), while in practice it remains relatively uncommon. Interestingly, euDKA was rarely reported in SGLT2i clinical trials (38). In a recent retrospective cohort study in Australia, those using SGLT2i had an odds ratio of 1.48 for DKA when compared with nonusers, and 41% of these patients had a peak glucose <13.8 mmol/L (<250 mg/dL), compared with 0.8% of nonusers (39). Precipitating factors include acute illness with poor oral intake, vomiting, fasting, and reductions in insulin doses related to improved glycemic control (or missing doses during illness). While several potential mechanisms have been proposed, it is likely a combination of factors with volume depletion and decreased glucose availability at the tissue level playing key roles. Management of euDKA includes the same triad approach as classical DKA, but with the clear distinction that dextrose containing fluids are required as an initial step rather than added later as glucose levels decline. The other key management point is that the glycosuria due to SGLT2i can persist for days (40), and fluid resuscitation may need to be extended beyond the stage when the patient is taking normal oral fluid intake.
Assessing DKA Severity
The ADA classifies the severity of DKA as mild, moderate, or severe based on the extent of acidemia as measured by pH and/or bicarbonate deficit and alteration in a patient’s mental status (Table 1). For all degrees of severity, the therapeutic triad is the same: fluid resuscitation, electrolyte repletion, and insulin therapy. While simple in concept, each of these therapies have the potential to pose unique risks to patients depending on the degree of medical complexity identified upon presentation. For example, fluid resuscitation and potassium repletion in the setting of severe AKI, and certainly end-stage renal disease, must be carefully considered. In these and other patients such as those with congestive heart failure or myocardial infarction, the risks and benefits of each therapy must be weighed often on an hourly basis and in an ICU setting. Due to the risk of hypoglycemia in critically ill patients, insulin treatment also requires frequent adjustment, thus forming the basis for IVI use in the ICU. Likewise, in severe COVID-19, the ICU is often needed for management, including mechanical ventilation and vasopressor support and hence IVI is often the most appropriate mode of therapy when needed. As discussed below, the overall medical complexity of the patient ultimately determines the treatment strategy and the appropriate use of lower intensity therapies including subcutaneous insulin.
Table 1.
Classification of hyperglycemic crisis severity (10) and insulin treatment options
| Mild DKA | Moderate DKA | Severe DKA | HHS | HK | |
|---|---|---|---|---|---|
| Blood glucose mg/dL (mmol/L) | > 250 (>13.8) | >250 (>13.8) | >250 (>13.8) | >600 (>33.3) | >600 (>33.3) |
| pH | 7.25-7.30 | 7.00-7.24 | <7.00 | >7.30 | |
| HCO2 (mmol/L) | 15-18 | 10-14 | <10 | >18 | |
| Urine/serum ketones | + | + | ± | ± | + |
| Serum osmolalitya (Osmeff) | 320 | 320 | |||
| Anion gap | Elevated | Elevated | Elevated | Elevated | Elevated |
| Mental status | Alert | Alert/drowsy | Stupor/coma | Stupor/coma | Stupor/coma |
| Insulin therapy | SC/IV | SC/IV | IV | IV | IV |
| Frequency of glucose monitoring | every 1-2 hours | every 1-2 hours | every 1 hour | every 1 hour | every 1 hour |
| Location of care | Intermediate care unit | Intermediate care unit/ICU | ICU | ICU | ICU |
Abbreviations: DKA, diabetic ketoacidosis; HCO2, bicarbonate; SC, subcutaneous; IV, intravenous; ICU, intensive care unit; BG, blood glucose; Na+, sodium.
aCalculated effective osmolality (Osmeff)= 2 [Na+] + BG/18 note: for calculation use measured (not corrected) Na+.
Management of Uncomplicated Mild/Moderate DKA in Patient with COVID-19
DKA management at home
Patients experiencing hyperglycemia who are armed with ketone testing at home are better able to communicate with their care team on the severity of their illness when inpatient care is being considered. However, given the imprecision of point of care ketone testing (41), any patient who has elevated ketones detected by any means should be assessed for other signs of possible DKA, for example, nausea, vomiting, and extreme thirst. In usual care, many patients who are clinically stable and can take oral fluids are able to self-manage early DKA with instructions and close communication with their care team. This has recently been described in COVID-19 patients by experienced diabetologists and in certain cases can prevent a hospitalization (42). However, in most patients known to be COVID-19 positive, given the concern for rapid clinical decline, it is reasonable to recommend patients with a positive ketone test be evaluated urgently for detailed assessment including laboratory testing with a basic metabolic chemistry panel.
The role of subcutaneous insulin use in DKA
As previously discussed there are unique considerations during a pandemic and utilizing strategies to provide intensive insulin therapy while minimizing nursing time at the bedside and preserving PPE has become a priority. Coordination of glucose monitoring and administration of insulin and other medications into 1 bedside interaction is strongly recommended whenever possible. Given available evidence, subcutaneous insulin therapy for mild/moderate uncomplicated DKA has been highlighted by many institutions as a useful strategy during a pandemic.
While IVI has been considered the treatment of choice in DKA, several studies have demonstrated use of subcutaneous rapid acting insulin every 1 to 2 hours is a safe and effective alternative in mild/moderate uncomplicated DKA (Table 2). A Cochrane review in 2016 evaluated 5 RCTs that randomized a total of 201 patients to DKA protocols using IVI versus subcutaneous rapid-acting insulin (43). The reviewers concluded that there are neither advantages nor disadvantages when comparing the effects of subcutaneous rapid-acting insulin analogues versus IVI for treating mild or moderate DKA. However, in the constraints of the COVID-19 pandemic, the advantages of reduced ICU utilization, exposure, and PPE use may well outweigh disadvantages if the hospital is able to adopt a safe and effective protocol.
Table 2.
Summary of subcutaneous insulin RCTs in DKA and potential strategies in COVID-19
| Population | Intervention vs conventional IVI protocol | Outcomes measured | Key findings | Notes for use in COVID-19 | |
|---|---|---|---|---|---|
| Della Manna, et al. (2005) (54) | Pediatric and adolescent patients with DKA (n = 60) | SC lispro 0.15 units/kg every 2 hours until BG < 13.8 mmol/L (250 mg/dL) then interval increased to every 4 hours until resolution of DKA | Time to resolution of DKA | Both groups reached BG < 13.8 mmol/L (<250 mg/dL) within 6 hours Metabolic acidosis and ketosis resolved faster in control group (IVI) 95% (57/60) patients were treated in ED and did not require admission | every 2-4 hours insulin dosing outside of ICU was effective but slightly slower to resolution |
| Ersöz, et al. (2006) (55) | Patients with mild/moderate DKA (n = 20) | Single bolus injection of 0.15 U/kg IV regular insulin then 0.075 units/kg every 1 hour until resolution of DKA | Time to resolution of DKA, amount of insulin used, mortality, hypoglycemia rate | No differences between groups with respect to time of resolution of DKA, amount of insulin use, rate of hypoglycemia or mortality | every 1 hour monitoring used in both groups |
| Hsia, et al. (2012) (36) | Patients with DM1 or DM2 (n = 61) | Glargine 0.25 units/kg within 12 hours of initiation of IV insulin | Rates of rebound hyperglycemia (BG >180 mg/dL) within 12 hours of discontinuation of IVI | Rebound hyperglycemia 33.3% in intervention group vs 93.5% in control (P < .001) Average lower glucose levels in intervention group (P < .01) | SC basal insulin during IVI can improve overall glucose control post DKA; may reduce rebound DKA |
| Karoli, et al. (2011) (56) | Patients with mild/moderate DKA (n = 50) | SC lispro initially 0.3 units/kg, followed by 0.2 units/kg 1 hour later then subsequently treated with 0.2 unit/kg every 2 hors until BG < 250 mg/dL, then dose reduced to 0.1 unit/kg every 1 hour | Duration of treatment and resolution of hyperglycemia and ketoacidosis. Other endpoints: total length of hospitalization, amount of insulin administration, hypoglycemia rate | No difference in the mean duration of treatment and amount of insulin required for correction of hyperglycemia and ketoacidosis. no differences in mortality or LOS | Could be adapted to allow every 2 hour monitoring and dosing until DKA resolution |
| Umpierrez, et al. (2004) (57) | Patients with mild/moderate DKA (n = 45) | SC aspart every 1 hour or every 2 hours 1-hour group: initial dose of 0.3 units/kg followed by 0.1 units/kg every 1 hour until BG <250 mg/dL dose reduced to 0.05 units/kg 2-hour group: initial dose of 0.3 units/kg followed by 0.2 units/kg every 2 hours until BG <250 mg/dL dose reduced to 0.1 units/kg | Duration of treatment and resolution of hyperglycemia and ketoacidosis. Other endpoints: total length of hospitalization, amount of insulin administration, hypoglycemia rate | No difference in mean duration of treatment until resolution of hyperglycemia or ketoacidosis or rate of hypoglycemia between group | Every 2 hour dosing was safe and effective |
| Umpierrez, et al. (2004) (58) | Patients with uncomplicated DKA (n = 40) | SC lispro, managed on medicine ward (n = 10) or an intermediate care unit (n = 10) initial dose of 0.3 units/kg followed by 0.1 units/kg every 1 hour until BG <250 mg/dL dose reduced to 0.05 units/kg | Duration of treatment and resolution of hyperglycemia and ketoacidosis. Other endpoints: total length of hospitalization, amount of insulin administration, hypoglycemia rate | No difference in mean duration of treatment until resolution of hyperglycemia or ketoacidosis or rate of hypoglycemia between group Treatment in ICU was associated with 39% higher hospitalization charges than was treatment with subcutaneous lispro in a non–intensive care setting ($14 429 ± $5243 vs $8801 ± $5549, P < .01) | Medical ward can be a safe environment for intensive SC protocol though every 1 hour monitoring used in all groups |
Abbreviations: IVI, intravenous insulin; DM1, diabetes mellitus type 1; DM2, diabetes mellitus type 2, SC, subcutaneous; IV, intravenous; DKA, diabetic ketoacidosis; LOS, length of stay.
Published studies of subcutaneous insulin use in DKA have used similar protocols but with important differences (Table 2). All studies defined the patient population as uncomplicated mild or moderate DKA. Four of 5 trials included only adults, and most subjects were reported as having poor compliance with diabetes therapy. In 1 study, patients were randomized to a regular IVI (0.1 unit/kg bolus followed by 0.1 units/kg/hour) versus an initial subcutaneous injection of 0.3 units/kg of lispro followed by 0.1 units/kg per hour until the glucose was <250 mg/dL. Although the subcutaneous insulin arm patients were managed on the general ward and the IVI group on the ICU, both protocols required every 1 hour glucose monitoring. The time required to achieve DKA resolution did not differ between the groups. In a second study by the same group, the subcutaneous arm dosed subcutaneous aspart every 1 or every 2 hours and reported no difference in time to DKA resolution across groups [40]. There was low-quality evidence to evaluate the effects of subcutaneous rapid-acting insulin analogues versus IVI on hypoglycemia, with both reporting no significant difference and overall low incidence with <10% of participants in both groups having a glucose <70 mg/dL
Of interest, none of these studies employed the use of an intermediate or long-acting basal insulin rapid-acting insulin analog-driven protocol to prevent rebound hyperglycemia and/or ketoacidosis (36). In the authors’ experience, the “tandem” use of glargine insulin can be useful along with rapid-acting insulin in the treatment of DKA. In a single-center study investigators tested the impact of adding early basal therapy with 0.25 unit/kg of long-acting insulin given within the first 12 hours of initiation of IVI. The outcome of interest was preventing rebound hyperglycemia after DKA resolution. With this method the authors reported a reduction in rebound hyperglycemia (33.3% in the intervention group versus 93.5% in the control (P < .001), which presumably led overall to better glycemic control during the hospitalization.
It is important to note that the subcutaneous insulin treatment modality has only been studied and recommended in uncomplicated patients with mild/moderate DKA as delineated above and in Table 1. Subcutaneous strategies are not recommended for patients with severe DKA and/or other complicated illness (end-stage renal disease, severe AKI, pregnancy, concomitant myocardial infarction, or stroke, requiring ICU level of care for other reasons, for example, mechanical ventilation and/or vasopressor support). For these patients, the critical care setting and use of IVI is required. Irrespective of the degree of acidosis and acidemia, patients with HHS or HK require close monitoring and therefore these patients require care in the ICU setting (10)
Management of Severe and/or Complicated DKA in Patients with COVID-19
Severe DKA, in addition to other forms of hyperglycemic crisis, requires management in an ICU with access to IVI, frequent blood, or capillary glucose monitoring (eg, every 1-2 hours), frequent laboratory monitoring (eg, for potassium and electrolytes), and access to respiratory support and cardiac monitoring as needed. Complicated patients regardless of DKA severity generally require IVI for part or most of the critical care course. Although substantive data on insulin requirements during COVID-19 are not yet published, clinicians have observed higher than usual insulin needs (up to 4 units/kg/day) in critically ill patients with COVID-19 (44). In addition, as described elsewhere (9), the concomitant use of vasopressors or corticosteroids can significantly impact insulin requirements as doses are adjusted over time. This requires an insulin delivery method that allows for rapid adjustment to dramatic changes in glycemic measures. Although the cautious use of rapid- or short-acting subcutaneous insulin dosed every 4 or 6 hours, respectively, in combination with long- or intermediate-acting insulin has been used successfully in some COVID-19 patients (44), IVI should remain standard practice for COVID-19-positive critically ill patients who have concomitant DKA. Given the unique needs during a pandemic, some institutions have decreased the frequency of glucose monitoring to every 4 or 6 hours in patients with stable insulin infusion rates and/or considered use of continuous glucose-monitoring devices.
As noted previously, the resolution of DKA is heralded by a rapid increase in insulin sensitivity, which is more dramatic in severe DKA. This requires hourly adjustment of the insulin rate while dextrose-containing fluids are infused to avoid hypoglycemia.
Prevention of Diabetic Ketoacidosis During COVID-19
The vast majority of people with diabetes who are infected with COVID-19 will not require hospitalization to manage their illness. Several authorities such as the Centers for Disease Control have advised against seeking care in the ED should individuals develop symptoms of COVID-19 but rather to contact their healthcare provider, seek testing in the community, and stay at home for mild to moderate symptoms using self-care whenever possible (45). For people with diabetes, self-care includes continuing their home insulin and to reassess their oral therapies when necessary. Insulin-deficient patients and those taking SGLT2 inhibitors should have ketone testing available to them at home should they develop illness. Many professional societies and patient advocacy groups have provided excellent resources for patients and providers alike to support a proactive approach to DKA prevention, including reminding individuals with diabetes what to do when they become ill, for example, “sick day rules” (46, 47). Additional key points specific to the pandemic include having all supplies on hand in the home in the event of illness or an emergency, for example, hypoglycemia. This should include a 3-month supply of medications if affordable, all supplies needed for insulin therapy include insulin pump infusion sets or pods, in addition to a method to test ketone bodies (blood or urine) as discussed above.
People with T1D are likely at the highest risk of DKA during the pandemic independent of COVID-19 due to potential lapses in access to medicines or their care team. As noted above, this decade has already seen a rise in DKA-related hospitalizations and hence additional attention has been paid to successful prevention programs (48, 49). In the COVID-19 environment, services that may have reduced DKA risk may not be available to patients in the same form, especially early diabetes screening/identification and family-level education. Telemedicine is already a proven modality for DKA prevention in adolescents, and overall an effective tool to deliver diabetes care (50, 51). During COVID-19 there is appropriate enthusiasm for enhanced telecommunication between adult patients and their clinicians, including texting and frequent communication through electronic health record portals. To prevent provider burnout, this preventive “checking in” should be shared among the healthcare team members including nurses, pharmacists, dieticians, and even administrative staff armed with standard communication tools. Finally, since DKA in T1D is often caused by omission of insulin and, perhaps more commonly now, rationing insulin, it is paramount that clinicians, clinical programs, hospital systems, and insurers as well as manufacturers come together to assist patients with diabetes to sustain their insulin regimens. Insulin manufacturers have recently established discount programs for those at highest risk in the United States, for example, uninsured and Medicare patients with high out-of-pocket requirements (52).
Patients taking SGLT2 inhibitors, as illustrated by this case, should be advised to discontinue the medication at the first sign of severe flu-like illness in order to prevent ketoacidosis (13). As described above, COVID-19-infected patients taking these medications may be at a uniquely high risk for DKA. As it is not always possible to predict the course of COVID-19 and who will develop severe disease, the most practical advice for patients is discontinue the drug at the onset of any symptoms consistent with a viral syndrome.
Lastly, since at least 10% of patients worldwide present with DKA as the initial presentation of diabetes (53), insulin starts and education should not be postponed during the pandemic. Patients identified as meeting criteria to start insulin should be referred for urgent education, either in-person or, whenever possible and practical, via video teleconferencing. This has been successful in the authors’ institutions.
Returning to Our Patients
Our 53-year-old female patient in Case 1 was critically ill requiring vasopressor support and mechanical ventilation for COVID pneumonia with ARDS. While requiring ICU level of care, she continued on IVI per DKA protocol until resolution of acidosis (pH normalized, bicarbonate stabilized >18 mmol/L, and anion gap closed). She did not receive bicarbonate as her blood pH was not lower than 6.9 (10). Given the euDKA, dextrose support was required at the start of insulin. Over the subsequent 12 days she clinically improved and supplemental oxygen was weaned. She was transitioned to a weight-based insulin regimen with 0.6 units/kg total daily dose (TDD) with half given as basal and half as bolus before meals. On hospital day 17 she was discharged home with recommendation to hold empagliflozin.
Our 45-year-old male patient in Case 2 remained hemodynamically and neurologically stable with good oxygenation by face mask. He was admitted to a non–critical care unit for management of moderate DKA. In attempt to minimize nursing time at the bedside and preserve PPE he was treated with a subcutaneous insulin strategy with every four hour glucose monitoring and insulin administration. He received 0.15 units/kg basal insulin (glargine) and 0.3 units/kg rapid-acting insulin (aspart) loading dose, followed by 0.2 units/kg rapid-acting insulin (aspart) every 4 hours until resolution of DKA. Dextrose was added to intravenous fluids once his glucose was lower than 250 mg/dL (13.8 mmol/L). His renal function improved with intravenous hydration, and oral potassium was administered to maintain normal levels. He did not require bicarbonate based on blood pH. When DKA resolved he transitioned to a weight-based basal bolus insulin regimen with 0.7 units/kg TDD. In the subsequent days, insulin was downtitrated to 0.6 unit/kg TDD and overall glycemic control remained at goal. GAD-65, islet cell and insulin autoantibodies were negative. He received diabetes education including insulin and glucose meter teaching prior to discharge. He was seen by a nurse educator via telemedicine within 48 hours of discharge and remains engaged in newly diagnosed diabetes.
Conclusion
Patients with diabetes are at increased risk of severe complications from COVID-19 which may include DKA. In the effort to balance the need to provide intensive insulin therapy with minimizing exposure to healthcare workers and preserving PPE, clinicians are actively seeking alternative and patient-tailored strategies for care delivery in the treatment of DKA. The cases above highlight important aspects to consider when determining the most appropriate way to treat DKA, including insulin method and location of care. For critically ill and medically complex patients, recognition and prompt treatment of DKA in the ICU setting is recommended. For those with uncomplicated mild/moderate DKA, strategic use of subcutaneous insulin is supported by high-quality evidence and can be useful to address the unique needs of care systems during a pandemic. Preventing DKA is critically important when possible, which may include maintaining key clinical services using telemedicine and proactively delivering standard prevention advice to patients. Further studies are needed to explore the incidence and pathogenesis of DKA in patients with SAR-CoV-2 infection.
Glossary
Abbreviations
- ACEI
angiotensin-converting enzyme inhibitor
- ADA
American Diabetes Association
- AKI
acute kidney injury
- ARB
angiotensin receptor blocker
- ARDS
acute respiratory distress syndrome
- DKA
diabetic ketoacidosis
- ED
Emergency Department
- euDKA
euglycemic DKA
- GAD-65
glutamic acid decarboxylase 65
- HbA1c
hemoglobin A1c
- HHS
hyperosmolar hyperglycemia syndrome
- HK
hyperosmolar ketoacidosis
- ICU
intensive care unit
- IL-6
interleukin-6
- IVI
intravenous insulin
- PPE
personal protective equipment
- RCT
randomized controlled trial
- SGLT2
sodium–glucose cotransporter 2
- TDD
total daily dose
Additional Information
Disclosure Statement: N.E. P. and A. R.S. have no relevant disclosures. M.E.M has received funding for research from NovoNordisk.
Data Availability: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Steenkamp DW, Alexanian SM, McDonnell ME. Adult hyperglycemic crisis: a review and perspective. Curr Diab Rep. 2013;13(1):130-137. [DOI] [PubMed] [Google Scholar]
- 2. Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes. 2004;53(8):2079-2086. [DOI] [PubMed] [Google Scholar]
- 3. Fayfman M, Pasquel FJ, Umpierrez GE. Management of hyperglycemic crises: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Med Clin North Am. 2017;101(3):587-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaiser UB, Mirmira RG, Stewart PM. Our response to COVID-19 as endocrinologists and diabetologists. J Clin Endocrinol Metab. 2020;105:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhong VW, Juhaeri J, Mayer-Davis EJ. Trends in hospital admission for diabetic ketoacidosis in adults with type 1 and type 2 diabetes in England, 1998–2013: a retrospective cohort study. Diabetes Care. 2018;41(9):1870-1877. [DOI] [PubMed] [Google Scholar]
- 6. Vellanki P, Umpierrez GE. Increasing hospitalizations for DKA: a need for prevention programs. Diabetes Care. 2018;41(9):1839-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benoit SR, Zhang Y, Geiss LS, Gregg EW, Albright A. Trends in diabetic ketoacidosis hospitalizations and in-hospital mortality – United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2018;67(12):362-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Umpierrez GE. Ketosis-prone type 2 diabetes: time to revise the classification of diabetes. Diabetes Care. 2006;29(12):2755-2757. [DOI] [PubMed] [Google Scholar]
- 9. Moghissi ES, Korytkowski MT, DiNardo M, et al. ; American Association of Clinical Endocrinologists; American Diabetes Association American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Munro JF, Campbell IW, McCuish AC, Duncan LJ. Euglycaemic diabetic ketoacidosis. Br Med J. 1973;2(5866):578-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. FDA. FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. FDA Drug Safety Communication https://www.fda.gov/drugs/drug-safety-and-availability/fda-revises-labels-sglt2-inhibitors-diabetes-include-warnings-about-too-much-acid-blood-and-serious. Accessed May 2, 2020.
- 13. Peters AL, Henry RR, Thakkar P, Tong C, Alba M. Diabetic ketoacidosis with canagliflozin, a sodium-glucose cotransporter 2 inhibitor, in patients with type 1 diabetes. Diabetes Care. 2016;39(4):532-538. [DOI] [PubMed] [Google Scholar]
- 14. Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S. Epidemiology of COVID-19 among Children in China. Pediatrics. 2020;145(6):e20200702. [DOI] [PubMed] [Google Scholar]
- 15. Diabetes ISoPaA https://www.ispad.org/page/CoronavirusinfectionCOVID-19-IIISPADSummary. Accessed May 2, 2020.
- 16. Singh AK, Gupta R, Ghosh A, Misra A. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr. 2020;14(4):303-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chee YJ, Ng SJH, Yeoh E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract. 2020;164:108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim NY, Ha E, Moon JS, Lee YH, Choi EY. Acute hyperglycemic crises with coronavirus disease-19: case reports. Diabetes Metab J. 2020;44(2):349-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stratigou T, Vallianou N, Vlassopoulou B, et al. DKA cases over the last three years: has anything changed? Diabetes Metab Syndr. 2019;13(2):1639-1641. [DOI] [PubMed] [Google Scholar]
- 20. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. [Published online ahead of print March 23, 2020]. JAMA. 2020. doi: 10.1001/jama.2020.4683 [DOI] [PubMed] [Google Scholar]
- 24. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. [Published online ahead of print March 13, 2020]. JAMA Intern Med. 2020. doi: 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petzold A, Solimena M, Knoch KP. Mechanisms of beta cell dysfunction associated with viral infection. Curr Diab Rep. 2015;15(10):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Masini M, Campani D, Boggi U, et al. Hepatitis C virus infection and human pancreatic beta-cell dysfunction. Diabetes Care. 2005;28(4):940-941. [DOI] [PubMed] [Google Scholar]
- 28. Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(3):193-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID-19 infection may cause ketosis and ketoacidosis. [Published online ahead of print April 20, 2020]. Diabetes Obes Metab. 2020. doi: 10.1111/dom.14057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen X, Zhao B, Qu Y, et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. [Published online ahead of print April 17, 2020]. Clin Infect Dis. 2020. doi: 10.1093/cid/ciaa449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nyenwe EA, Kitabchi AE. The evolution of diabetic ketoacidosis: An update of its etiology, pathogenesis and management. Metabolism. 2016;65(4):507-521. [DOI] [PubMed] [Google Scholar]
- 32. Gamba G, Oseguera J, Castrejón M, Gómez-Pérez FJ. Bicarbonate therapy in severe diabetic ketoacidosis. A double blind, randomized, placebo controlled trial. Rev Invest Clin. 1991;43(3):234-238. [PubMed] [Google Scholar]
- 33. Bevilacqua S, Bonadonna R, Buzzigoli G, et al. Acute elevation of free fatty acid levels leads to hepatic insulin resistance in obese subjects. Metabolism. 1987;36(5):502-506. [DOI] [PubMed] [Google Scholar]
- 34. Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care. 1990;13(6):610-630. [DOI] [PubMed] [Google Scholar]
- 35. Karajgikar ND, Manroa P, Acharya R, et al. Addressing pitfalls in management of diabetic ketoacidosis with a standardized protocol. Endocr Pract. 2019;25(5):407-412. [DOI] [PubMed] [Google Scholar]
- 36. Hsia E, Seggelke S, Gibbs J, et al. Subcutaneous administration of glargine to diabetic patients receiving insulin infusion prevents rebound hyperglycemia. J Clin Endocrinol Metab. 2012;97(9):3132-3137. [DOI] [PubMed] [Google Scholar]
- 37. Limenta M, Ho CSC, Poh JWW, Goh SY, Toh DSL. Adverse drug reaction profile of SGLT2 inhibitor-associated diabetic Ketosis/Ketoacidosis in Singapore and their precipitating factors. Clin Drug Investig. 2019;39(7):683-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zinman B, Wanner C, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. [DOI] [PubMed] [Google Scholar]
- 39. Hamblin PS, Wong R, Ekinci EI, et al. SGLT2 inhibitors increase the risk of diabetic ketoacidosis developing in the community and during hospital admission. J Clin Endocrinol Metab. 2019;104(8):3077-3087. [DOI] [PubMed] [Google Scholar]
- 40. Alhassan S, Rudoni M, Alfonso-Jaume MA, Jaume JC. Protracted glycosuria after discontinuation of sodium-glucose cotransporter 2 inhibitors: implications for weekly dosing and extended risk of euglycemic diabetes ketoacidosis. J Diabetes. 2019;11(5):410-411. [DOI] [PubMed] [Google Scholar]
- 41. Misra S, Oliver NS. Utility of ketone measurement in the prevention, diagnosis and management of diabetic ketoacidosis. Diabet Med. 2015;32(1):14-23. [DOI] [PubMed] [Google Scholar]
- 42. Peters AL, Garg SK. The silver lining to COVID-19: avoiding diabetic ketoacidosis admissions with telehealth. Diabetes Technol Ther. 2020;22(6):449-453. [DOI] [PubMed] [Google Scholar]
- 43. Andrade-Castellanos CA, Colunga-Lozano LE, Delgado-Figueroa N, Gonzalez-Padilla DA. Subcutaneous rapid-acting insulin analogues for diabetic ketoacidosis. Cochrane Database Syst Rev. 2016:2016;(1):CD011281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Korytkowski M. Antinori-Lent K, Drincic A, et al. A pragmatic approach to inpatient diabetes management during the COVID-19 pandemic. [Published online ahead of print June 4, 2020]. J Clin Endocrinol Metab. 2020. doi: 10.1210/clinem/dgaa342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. CDC. Stay home if you’re sick. https://www.cdc.gov/nonpharmaceutical-interventions/pdf/stay-home-youre-sick-item5.pdf. Accessed May 2, 2020.
- 46. Foundation JDR. Coronavirus and type 1 diabetes: what you need to know. https://www.jdrf.org/coronavirus/. Accessed May 25, 2020.
- 47. Laffel LM, Limbert C, Phelan H, Virmani A, Wood J, Hofer SE. ISPAD Clinical Practice Consensus Guidelines 2018: Sick day management in children and adolescents with diabetes. Pediatr Diabetes. 2018;19(Suppl 27):193-204. [DOI] [PubMed] [Google Scholar]
- 48. Barker JM, Goehrig SH, Barriga K, et al. ; DAISY study Clinical characteristics of children diagnosed with type 1 diabetes through intensive screening and follow-up. Diabetes Care. 2004;27(6):1399-1404. [DOI] [PubMed] [Google Scholar]
- 49. Elding Larsson H, Vehik K, Bell R, et al. ; TEDDY Study Group; SEARCH Study Group; Swediabkids Study Group; DPV Study Group; Finnish Diabetes Registry Study Group Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care. 2011;34(11):2347-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wagner DV, Barry SA, Stoeckel M, Teplitsky L, Harris MA. NICH at its best for diabetes at its worst: texting teens and their caregivers for better outcomes. J Diabetes Sci Technol. 2017;11(3):468-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McDonnell ME. Telemedicine in complex diabetes management. Curr Diab Rep. 2018;18(7):42. [DOI] [PubMed] [Google Scholar]
- 52. Novonordisk Savings Program. COVID-19 Novo Nordisk information and resources. https://www.novonordisk-us.com/media/COVID19.html. Accessed June 25, 2020.
- 53. Jefferies CA, Nakhla M, Derraik JG, Gunn AJ, Daneman D, Cutfield WS. Preventing diabetic ketoacidosis. Pediatr Clin North Am. 2015;62(4):857-871. [DOI] [PubMed] [Google Scholar]
- 54. Della Manna T, Steinmetz L, Campos PR, et al. Subcutaneous use of a fast-acting insulin analog: an alternative treatment for pediatric patients with diabetic ketoacidosis. Diabetes Care. 2005;28(8):1856-1861. [DOI] [PubMed] [Google Scholar]
- 55. Ersöz HO, Ukinc K, Köse M, et al. Subcutaneous lispro and intravenous regular insulin treatments are equally effective and safe for the treatment of mild and moderate diabetic ketoacidosis in adult patients. Int J Clin Pract. 2006;60(4):429-433. [DOI] [PubMed] [Google Scholar]
- 56. Karoli R, Fatima J, Salman T, Sandhu S, Shankar R. Managing diabetic ketoacidosis in non-intensive care unit setting: role of insulin analogs. Indian J Pharmacol. 2011;43(4):398-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Umpierrez GE, Cuervo R, Karabell A, Latif K, Freire AX, Kitabchi AE. Treatment of diabetic ketoacidosis with subcutaneous insulin aspart. Diabetes Care. 2004;27(8):1873-1878. [DOI] [PubMed] [Google Scholar]
- 58. Umpierrez GE, Latif K, Stoever J, et al. Efficacy of subcutaneous insulin lispro versus continuous intravenous regular insulin for the treatment of patients with diabetic ketoacidosis. Am J Med. 2004;117(5):291-296. [DOI] [PubMed] [Google Scholar]