Summary
A method for the electrophoretic transfer of high and low molecular weight proteins to nitrocellulose membranes following sodium dodecyl sulfate (SDS) polyacrylamide gel is described here. The transfer was performed with heated (70-75°C) normal transfer buffer from which methanol had been omitted. Complete transfer of high and low molecular weight antigens (molecular weight protein standards, a purified protein, and proteins from a human tissue extract) could be carried out in 10 minutes for a 7% (0.75 mm) SDS polyacrylamide gel. For 10 and 12.5% gels (0.75 mm) the corresponding time was 15 minutes. A complete transfer could be carried out in 20 minutes for 7, 10 and 12.5% gels (1.5 mm gels). The permeability of the gel is increased by heat, such that the proteins trapped in the polyacrylamide gel matrix can be easily transferred to the membrane. The heat mediated transfer method was compared with a conventional transfer protocol, under similar conditions. The conventional method transferred minimal low molecular weight proteins while retaining most of the high molecular weight proteins in the gel. In summary, this procedure is particularly useful for the transfer of high molecular weight proteins, very rapid and avoids the use of methanol.
Keywords: SDS-PAGE, Western blotting, Nitrocellulose, High molecular weight protein
1. Introduction
Proteins transfer from sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels to nitrocellulose or polyvinylidene difluoride membranes have been achieved by (a) simple diffusion (1); vacuum-assisted solvent flow (2); and (c) electrophoretic elution (3). There is considerable interest in diffusion mediated transfer of proteins, since it was showed that (1) multiple immunoblots can be generated following non-electrophoretic bidirectional transfer of a single SDS-PAGE gel with multiple antigens. The lifts from SDS-PAGE gels, for immunoblotting, using this method are particularly useful in identification of proteins by mass spectrometry (4,5). However, electrophoretic elution is still the method of choice for protein transfer to membranes widely in most laboratories.
Electrophoretic transfer of proteins, resolved by SDS-PAGE, to nitrocellulose is a fundamental step prior to detection of specific proteins with specific antibodies (6–8). The protein transfer procedure normally takes about 2-4 hours at about 70 volts or an overnight transfer at 30 volts. High molecular weight proteins are often stubbornly resistant to transfer (9) in spite of these prolonged runs and this problem is accentuated when higher percentage gels are used. Prolonged electrotransfer (16-20 h) at high current density coupled with inclusion of 0.01% sodium dodecyl sulfate, to enhance protein elution, has been used to efficiently transfer high-molecular weight proteins (9).
In order to determine the efficiency of heat-mediated electro-blotting, we have used purified Ro 60 autoantigen, prestained molecular weight standards and a human cell extract using gels of two different thicknesses (0.75 mm and 1.5 mm) and gels with three different amounts of acrylamide (7, 10 and 12.5%). In addition, transfer of proteins from a 4-20% gradient SDS-PAGE gel and a 12.5% (1.5 mm) regular gel was also investigated and compared to that obtained using a conventional transfer method under similar conditions.
Prestained high and low molecular weight protein standards (Fig. 1, 2; Lane 4) and bovine Ro 60 (Fig. 1,2; Lane 2) could be transferred completely to nitrocellulose. All the protein markers (5 μl of the marker) could be transferred in about 10 min from a 0.75 mm, 7% gel (Fig. 1A, Lane 4). All the protein markers, ranging from 184 kD to 9 kD, could be transferred to membranes from 7, 10 and 12.5% gels (Fig. 1B, 1C - Lane 4) in 15 min. The post-transfer polyacrylamide gels were clean, without any sign of residual non-transferred protein markers. It took 20 min to transfer all the protein markers in the case of the 1.5 mm gels (7, 10 and 12.5% gels) (Fig. 2, Lane 4).
Figure 1:
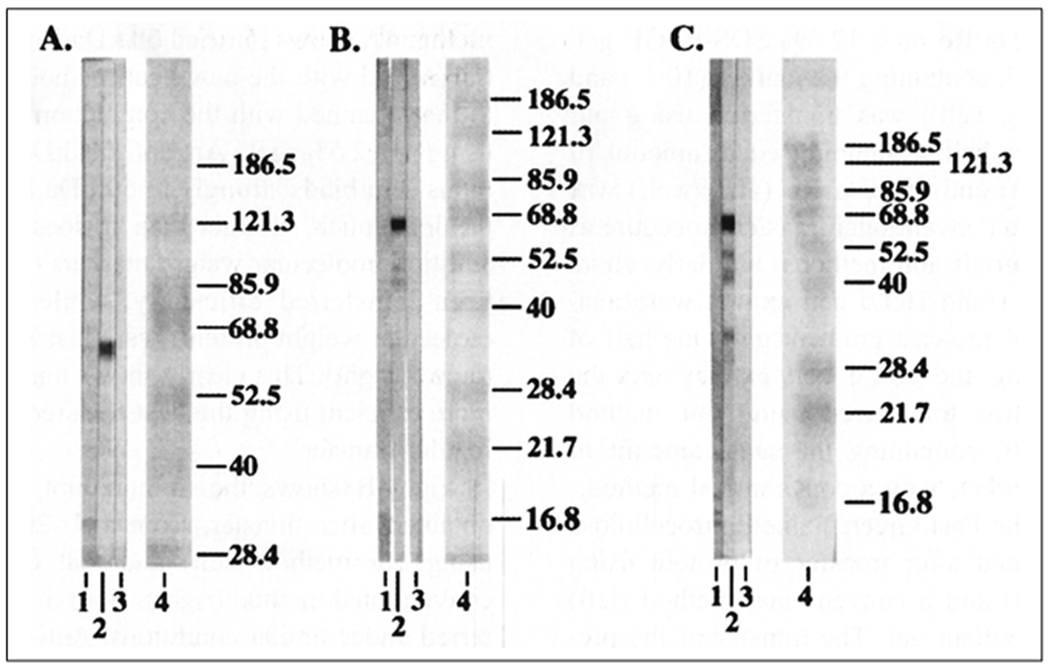
Western blot transfer and immunoblotting of bovine Ro 60 and prestained molecular weight standards using 7, 10 and 12.5% gels (0.75 mm gels).
Fig. 1A, 1B and 1C- Proteins on a 7, 10 and 12.5% SDS-PAGE gels respectively transferred using heat. Lane 1-Anti-Ro 60 negative control; Lane 2- Anti-Ro 60 positive control; Lane 3-Conjugate control; Lane 4-Prestained molecular weight standards. (Reproduced from Ref 8 with permission from Elsevier).
Figure 2:
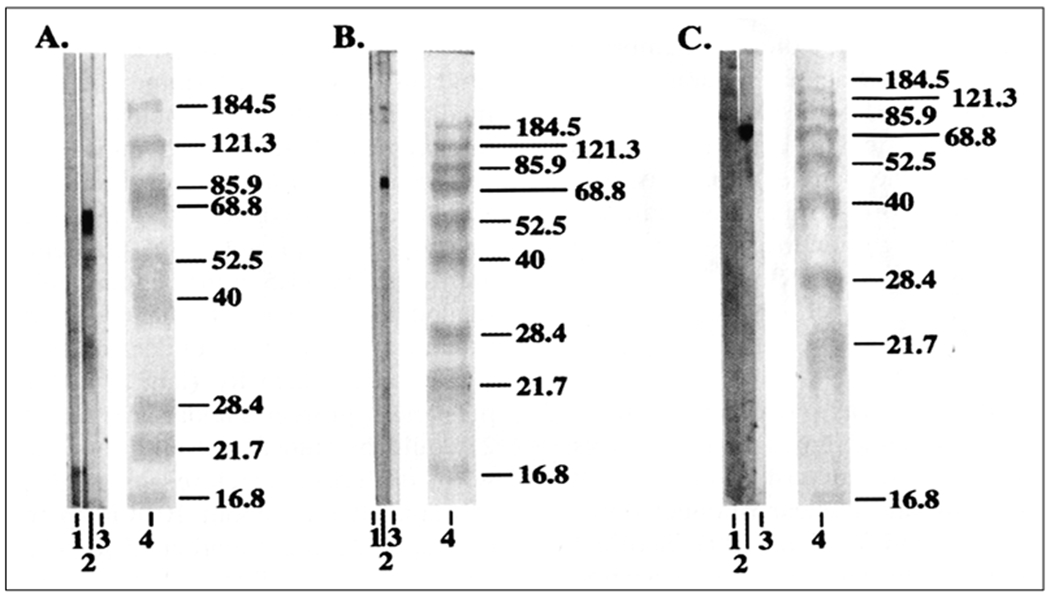
Western blot transfer and immunoblotting of bovine Ro 60 and prestained molecular weight standards using 7, 10 and 12.5% gels (1.5 mm gels). Fig. 2A, 2B and 2C- Proteins on a 7, 10 and 12.5% SDS-PAGE gels transferred using heat. Lane 1-Anti-Ro 60 negative control; Lane 2- Anti-Ro 60 positive control; Lane 3-Conjugate control; Lane 4-Prestained molecular weight standards. (Reproduced from Ref 8 with permission from Elsevier).
Figure 3 shows the fast green stained nitrocellulose membranes following transfer of protein using our method (Fig. 3, right panel) and a conventional method (Fig. 3, left panel). The protein transfer was found to be efficient with heat mediated transfer, while even the low molecular weight proteins can be barely seen following 20 min of transfer using a conventional method. Similarly only low levels of proteins could be detected immunologically following a conventional electrotransfer compared to that obtained following heat mediated transfer (Fig. 4).
Figure 3:
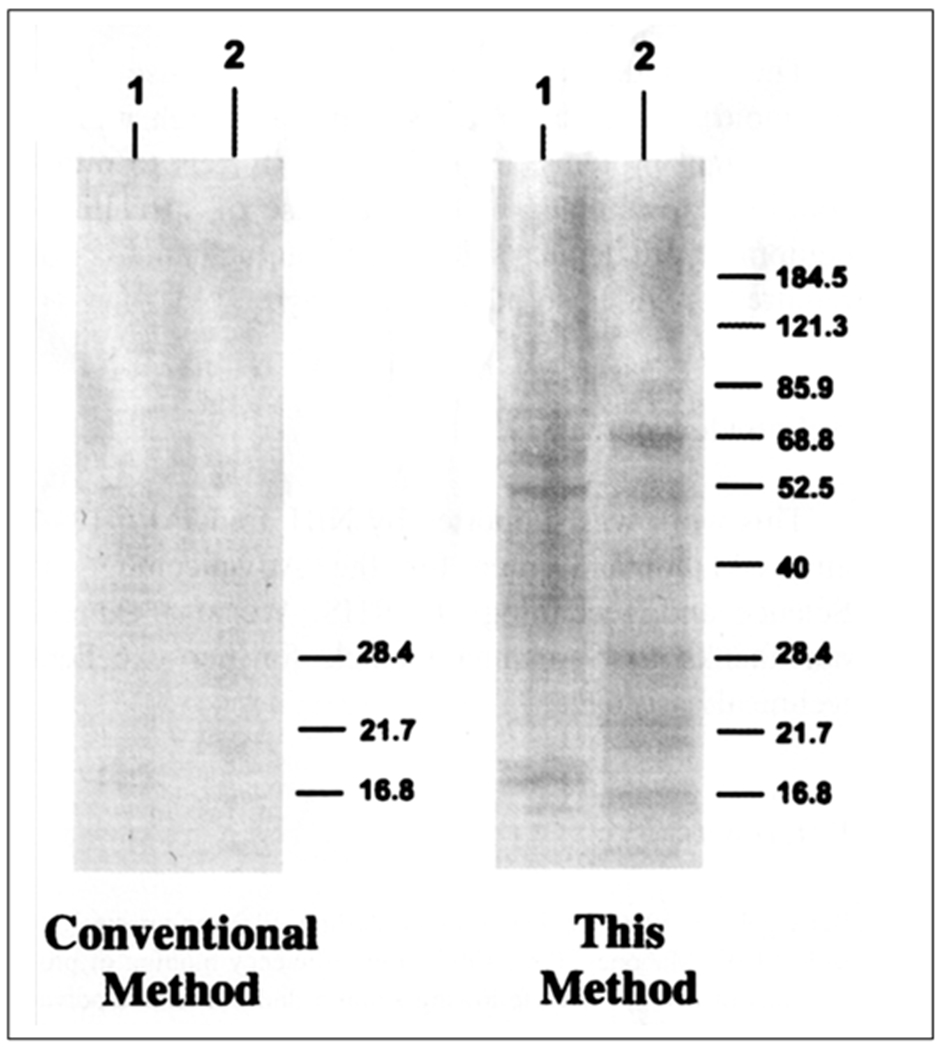
Fast Green staining of proteins from a HeLa cell extract transferred to nitrocellulose membrane by a conventional method (left) and using the heat transfer method (right) from a 4-20% SDS-PAGE gradient gel. Lane 1- HeLa cell extract; Lane 2 – 10 μL of prestained molecular weight marker. (Reproduced from Ref 8 with permission from Elsevier).
Figure 4:
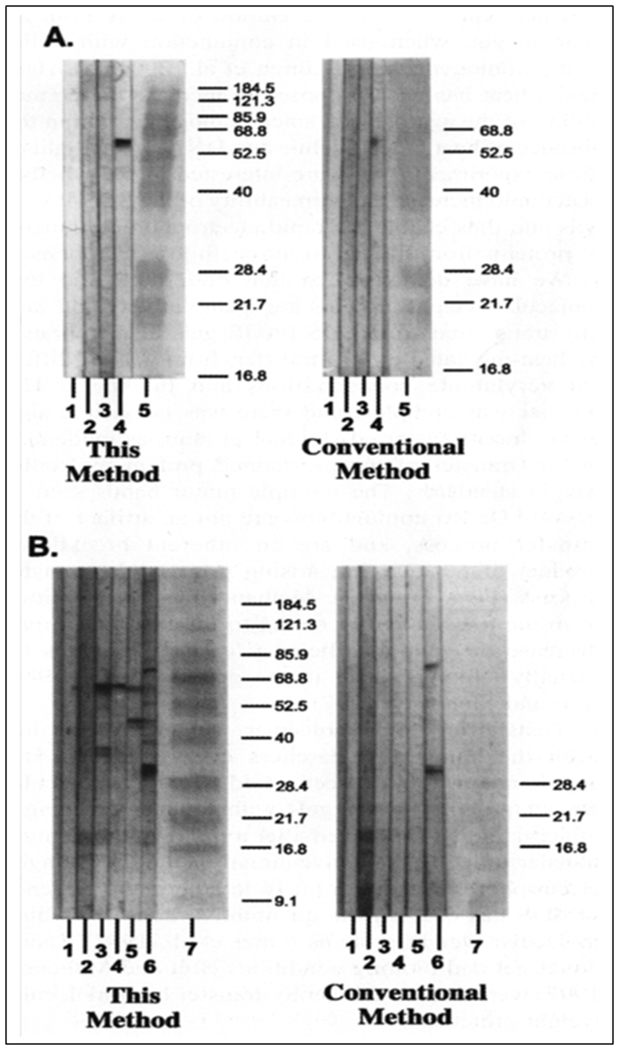
Immunoblots of purified Ro 60 and proteins derived from a HeLa cell extract transferred to nitrocellulose membrane using the heat transfer method (left) and by a conventional method (right).
Fig. 4A: Purified Ro 60 autoantigen immunoblot obtained from a 12.5% SDS-PAGE gel using the heat transfer method (left) and by a conventional method (right). Lane 1 – conjugate control; lanes 2 and 3 – normal controls; lane 4 – anti- Ro 60 SLE sera; lane 5 – prestained protein molecular weight standards.
Fig. 4B: HeLa cell extract immunoblot obtained from a 4-20 % gradient SDS-PAGE gel using the heat transfer method (left) and a conventional transfer method (right). Lane 1-conjugate control; lane 2 - normal control; lane 3-anti- Ro 60 SLE sera; lane 4 - anti-La sera; lane 5 - anti- Ro 52 sera; lane 6 - anti-Sm/nRNP sera; lane 7-prestained protein molecular weight markers (10 μL). (Reproduced from Ref 8 with permission from Elsevier).
This method clearly demonstrates that both low and high molecular weight proteins can be transferred very efficiently to nitrocellulose membranes in a very short time using heated transfer buffer without methanol (10).
2. Materials
Prepare all solutions using ultrapure water (prepared by purifying deionized water, to attain a sensitivity of 18 M Ω cm2 at 25 °C) and analytical grade reagents. Prepare and store all reagents at room temperature (unless indicated otherwise). Diligently follow all waste disposal regulations when disposing waste materials. We do not add sodium azide to our reagents.
10% SDS-PAGE precast gels (10-well).
SDS lysis buffer (5x): 0.3 M Tris-HCl (pH 6.8), 10% SDS, 25% β-mercaptoethanol, 0.1% bromophenol blue, 45% glycerol. Leave one aliquot at 4°C for current use and store remaining aliquots at −20°C (see Note 1).
SDS-PAGE running buffer: 0.025 M Tris, pH 8.3, 0.192 M glycine, 0.1% SDS.
Phosphate buffered saline (PBS), pH 7.4.
Purified bovine Ro 60 autoantigen (11,12) was a gift from Immunovision, Springdale, AK, USA.
BenchMark pre-stained molecular weight standards.
Nitrocellulose membranes.
Transfer buffer (with methanol): 0.025 mM Tris, 192 mM glycine, 20% methanol.
Transfer buffer (without methanol): 0.025 mM Tris, 192 mM glycine.
Western blot transfer apparatus with capability of circulating hot or cold water at the base.
Refrigerator bath/circulating hot water bath.
Branson Sonifier Cell Disruptor 185.
Tris buffered saline (TBS; 10x): 1.5 M NaCl, 0.1 M Tris-HCl, pH 7.4.
TBS containing 0.05% Tween-20 (TBST).
Blocking solution: 5% milk in TBS, pH 7.4. Store at 4°C (see Note 2).
Diluent solution: 5% milk in TBS, pH 7.4 containing 0.05% Tween. Store at 4°C (see Note 2).
Mini PROTEAN® 3 System Glass plates.
FB300 power supply.
Corning PC-351 magnetic stirrer.
Nitro blue tetrazolium (NBT)/5-bromo-4-chloro-3-indolyl phosphate (BCIP): Dissolve 1 g NBT in 20 mL of 70% dimethylformamide (DMF). Disolve 1 g BCIP in 20 mL of 100% DMF. Add 33 μL of BCIP and 66 μL of NBT to 10 mL of alkaline phosphatase buffer just before adding to membrane. Alternatively, use 1-Step™ premixed NBT/BCIP.
3. Methods
All procedures are carried out at room temperature unless otherwise specified.
3.1. Preparation of HeLa cell lysate
Harvest freshly cultured HeLa cells by centrifuging at 800 x g and wash twice with PBS.
Lyse cells by sonication in SDS-PAGE lysis buffer using a Branson sonicator (setting 4) and spin at 10, 000 x g for 10 min.
Use an aliquot of the supernatant for SDS-PAGE.
3.2. SDS-PAGE
Carry out SDS-PAGE (13) (see Chapters 11 and 34) on 7, 10 or 12.5% gels (0.75 mm or 1.5 mm thickness).
3.3. Conventional Electrophoretic Transfer
Carry out conventional electrotransfer of proteins (3) separated on gradient and regular gels (see Chapters 22 and 34) at 4°C using standard transfer buffer with methanol) for 20 min.
Save the nitrocellulose membrane for use with the membrane to be obtained following heat mediated electrophoretic transfer for 20 min also. Stain the polyacrylamide gel with Coomassie brilliant blue stain to ensure the efficiency of transfer.
3.4. Heat Mediated Electrophoretic Transfer
Turn on the circulating hot water bath and set temperature at 70°C.
Following SDS PAGE, pry the gel plates open with the use of a spatula. The gel remains on one of the glass plates. Rinse the gel gently with deionized water and transfer carefully to a container with transfer buffer (without methanol).
Heat the transfer buffer (without methanol) to about 70–75°C in a one-liter glass beaker. Cover the beaker with clear plastic wrap.
Cut a nitrocellulose membrane to the size of the gel and immerse in transfer buffer (see Note 3).
Cut four sheets of Whatman 3MM filter paper to the size of the gel and transfer to the transfer buffer (see Note 4). Place 2 adsorbent pads also in the buffer and remove air bubbles by pressing down on it with help of fingers.
Place clear plastic wrap (12 inches in length) on the work-bench. Place two filter papers on top of the plastic wrap. Position the membrane on top of the filter papers. Transfer the gel to the top of the membrane in such a way that there are no air bubbles between the gel and the membrane (see Note 5). Place the remaining two filter papers on top of the gel. Place in transfer cassette. Ensure that the nitrocellulose membrane is between the gel and the anode (see Note 6).
Place the transfer apparatus on a magnetic stirrer and connect it to the hot water bath maintained at 70°C. Transfer the hot transfer buffer into the apparatus (see Note 7). Place a magnetic stir bar inside the transfer apparatus to circulate the buffer.
Carry out the transfer at 40 volts (see Note 8) for periods ranging from 10 min to 20 min, depending upon the type of SDS polyacrylamide gel used (see Note 9).
Disconnect power supply and disassemble the sandwich. Save the membrane along with one obtained using conventional electrotransfer.
Stain the polyacrylamide gel with Coomassie brilliant blue stain to ensure the efficiency of transfer.
3.5. Immunoblotting
Carry out standard immunoblotting (3) (see Chapters 16 and 25) for both sets of membranes.
Use human systemic lupus erythematosus (SLE) sera with autoantibodies against 60 kD Ro, 48 kD La, 52 kD Ro, Sm or nuclear ribonucleoprotein autoantigens (11,12) to identify the respective antigens transferred to nitrocellulose from various gels (see Note 9).
Notes
SDS precipitates at 4°C. Therefore, the lysis buffer needs to be warmed prior to use.
Add 100 ml of 10X TBS to a 1L graduated cylinder and make it to about 800 ml with water. Transfer 50 g skim milk powder into the cylinder and mix stir until dissolved. Make to 1L with water. Separate 500 ml as the blocking solution. To the remaining 500 ml add 250 μL of Tween-20 (cut end of blue tip to aspirate Tween-20 easily), dissolve and use it as the diluent.
Moistening the nitrocellulose completely with the transfer buffer (without methanol) takes about ten min. Alternately, the nitrocellulose membrane can be moistened almost instantaneously with regular transfer buffer that contains methanol. The membrane can then be rinsed with water and the transfer buffer (without methanol).
Preferably, the buffer used to assemble the sandwich should be at room temperature or just slightly warm. Using the hot buffer to assemble the sandwich causes the 3 MM filter paper, used as a part of the gel sandwich, to disintegrate partially.
Hold the two top corners of the gel with each hand. Lower the bottom part of the gel first of the membrane and gently release the gel little by little to lay the complete gel on the membrane. This will prevent trapping of bubbles in between the gel and the membrane. We use clear plastic wrap. Part of the wrap was folded over the sandwich. A 10 mL pipette was used to roll out the air bubbles from the gel membrane sandwich prior to placing in transfer cassette.
In the Bio-Rad transfer apparatus, we place the gel side of the transfer cassette facing the black side of the transfer cassette holder and the membrane side facing the red side.
Exercise caution in handling the hot contents. Use thermo gloves to hold the hot beaker and pour carefully.
Forty Volts was close to the upper limit possible using the FB300 power supply. The current was about 500 mA at this point.
Transfer from a 7.5% SDS polyacrylamide gel (0.75 mm thick) can be carried out in 10 minutes. However, it takes longer (20 min) for a 12.5% gel (1.5 mm thick). If a circulating water bath is not available, it is still possible to transfer using just the heated buffer, by increasing the time of transfer. The main advantage of this procedure that it is possible to transfer high molecular weight protein, in addition to saving time.
Exercise universal precaution when handling human sera. Treat each serum sample as potentially dangerous.
Acknowledgement
This work was supported by NIH grant ARO1844 and Oklahoma Center for the Advancement of Science and Technology to RHS. We also express our thanks to Samantha Ganick for her excellent technical assistance.
References
- 1.Kurien BT, and Scofield RH (1997) Multiple immunoblots after non-electrophoretic bidirectional transfer of a single SDS-PAGE gel with multiple antigens. J Immunol Methods 205, 91–94. [DOI] [PubMed] [Google Scholar]
- 2.Peferoen M, Huybrechts R, and De Loof A (1982) Vacuum-blotting: a new simple and efficient transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to NC. FEBS Lett. 145, 369–372. [Google Scholar]
- 3.Towbin H, Staehelin T, and Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to NC sheets: procedure and applications. Proc Natl Acad Sci USA 76, 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurien BT, and Scofield RH (2000) Association of neutropenia in systemic lupus erythematosus with anti-Ro and binding of an immunologically cross-reactive neutrophil membrane antigen. Clin Exp Immunol. 120, 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurien BT, Matsumoto H and Scofield RH (2001) Purification of tryptic peptides for mass spectrometry using polyvinylidene fluoride membrane. Indian J Biochem Biophys. 38, 274–276. [PubMed] [Google Scholar]
- 6.Bolt MW, and Mahoney PA (1997) High efficiency blotting of proteins of diverse sizes following sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal Biochem. 247, 185–192. [DOI] [PubMed] [Google Scholar]
- 7.MacPhee DJ (2010). Methodological considerations for improving Western blot analysis. J Pharmacol Toxicol Methods 61, 171–177. [DOI] [PubMed] [Google Scholar]; Review. Methods Mol Biol 536, 181–90. [DOI] [PubMed] [Google Scholar]
- 8.Kurien BT, and Scofield RH (2000) Ultrarapid electrophoretic transfer of high and low molecular weight proteins using heat. Methods Mol Biol 536, 181–90. [DOI] [PubMed] [Google Scholar]
- 9.Otter T, King SM, and Witman GB (1987) A two-step procedure for efficient electrotransfer of both high-molecular weight (greater than 400,000) and low- molecular weight (less than 20,000) proteins. Anal Biochem. 162, 370–377. [DOI] [PubMed] [Google Scholar]
- 10.Kurien BT, and Scofield RH (2002) Heat mediated, ultra-rapid electrophoretic transfer of high and low molecular weight proteins to NC membranes. J Immunol Methods 266,127–133. [DOI] [PubMed] [Google Scholar]
- 11.Deutscher SL, Harley JB, and Keene JD (1988) Molecular analysis of the 60 kD human Ro ribonucleoprotein. Proc Natl Acad Sci USA 85, 9479–9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben-Chetrit E, Gandy BJ, Tan EM, and Sullivan KF (1989) Isolation and characterization of a cDNA clone encoding the 60 kDa component of the human SS-A/Ro ribonucleoprotein autoantigen. J Clin Invest. 83, 1284–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]


