Abstract
Aims
Neuromodulation (nerve stimulation) can produce analgesia. One form, bilateral pudendal nerve stimulation (bPNS), suppresses responses to urinary bladder distension (UBD) in hypersensitive rats. Drugs can modify this effect (e.g., benzodiazepines, but not opioids, suppress bPNS effects). Prior to a clinical trial of bPNS effects on bladder pain, we felt it was prudent to survey the effects of medications commonly used in patients with bladder disorders.
Methods
Bladder hypersensitivity was produced by neonatal bladder inflammation in rat pups coupled with a second inflammatory insult as an adult. Antimuscarinic (oxybutynin), β3-adrenoceptor agonist (mirabegron, CL316243), α1-adrenoceptor antagonist (tamsulosin), antidepressant (amitriptyline), muscle relaxing (baclofen), and sedative (propofol) agents were administered and effects of bPNS on responses to UBD assessed. bPNS consisted of bilateral biphasic electrical stimulation of the mixed motor/sensory component of the pudendal nerves. Visceromotor responses (VMRs; abdominal muscle contractile responses) were used as nociceptive endpoints.
Results
Many of these drugs directly inhibited the VMRs to UBD, but only mirabegron, at the doses employed, significantly reduced inhibitory effects of bPNS. In the presence of the other drugs, bPNS continued to produce statistically significant inhibition of VMRs to UBD.
Conclusions
This study suggests that concurrent therapy with drugs used to treat bladder disorders could affect assessment of the effects of bPNS on bladder hypersensitivity. This study gives guidance to clinical trials using bPNS for the treatment of painful bladder syndromes and suggests potential clinical use of some of these medications in the treatment of these same disorders.
Introduction
Neuromodulation, in the form of peripheral nerve stimulation, has been utilized to treat many chronic pain disorders and has found increasing utilization for the treatment of painful bIadder syndromes1,2. An animal model utilizing one form of peripheral nerve stimulation, bilateral electrical pudendal nerve stimulation (bPNS), produces inhibition of nociceptive responses to urinary bladder distension (UBD) in rats made hypersensitive to bladder stimuli3. There are multiple anecdotal and case series reports in which the use of nerve stimulation resulted in improved pain control in people with the diagnosis of interstitial cystitis/bladder pain syndrome (IC/BPS)4–8. However, bPNS is not FDA-approved as a treatment for pain which suggests the need for a controlled clinical trial of this type of neuromodulation.
Recently, we reported, that the benzodiazepines diazepam and midazolam, administered systemically9, as well as numerous neurotransmitter antagonists administered intraspinally10 suppressed the inhibitory effects of bPNS. Concomitant medication use is common in patients with IC/BPS and it is likely that centrally-acting drugs could alter responses to therapeutic treatments such as bPNS. With the exception of our reports, cited above, there is almost a complete absence of relevant literature that has examined the role of concomitant medications on neuromodulatory effects. Therefore, a focused preclinical investigation into interactions between bPNS and medications used to treat bladder overactivity and/or pelvic pain symptoms appears warranted as their concomitant use could potentially alter clinical responses to the pain-relieving effects of this neuromodulatory manipulation.
In our previous studies in hypersensitive rats3, we identified optimal stimulation parameters and sites of electrical stimulation for the pudendal nerves (which arise from the same spinal segments as the lumbosacral nerves which contain afferents from the bladder). We studied these effects in a model of bladder hypersensitivity in which rats experience neonatal bladder inflammation and then receive a second bladder inflammatory challenge as adults11,12. It is thought that this model may be particularly relevant to IC/BPS13 since it is associated with multiple phenotypic features of IC/BPSe.g.14 and therefore is an appropriate model for study of the effects of medications and their potential interaction with neuromodulation. The aim of the present study was to use this model to test whether drugs commonly used to treat bladder and pelvic pain disorders altered responses to neuromodulation produced by bPNS.
Materials and Methods.
Animal subjects
All studies were approved by the UAB Institutional Animal Care and Utilization Committee. Subjects were adult, female Sprague-Dawley rats (n=81), raised from birth; the maternal animal source was Harlan Laboratories (Sprattville, AL). Rat pups, on three consecutive days (P14-P16), were anesthetized with inhaled isoflurane (1–2%), treated with 50 mg/kg s.c. ampicillin, their urethral orifices were swabbed with an iodine/povidone solution and they had their urethras cannulated using a 24 gauge angiocatheter. Intravesical zymosan (1% in saline, 0.1 ml) was then instilled for 30 minutes. They were kept warm on a heating pad and returned to their mothers following this treatment. As adults (12–15 weeks of age) these same rats received additional pretreatments one day before additional testing by being anesthetized with inhaled isoflurane (1–5%), treated with 50 mg/kg s.c. ampicillin, their urethral orifices were swabbed with an iodine/povidone solution, they had their urethras cannulated using a 22 gauge angiocatheter and intravesical zymosan (1% in saline, 0.5 ml) was again instilled for 30 minutes. They were allowed to recover from anesthesia and studied 24 hours later. Individual group sample numbers are described in tables and figure legends. There was no attempt to control for estrous cycle, as the focus of this study was not on estrous-related changes in pain, and we have previously shown that hormone fluctuations due to the estrous cycle do not alter the vigor of UBD-evoked responses when the current methodology is employed15.
Assessment of visceromotor responses (VMRs) to urinary bladder distension (UBD)
The abdominal muscle electromyographical (EMG) activity to UBD has been widely used to evaluate bladder pain-related responses16. These VMRs remain remarkably stable over time although a general drift upwards in EMG activity over the period of study has been noted, so similar to our previous studies3, bPNS treatments were performed in a randomized order of intensity so that ordering effects might be avoided. To measure VMRs, rats were maintained in an anesthetized state (initially 1–2% isoflurane in oxygen delivered by tight-fitting mask within a ventilation hood followed by urethane 1.2 gm/kg s.c. with subsequent reduction in isoflurane to <0.5%.) Silver wire electrodes were inserted into the left lower external oblique musculature immediately superior to the inguinal ligament. EMG activity was measured via these electrodes using standard differential amplification (Grass Inc., P511 AC amplifiers; 50× amplification, 60 Hz clipping, low filter setting 10 Hz–high filter setting 3 kHz) and digitally quantified VMR measures were saved on a computer (Cambridge Experimental Design, Spike 2 system). Electronic rectification of EMG voltage measures to all positive values allowed for calculation of a mean mV measure of the EMG activity during selected time periods such as before or during UBD as previously describede.g.3. Surgery for bPNS was performed as described below and a 22-gauge polytetrafluoroethylene angiocatheter was placed into the bladder via the urethra and held in place by a tight suture around the distal urethral orifice. Following surgery, isoflurane anesthesia was lowered until flexion reflexes were present in the hind limbs, but spontaneous escape behaviors were absent (approximately 0–0.5% isoflurane). UBDs for 20 seconds were produced using compressed air, and intravesical pressure was monitored using an in-line pressure transducer.
Bilateral Pudendal Nerve Stimulation (bPNS)
Using a posterior approach, the mixed motor/sensory pudendal nerves were exposed immediately after they exited the lumbosacral plexus as they started to pass anteriorly into the pelvis. Both pudendal nerves had double hook electrode assemblies placed around each nerve which were held in place with polysiloxane gel. Grounding electrodes were placed dorsally. Electrical stimuli consisted of trains of biphasic pulses (100 μsec; 10 Hz) delivered at 1× or 3× the motor threshold (1T or 3T respectively), the minimal current needed to evoke any observable muscle contraction. Stimulation intensities to each side were adjusted independently.
Protocol for Studying Effects of Acute Drug Administration on bPNS-Produced Inhibition of VMRs
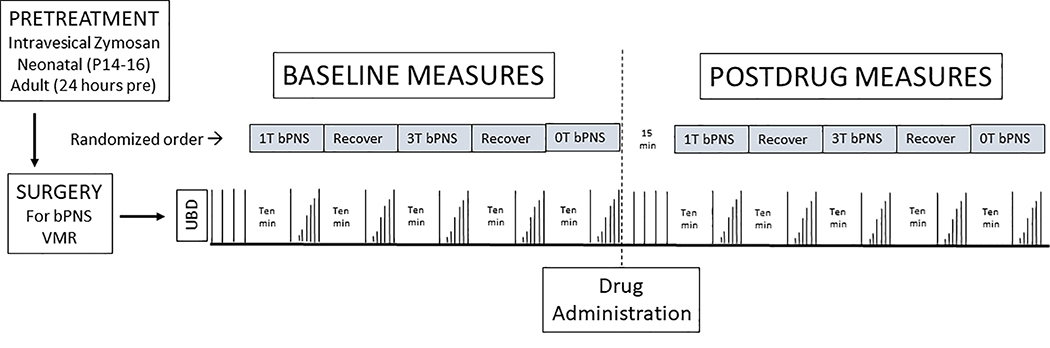
Rats treated early-in-life and as adults with intravesical zymosan were surgically prepared for measurement of VMRs and administration of bPNS as described above. Following surgery, 60 mm Hg UBDs were administered with an intertrial interval (ITI) of 3 minutes until responses were stable (+/− 20%). The VMRs to graded UBD (10–20-40–60 mm Hg, 20 s) were then obtained in succession. As previously reported3 the effect of No Stimulation, 1T bPNS and 3T bPNS were determined in randomized order using a 10 minute stimulation period and a 10 minute recovery period. After baseline effects of bPNS were measured, oxybutynin (1 mg/kg), mirabegron (1 mg/kg), CL316243 (3 mg/kg), tamsulocin (0.02 mg/kg), baclofen (2 mg/kg) or normal saline were injected in a volume of 1 ml/kg i.p. Doses of drug utilized represent those utilized in the literature for study of their effects on bladder function. After an additional 15 minutes, VMRs evoked by 60 mm Hg, 20 second UBD with 3 minute ITI were measured three times and then the effect of bPNS re-determined in a fashion identical to that done before the acute drug/saline dosing. Figure 1 gives a diagrammatic description of the primary study design. In the case of propofol, which has a high first pass clearance by the liver, a jugular venous line was placed and the drug infused at a rate of 100 μg/kg/min at the time of drug administration and the infusion continued throughout the period of testing.
Figure 1.
Diagrammatic description of study protocol. See text for details.
Protocol for Studying Effects of Amitriptyline on bPNS-Produced Inhibition
An alternative methodology for drug administration and measurement of effects was utilized in studies of amitriptyline since the clinical effects of that drug do not have onset until after two weeks of treatment. Adult rats which had been treated with zymosan early-in-life were given daily s.c. injections of vehicle or amitriptyline (30 mg/kg) in a volume of 1 ml/kg for 14 consecutive days. On the 13th day of chronic amitriptyline or saline dosing these same rats were anesthetized with isoflurane and treated with intravesical zymosan (1% in saline, 0.5 ml x 30 min) and ampicillin (50 mg/kg s.c.). The following day (Day 14), rats received a final 30 mg/kg s.c. injection of amitriptyline or vehicle three hours before being anesthetized and prepared surgically for the measurement of VMRs and administration of bPNS. Following surgery, 60 mm Hg UBDs were administered with an ITI of 3 minutes until responses were stable (+/− 20%). Then, VMRs to graded UBD (10–20-40–60 mm Hg, 20 seconds) were obtained in succession and then the effects of No Stimulation, 1T bPNS and 3T bPNS were determined in randomized order using a 10 minute stimulation period and a 10 minute recovery period.
Statistics and sample size calculations
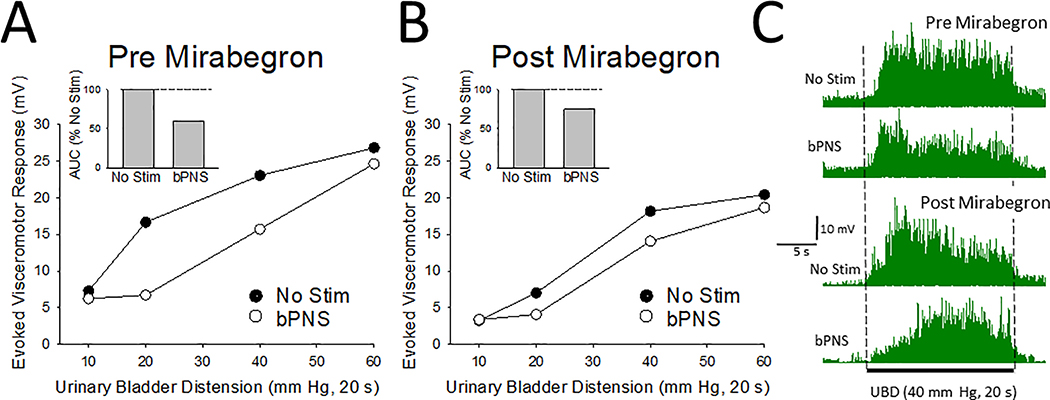
All data represents mean + SEM unless otherwise stated. Repeated measures one and two-way ANOVAs with Students-t tests used for post hoc evaluations were used to characterize responses to bPNS. SYSTAT 12 (SYSTAT Software Inc., San Jose, CA) was used for statistical analysis. In this study, the different VMRs obtained during the period of bPNS (or No Stimulation) were also compared in the same animal by calculating Areas-Under-the-Curve (AUCs) for responses to graded VMRs. This approach allows for an efficient use of animal resources since the baseline variability of the vigor of the VMRs between individual rats can be dissociated from the bPNS effect. Data related to the bPNS effect, although determined at two different intensities of stimulation (1T and 3T), were combined into a single bPNS statistic for each subject-condition since paired data from the present study, as well as three recently published studies9,10,17 failed to observe any statistically significant difference in the magnitude of inhibition produced by the two different stimulation intensities. Sample size calculations were performed using an online calculator (http://statulator.com/SampleSize/ss2PM.html) and data from rats in which the same technician had performed surgery and data collection9. That study demonstrated a 25% reduction in AUC by bPNS with a SD=16.4 and an n=64). It was determined that a sample size of n=7 was needed to achieve a power of 80% and a two-tailed level of significance of 5% using a paired t-test for analysis. An individual example of the response to drug treatment, in this case mirabegron, and the method of quantification are presented in Figure 2.
Figure 2.
Typical example from a single rat of bPNS and drug treatment effects as measured in the present study. In this example, visceromotor responses to graded urinary bladder distension (UBD, 10–60 mm Hg, 20 s) in the presence (bPNS) and absence (No Stim) of bilateral pudendal nerve stimulation (bPNS, see text for more description) were determined, before [panel A] and after [panel B] administration of mirabegron (1 mg/kg i.p.) as per the protocol described in Figure 1. Insets in [A] and [B] indicate Area-Under-the-Curve (AUC) values normalized to the No Stim measures for each set. Panel C presents rectified electromyograms of the external oblique musculature from the same rat before-during-after a 40 mm Hg, 20 s UBD stimulus in the absence (No Stim) and presence (bPNS) of neuromodulation before (top two tracings) and after (bottom two tracings) administration of mirabegron.
Results
Effects of acute drug administration on VMRs
Acute drug administration, in some cases, produced inhibition of VMRs to UBD when compared to saline administration when using AUC measures as endpoints. Data for all acutely administered drugs is presented in Table 1. Drugs that produced statistically significant reductions in baseline VMR responses in comparison with saline treatment include the β3-adrenoceptor agonists mirabegron and CL316243, the α1-adrenoceptor antagonist tamsulocin, the muscle relaxant baclofen, and the procedural sedative propofol. The muscarinic antagonist oxybutynin, at the dose employed, did not have statistically significant effects.
Table 1.
Effect of Drugs on Baseline VMRs to UBD
| DRUG (dose) | n | % AUC of No Stimulation condition measured pre-drug | Statistic |
|---|---|---|---|
| Normal Saline (1 ml/kg i.p.) | 12 | 120.6±8.7 | -- |
| Oxybutynin (1 mg/kg i.p.) | 8 | 108.0±9.2 | p=0.348 |
| Mirabegron (1 mg/kg i.p.) | 8 | 70.4±7.7* | p<0.001 |
| CL316243 (3 mg/kg i.p.) | 7 | 86.8±4.0* | p=0.011 |
| Tamsulocin (0.02 mg/kg i.p.) | 9 | 31.8±6.1* | p<0.001 |
| Baclofen (2 mg/kg i.p.) | 8 | 55.8±7.2* | p<0.001 |
| Propofol (100 μg/kg/min i.v.) | 7 | 68.9±11.1* | p=0.002 |
Data represents mean ± SEM
indicates statistically different from normal saline group; p value at right
Acute drug administration effects on inhibition produced by bPNS
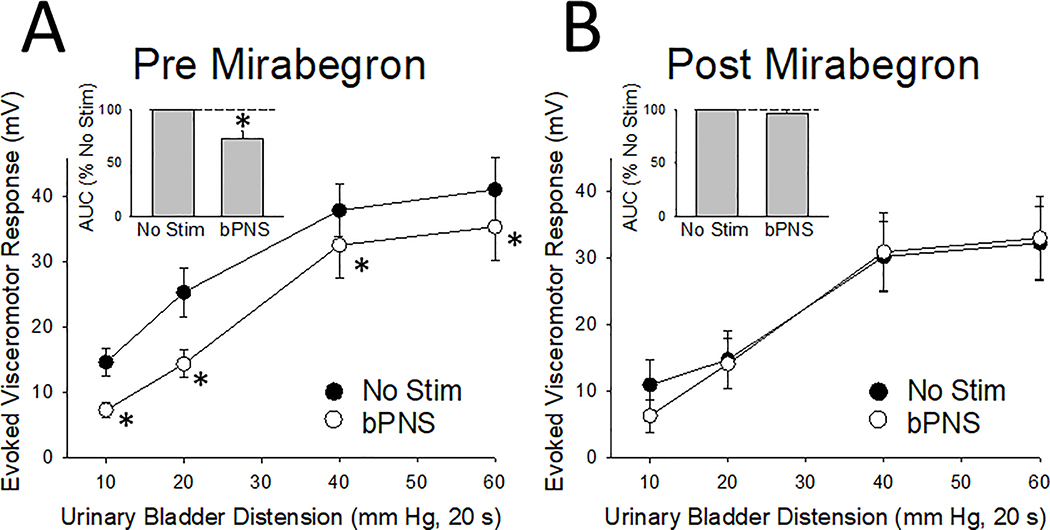
When using AUC assessments of effects, statistically significant inhibition of VMRs to UBD continued to be produced by bPNS following most acute drug treatments including oxybutynin, tamsulocin, baclofen and propofol. Results are summarized in Table 2. The exception to this was treatment with mirabegron which reduced the 27.2% inhibition of VMRs produced by bPNS (as measured by AUC calculations) to only a 3.4% inhibition. An individual example of the effect of mirabegron is given in Figure 2 and grouped data in Figure 3. A repeated measures, two-way ANOVA indicated an overall drug effect of mirabegron (F=7.68, p<0.01) and an interaction effect of the drug with the effects of bPNS (F=2.77, p=0.045). To examine for the role of the β3-adrenoceptor in this effect, the more specific agonist, CL316243, was also tested in this model and although it had inhibitory effects on baseline VMRs to UBD (as noted in Table 1), it did not reduce the bPNS-induced inhibition (as noted in Table 2).
Table 2.
Effect of Medications on Inhibition Produced by Bilateral Pudendal Nerve Stimulation (bPNS) as %Reduction from No Stimulation AUC data
| DRUG (dose) | n | % Effect bPNS Before Drug1 | % Effect bPNS After Drug1 | Statistic2 |
|---|---|---|---|---|
| Normal Saline (1 ml/kg) | 12 | 28.1±5.2* | 24.1±5.4* | p=0.730 |
| Oxybutynin (1 mg/kg) | 8 | 37.0±8.6* | 29.4±8.9* | p=0.5976 |
| Mirabegron (1 mg/kg) | 8 | 27.2±7.0* | 3.4±5.5 | p=0.007* |
| CL316243 (3 mg/kg) | 7 | 36.0±8.8* | 44.9±10.3* | p=0.2858 |
| Tamsulocin (0.02 mg/kg) | 9 | 37.1±8.7* | 38.1±9.8* | p=0.9866 |
| Baclofen (1 mg/kg) | 8 | 31.3±7.9* | 38.2±10.1* | p=0.8980 |
| Propofol (100 μg/kg/min i.v.) | 7 | 40.3±12.2* | 45.3±10.9* | p=0.6371 |
Data is presented as mean ± SEM
Data represents percentage reduction from No Stimulation measure in that data set
indicates significant effect of bPNS; p<0.05 – paired t-test comparison with No Stim measures
probability of difference from predrug % effect measure, paired t-test
Figure 3.
Grouped data related to effect of mirabegron on bilateral pudendal nerve stimulation (bPNS, see text for more description) effect in 8 rats. [A] Pre-drug responses to graded urinary bladder distension (UBD, 10–60 mm Hg, 20 s) in the presence (bPNS) and absence (No Stim) of bPNS. [B] Post-drug responses to graded UBD in the presence (bPNS) and absence (No Stim) of bPNS. Insets in [A] and [B] indicate Area-Under-the-Curve (AUC) values normalized to the No Stim measures for each set. Data represent mean + SEM. * indicates significant difference from the No Stim measurement with p<0.05.
Effects of chronic amitriptyline
The effects of 14 days of amitriptyline treatment on baseline VMRs was determined by comparing the overall vigor of the raw evoked EMG responses to UBD in amitriptyline-treated (n=11) versus saline-treated (n=11) rats on Day 14 of treatment. This was necessary since no baseline, pre-drug measures were obtained as in the other studies. The AUC for the SRFs of the VMRs to UBD demonstrated the vigor of the VMRs in amitriptyline-treated rats to be 929+125 mV*mm Hg units which was not statistically different from the 879+141 mV*mm Hg units measured in saline-treated rats. An overall ANOVA analysis including interactions comparing the two sets of data also demonstrated that the groups were not statistically different. An assessment of the effect of bPNS on these VMRs demonstrated a 23.6+5.6% decrease in the VMRs during bPNS versus No Stimulation measures in amitriptyline-treated rats and a 23.0+4.1% decrease in the VMRs during bPNS versus No Stimulation measures in saline-treated rats indicating there was no statistically significant effect of amitriptyline on bPNS-related inhibition.
Discussion
The most important finding of the present study is that many medications used to treat overactive bladders and/or pelvic pain did not abolish the inhibition of VMRs produced by bPNS in rats with bladder hypersensitivity. Two drug classes have been previously tested in a similar fashion: morphine, which had no effect on the bPNS-related inhibition17 and the benzodiazepines diazepam and midazolam which both abolished the bPNS-induced inhibitory effect9. That particular finding suggested that use of drugs such as benzodiazepines should therefore be discontinued prior to clinical use of bPNS for the treatment of bladder pain. The findings of the present study suggest that it might also be advisable to stop the medication mirabegron prior to any clinical trial of neuromodulation. It is not wholly clear what pharmacological effect of mirabegron may be interacting with the bPNS effects since the “cleaner” β3-adrenoceptor agonist, CL316243 did not appear to interfere with the bPNS effect. Mirabegon also has been noted to have α1-adrenoceptor antagonist effects in urological systems (via α1A and α1D receptors) that CL316243 does not have, however the α1-adrenoceptor antagonist tamsulocin, at the doses employed, did not suppress bPNS effects, but had profound inhibitory effects on VMRs to UBD. It is therefore likely that subtleties of receptor subtype action and central nervous system penetration may also explain differential effects of the different drugs. The pragmatic take-home message is that the present studies suggest that it would be best if mirabegon was not being utilized at the time of neuromodulation testing and potentially after formal implantation.
The present study also identified potential clinical utility for the treatment of bladder pain of several drug classes since baclofen (a GABAB agonist), C316243 and tamsulocin all attenuated the baseline VMRs to UBD independent of neuromodulatory interventions. In relation to potential clinical trials, an attenuation of baseline pain measures may make it more difficult for patients to ascertain the magnitude of bPNS effects by themselves, and so it may be prudent to avoid these drugs when determining efficacy of bPNS neuromodulation. That said, if a high intensity of clinical pain was still present in a potential research subject even in the presence of these medications, it would appear that a tapering of their use might not be necessary. The use of α1-adrenoceptor antagonists for analgesic purposes in pelvic pain syndromes related to urogenital structures is not a wholly new idea as both theye.g.19 and β3-adrenoceptor agonistse.g.20 have had clinical trials probing their potential urological benefits. The drug which we find particularly novel in relation to urogenital pain treatment is baclofen. There is evidence of analgesic effects of baclofen in the setting of postoperative pain (particularly in the presence of opioids)21 and preclinical as well as anectdotal evidence of efficacy in urogenital pain disorders22,23 so future studies of that drug as an analgesic treatment would appear warranted. Pudendal nerve stimulation, in preclinical studies, has also been demonstrated to have effects on endpoints other than bladder nociception, which was the focus of the present study. This includes effects on bladder capacity24 and improvement in the symptoms of stress incontinence25. One would expect that the medications studied here might also affect those endpoints, although in what direction would be pure conjecture.
The procedural sedative propofol was also tested in these studies since sedation is commonly used during “trials” of neuromodulatory interventions. Midazolam, although commonly used for periprocedural sedation, as noted above, has been previously been demonstrated to suppress the inhibitory effects of bPNS9. The present study would suggest that use of propofol would likely have minimal problems in this setting and so be of clinical utility for sedation during neuromodulatory trials of bPNS.
There are obvious limitations to this study’s interpretation since the data represents the responses of a non-human species in a lightly anesthetized state and for a very specific case of neuromodulation. Further, the doses of drugs used in these studies may be relevant for rats, but of uncertain effectiveness in humans. As a consequence, direct extrapolation to the human condition should be cautious. However, since avoiding particular medications, if done appropriately, is not life-threatening. It would seem prudent that use of some of these drugs (such as mirabegon) should be minimized when assessing neuromodulatory effects. Future studies should assess whether the present findings are unique to bPNS or more generalizable to other forms of neuromodulation such as spinal cord stimulation. Likewise, there may be a need to study additional drugs classes which may alter the effects of neuromodulation. Specific to the present study, it would also be desirable to determine whether the effects of an acute administration of mirabegron continue when administered chronically.
Conclusions
This study suggests that inhibitory effects of bPNS may be suppressed by medications such as mirabegron and so these drugs may need discontinuation prior to testing the clinical effectiveness of this neuromodulation. Most other drugs used to treat overactive bladders and/or pelvic pain leave the bPNS effect intact and so may not need discontinuation. It appears that some of these drugs may blunt primary nociceptive responses which, in turn indicates that future studies of these drug classes should be undertaken to assess their potential clinical utility in the treatment of bladder pain.
Acknowledgements
Supported by Medtronic, Inc. and by NIH-DK51314
FUNDING. This research was supported by Medtronic, Inc. and by the United States National Institutes of Health: DK51413
Footnotes
Site: This work was performed at the University of Alabama at Birmingham.
References
- 1.Ammirati E, Giammo A, Manassero A, Carone R. Neuromodulation in urology, state of the art. Urologia 2019; 86: 177–182. [DOI] [PubMed] [Google Scholar]
- 2.Mahran A, Baaklini G, Hassanai D, Abolella HA, Safwat AS, Neudecker M, Hijaz AK, Mahajan ST, Siegel SW and El-Nashar SA. Sacral neuromodulation treating chronic pelvic pain: a meta-analysis and systematic review of the literature. Int Urogynecol J 2019; 39: 1023–1035. [DOI] [PubMed] [Google Scholar]
- 3.Ness TJ, Randich A, Nelson DE and Su X. Screening and optimization of nerve targets and parameters reveals an inhibitory effect of pudendal stimulation on rat bladder hypersensitivity. Reg Anesth Pain Med. 2016; 41: 737–743. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Chen Y, Chen J, Zhang G and Wu P. Sacral neuromodulation for refractory bladder pain syndrome/interstitial cystitis: a global systemactic review and meta-analysis. Scientific Reports 2017; 7: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters KM, Killinger KA, Boguslawski BM and Boura JA. Chronic pudendal neuromodulation: expanding available treatment options for refractory urological symptoms. Neurourol Urodyn 2010; 29: 1267–1271. [DOI] [PubMed] [Google Scholar]
- 6.Siegel S and Kaula N. Pudendal nerve neuromodulation: a new option for refractory bladder overactivity and pain. Curr Bladder Dysfunct Rep 2010; 5: 102–106. [Google Scholar]
- 7.Srivastava D Efficacy of sacral neuromodulation in treating chronic pain related to painful bladder syndrome.interstitial cystitis in adults. J Anaesthesiol Clin Pharmacol. 2012; 28: 428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tirlapur SA, Vlimas A, Ball E and Khan KS. Nerve stimulation for chronic pelvic pain and bladder pain syndrome: a systematic review. Acta Obstet Gyn Scand 2013; 92: 881–887. [DOI] [PubMed] [Google Scholar]
- 9.Ness TJ, Clodfelder-Miller B, McNaught J, Miller DE and Su X. Benzodiazepines suppress neuromodulatory effects of pudendal nerve stimulation on rat bladder nociception. Anesth Analg, in press, 2019. PMID:31490256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ness TJ, DeWitte C, McNaught J, Clodfelder-Miller B and Su X. Spinal mechanisms of pudendal nerve stimulation-induced inhibition of bladder hypersensitivity in rats. Neurosci Lett 2018; 686: 181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Randich A, Uzzell TW, DeBerry JJ and Ness TJ. Neonatal urinary bladder inflammation produces adult bladder hypersensitivity. J Pain 2006; 7: 469–79. [DOI] [PubMed] [Google Scholar]
- 12.DeBerry J, Ness TJ, Robbins MT, Randich A. Inflammation-induced enhancement of the visceromotor reflex to urinary bladder distention: modulation by endogenous opioids and the effects of early-in-life experience with bladder inflammation. J Pain 2007; 8: 914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanno PM, Burks DA, Clemens JQ et al. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J.Urol. 2011; 185: 2162–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeBerry JJ, Randich A, Shaffer AD, Robbins MT and Ness TJ. Neonatal bladder iniflammation produces functional changes and alters neuropeptide content in bladders of adult female rats. J Pain 2010; 11: 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ball CL, Ness TJ, Randich A Opioid blockade and inflammation reveal estrous cycle effects on visceromotor reflexes evoked by bladder distention. J Urol 2010;184:1529–35. [DOI] [PubMed] [Google Scholar]
- 16.Su X, Riedel ES, Leon LA and Laping NJ. Pharmacological evaluation of pressor and visceromotor reflex responses to bladder distension. Neurourol Urodynam 2008; 27: 249–253. [DOI] [PubMed] [Google Scholar]
- 17.Ness TJ, Clodfelder-Miller B, McNaught J, Miller DE and Su X. Neuromodulatory Effects of Pudendal Nerve Stimulation on Bladder Hypersensitivity Are Present in Opioid-Pretreated Rats” Reg Anesth Pain Med 2019; 44: 1015–1020. [DOI] [PubMed] [Google Scholar]
- 18.Alexandre EC, Kiguti LR, Calmasini FB, Silva FH, da Sivla KP, Ferreira R, Ribeiro CA, Monica FZ, Pupo AS and Antunes E. Mirabegron relaxes urethral smooth muscle by a dual mechanism involving β3-adrenoceptor activation and α1-adrenoceptor blockade. Br J Pharmacol 2016; 173: 415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nickel JC, Touma N. α-Blockers for the Treatment of Chronic Prostatitis/Chronic Pelvic Pain Syndrome: An Update on Current Clinical Evidence. Rev Urol. 2012;14: 56–64. [PMC free article] [PubMed] [Google Scholar]
- 20.Di Lena M1, Tolls V1, Kelly KL1, Nickel JC. Mirabegron as adjuvant treatment for patients with interstitial cystitis/bladder pain syndrome. Can Urol Assoc J. 2018; 12:E100–E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon NC, Gear RW, Heller PH, Paul S, Miaskowski C, Levine JD. Enhancement of morphine analgesia by the GABAB agonist baclofen. Neuroscience 1995; 69:345–9. [DOI] [PubMed] [Google Scholar]
- 22.Abelli L, Conte B, Somme V, Maggi CA, Giuliani S and Meli A. A method for studying pain arising from the urinary bladder in conscious, free-moving rats. J Urol 1989; 141: 148–151. [DOI] [PubMed] [Google Scholar]
- 23.Chen CH, Hsiao SM, Chang TC, Wu WY and Lin HH. Clinical and urodynamic effects of baclofen in women with functional bladder outlet obstruction: preliminary report. J Obstet Gynaecol Res 2016; 42: 560–565. [DOI] [PubMed] [Google Scholar]
- 24.Gonzales EJ and Grill WM. Sensory pudendal nerve stimulation increases bladder capacity through sympathetic mechanisms in cyclophosphamide-induced cystitis rats. Neurourol Urodyn 2019; 38: 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang H-H, Song Q-X, Gill BC, Balog BM, Juarez R, Cruz Y and Damaser MS. Electrical stimulation of the pudendal nerve promotes neuroregeneration and functional recovery from stress urinary incontinence in a rat model. Am J. Physiol Renal Physiol 2018; 315: F1555–F1564. [DOI] [PMC free article] [PubMed] [Google Scholar]