Abstract
Previous research demonstrated that providing qualitative and quantitative information in a “drug facts box” may help individuals understand prescription drug information in print direct-to-consumer (DTC) advertisements. We sought to determine whether qualitative, quantitative, or a combination of the two best communicates benefit and risk information. To replicate and extend previous research, we used simple quantitative drug information. A randomized controlled study was conducted with 5,067 Internet panelists with heartburn. Participants viewed a drug facts box with benefit and risk information that varied the presence or absence of qualitative summaries and absolute frequencies, percentages, and absolute differences. Measures included knowledge of drug benefits and risks, perceptions, and intentions. Providing absolute frequencies and percentages most improved participants’ drug knowledge and affected perceptions and intentions. This suggests that, for simple drug information, adding absolute frequencies and percentages to DTC ads may benefit consumers. Absolute differences and qualitative labels may not be needed.
Keywords: direct-to-consumer, advertising, prescription drugs, risk communication, quantitative information
Introduction
Direct-to-consumer (DTC) ads for prescription drugs may improve patients’ decisions about prescription drugs or confuse patients trying to make these decisions. By law, ads for prescription drugs must provide information “in brief summary” about the advertised product’s “side effects, contraindications, and effectiveness” (1). This brief summary has traditionally been dense with information and difficult to read (2, 3). Recently, there have been calls to improve this information to enhance patients’ understanding of prescription drug information (2, 4–8). Specifically, some have advocated for a “drug facts box” similar to the one required for over-the-counter (OTC) medication. A drug facts box differs from a traditional brief summary in both formatting (a box with bullet points instead of paragraphs of text) and content (the addition of qualitative and quantitative information about benefits and risks).
Evidence suggests that the format in which information is presented influences individuals’ understanding. For instance, research on the OTC Drug Facts box (9, 10), nutrition facts labeling (11), and other formats (12) demonstrates that information presented with section headings, graphics, and other design elements is more easily read than information presented in paragraphs. A study of the brief summary found that a format modeled on the OTC Drug Facts box—with similar content as a traditional brief summary—was well-received by participants (2).
Adding content to the brief summary may also improve understanding. The benefits and risks of drugs can be described using words such as “rare,” or qualitative information. They may also be described using numbers such as 1%, or quantitative information. Research has shown that quantitative information in particular can be used to communicate to individuals about risks in general (13, 14) and prescription drug benefits specifically (15, 16). Several studies suggest that benefits and risks are better understood when presented quantitatively rather than qualitatively (17–19). One reason quantitative information may more accurately convey information about risks and benefits than qualitative information is that the meaning of qualitative labels (e.g., “rare”) varies widely between individuals and contexts (14, 20–22).
Combining format and content changes to the brief summary, Schwartz, Woloshin, and Welch (23, 24) designed a prescription drug facts box similar in format to both the Nutrition Facts panel found on most packaged food and the OTC Drug Facts box. This box contained qualitative (e.g., “fewer people… had heartburn”) and quantitative information (e.g., absolute frequencies, percentages, and absolute differences) about benefits and risks. Schwartz et al. (23, 24) demonstrated that individuals who were provided benefit information in a prescription drug facts box could use the information and were more likely to correctly choose the higher-efficacy product than were individuals who saw a traditional brief summary that used medical language and did not include qualitative or quantitative information.
Although these studies suggest that a drug facts box is more effective at communicating information than the traditional brief summary, it is unclear which elements of the drug facts box contribute to improved understanding. One study found that percentages outperformed natural frequencies and a combination of the two (25), suggesting that the drug facts box could be simplified. However, the drug facts boxes tested in that study all included qualitative information and absolute differences. Thus, it is not known whether qualitative labels, absolute differences, and absolute frequencies are all needed. Previous research has suggested that the simpler the presentation, the more likely more people will be to understand it (26). Determining what elements are critical to improving understanding in a document directed at potential patients is essential.
The current study addresses the communication of medical information to patients, adding to the literature by systematically examining the qualitative and quantitative elements used previously to determine whether and how to add qualitative and quantitative drug information to DTC ads. We developed hypotheses in the following five areas:
We first sought to replicate and extend past research by testing how a drug facts box with no quantitative information compared to the previously-tested box (which had both qualitative and quantitative elements) and various versions of the previously-tested box. We also predicted that, in the absence of quantitative efficacy or risk information, people may assume a high level of efficacy and a low level of risk.
Next we tested how a drug facts box with only a qualitative label compared to drug facts boxes with quantitative information, predicting that adding quantitative information would help participants understand the qualitative label.
To examine the utility of qualitative and quantitative information, we tested how different versions of the drug facts box with quantitative information compared to each other, specifically predicting that adding a qualitative label to absolute frequencies may help participants understand the information.
We predicted that participants given information about the drug’s efficacy or risk level would be able to use the information to form perceptions and intentions but that their knowledge of the quantitative information would not be affected by seeing a low or high efficacy (or risk) drug.
Because quantitative information may be less useful for individuals with low numeracy (27), we also measured this characteristic.
Materials and Methods
Design Overview
We had two separate designs: one that varied the level of benefit presented in the ad (low or high) while keeping the level of risk constant, and one that varied the level of risk presented in the ad (low or high) while keeping the level of benefit constant (Figure 1). Each design consisted of a 2 (level: low or high) by 4 (information type: numeric, numeric + qualitative, absolute difference + qualitative, or full) design plus two control conditions (no-information and qualitative only). This resulted in a total of 10 conditions per design.
Figure 1.
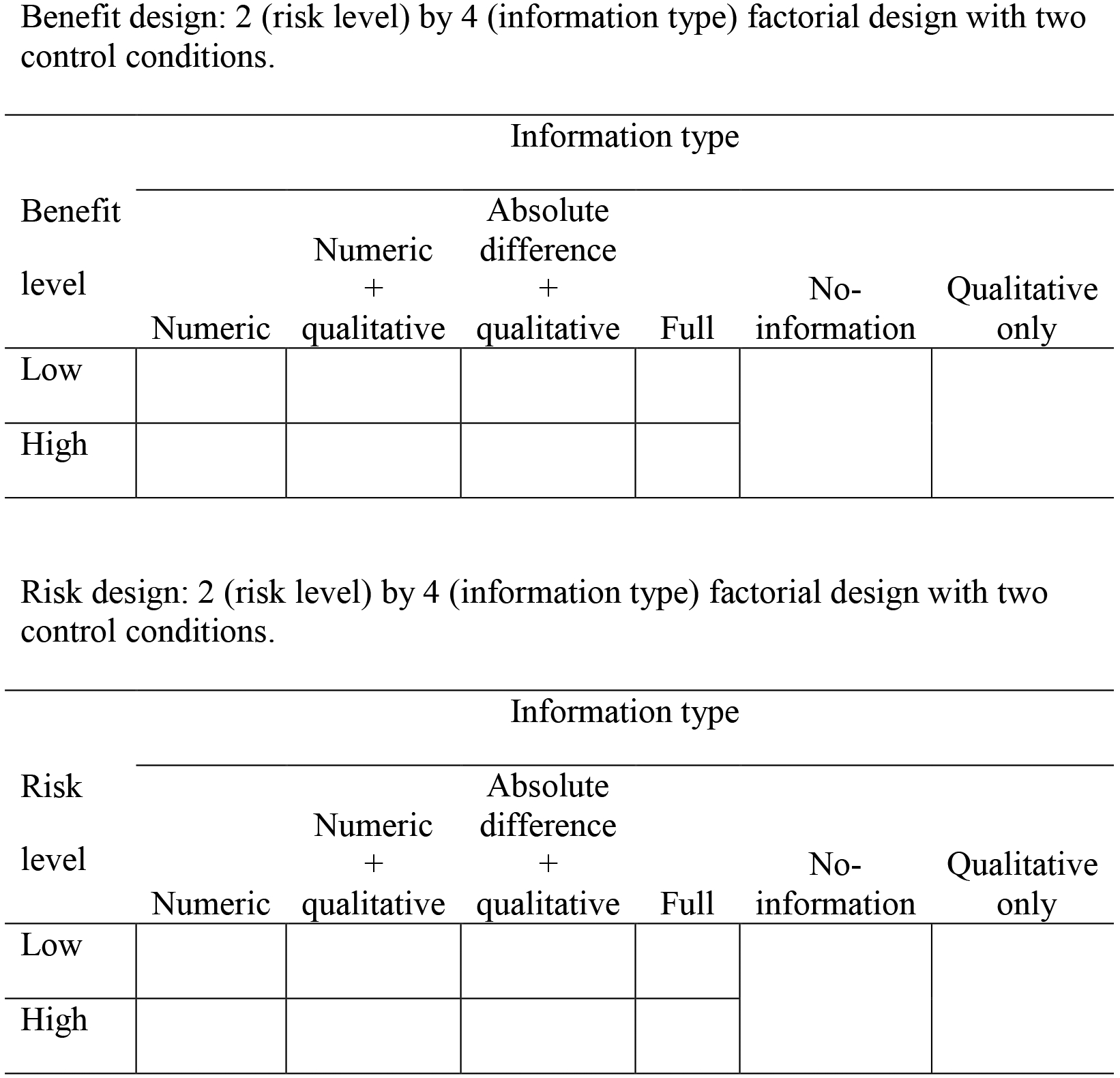
Factorial designs—(A) benefit and (B) risk—with 2 control conditions each (no information and qualitative only): 2 (benefit/risk level) × 4 (information type).
Participants
Online panelists (5,068, based on a priori power analyses) consented to and completed the study (Table 1). Eligibility included self-reported symptoms of heartburn or acid reflux in the prior 3 months; 18 or more years of age; ability to read, understand, and speak English; not working for a pharmaceutical, advertising, or market research company; and not being a healthcare professional. We used 2007 National Health Interview Survey data (28) to establish recruitment targets and stratify sampling.
Table 1.
Participant Characteristics
| Benefit design | Risk design | |
|---|---|---|
| Self-reported heartburn or acid reflux in past 3 months | 2,537 (100%) | 2,531 (100%) |
| Sex | ||
| Male | 1,053 (41.6%) | 1,077 (42.5%) |
| Female | 1,478 (58.4%) | 1,459 (57.5%) |
| Race/Ethnicity | ||
| White, non-Hispanic | 1,883 (74.2%) | 1,914 (75.6%) |
| Black, non-Hispanic | 203 (8.0%) | 191 (7.5%) |
| Other, non-Hispanic | 147 (5.8%) | 140 (5.5%) |
| Hispanic | 304 (12.0%) | 286 (11.3%) |
| Education | ||
| Less than high school | 32 (1.3%) | 25 (1.0%) |
| High school degree | 415 (16.4%) | 377 (14.9%) |
| Some college | 638 (25.2%) | 678 (26.8%) |
| Associate’s degree | 255 (10.1%) | 299 (11.8%) |
| Bachelor’s degree | 733 (28.9%) | 686 (27.1%) |
| Advanced degree | 463 (18.3%) | 466 (18.4%) |
| Age | ||
| 18–40 | 594 (23.4%) | 617 (24.4%) |
| 41–52 | 639 (25.2%) | 649 (25.6%) |
| 53–64 | 683 (26.9%) | 643 (25.4%) |
| 65+ | 621 (24.5%) | 622 (24.6%) |
| Taking prescription heartburn medication | 917 (36.1%) | 945 (37.3%) |
Procedure
Participants were randomized to view 1 of 20 DTC print ads containing a drug facts box for a fictitious prescription heartburn drug (Fentiva). Participants viewed the print ad and then answered a series of questions. They could return to the ad any time during the study.
All conditions featured a brief prose introduction entitled “Prescription Drug Facts: Fentiva.” In a box entitled “Fentiva Study Findings” underneath the introduction, we created six variations of information that appeared for both benefit and risk designs. We created a full condition (see Figure 2) in which all elements of the drug facts box tested previously (24) were present: absolute frequency and percentage, or numeric, information (e.g., “18% [180 in 1,000]”); absolute difference (e.g., “32 percentage points more”); and qualitative labeling (e.g., “more people had heartburn relief”). We then removed information from the full condition box to create five additional conditions: numeric, numeric + qualitative, absolute difference + qualitative, qualitative-only, and no-information. In the no-information condition, the box was deleted; participants saw only the prose introduction and the information below the box. All participants saw the same display page, which included the drug’s indication and side effects.
Figure. 2.
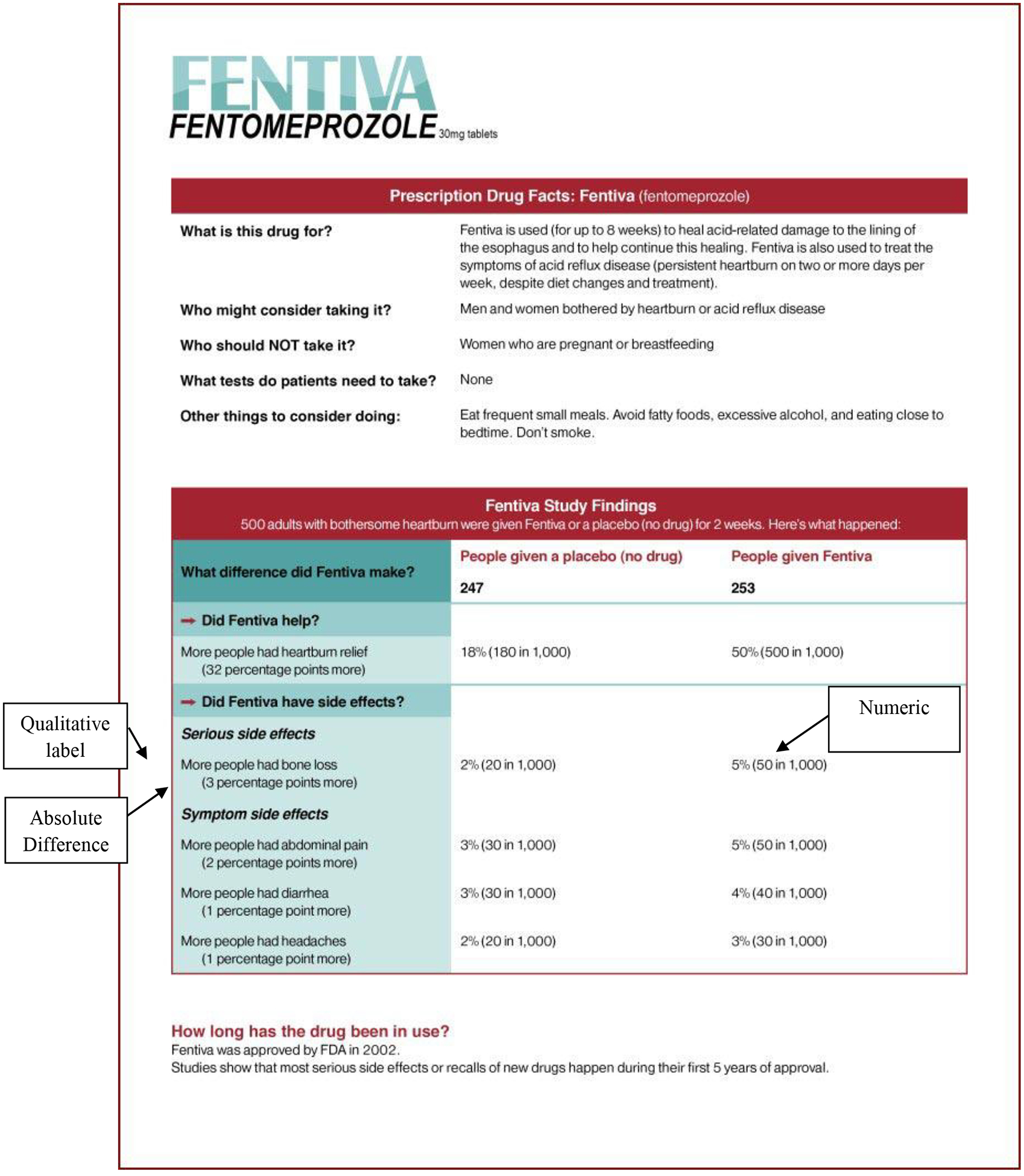
Example drug facts box used in the low risk/full condition.
Note. Call-out boxes are included in the figure to identify components of the drug facts box.
The benefit design contained two levels of drug benefit (low: 20% had heartburn relief, high: 85% had heartburn relief), with one constant level of drug risk (medium: 15% had bone loss). The risk design contained two levels of drug risk (low: 5% had bone loss, high: 20% had bone loss), with one constant level of drug benefit (medium: 50% had heartburn relief). The placebo rates were constant across conditions (18% had heartburn relief, 2% had bone loss). These numbers were selected based on pretesting in which general population participants were asked to estimate how many people would have heartburn relief or side effects at various levels of benefit or risk (data not shown). Because the qualitative-only and no-information conditions did not contain any quantitative information, they did not vary by benefit or risk level.
Although we modeled our design on the DTC print-ad format used previously (24), we made some changes based on cognitive interviews, public and peer reviewer comments on the study design, and recent research (25). These changes include: adding a serious risk and giving the drug and placebo different risk profiles, wording changes (e.g., “X% fewer” was changed to “X percentage points fewer,” and “a decrease in heartburn” was changed to “an increase in heartburn relief”), using a DTC rather than OTC display page, and using an indication statement similar to currently approved labeling for proton pump inhibitors.
Measures
We measured whether participants could accurately report how well the drug worked and what the drug’s serious risks were using three questions each: “What percentage (%) of people who took Fentiva had heartburn relief at the end of the 2 week study?” (drug benefit), “What percentage (%) of people who took a placebo had heartburn relief at the end of the 2 week study?” (placebo benefit), “Compared to taking placebo, Fentiva increased the chance of heartburn relief by [2, 12, 32, 57, 67, no effect] percentage points” (comparative benefit), “What percentage (%) of the people who took Fentiva had bone loss at the end of the 2 week study?” (drug risk), “What percentage (%) of people who took a placebo had bone loss at the end of the 2 week study?” (placebo risk), and “Compared to taking placebo, Fentiva increased the chance of bone loss by [3, 8, 13, 18, 23, no effect] percentage points” (comparative risk).
Drug benefit, placebo benefit, drug risk, and placebo risk were open-ended measures. Comparative benefit and comparative risk were closed-ended measures. We coded responses as correct or incorrect. To enable comparisons, we measured the number of participants in the no-information and qualitative-only conditions who guessed the correct answers from the small or large benefit (or risk) conditions.
We measured perceived benefit by averaging two items that assessed how likely it was that Fentiva would work and how effective it would be for them (1 = very unlikely/would eliminate very little of my heartburn, 7 = very likely/would eliminate all of my heartburn; α = .85 and .83 in the benefit and risk designs, respectively). We measured perceived risk by averaging two items that assessed how likely it was that participants would have side effects on Fentiva and how serious those side effects would be (1 = very unlikely/not at all serious, 7 = very likely/very serious; α = .80 and .79 in the benefit and risk designs, respectively).
We measured intention by averaging items that asked participants how likely they were to do three behaviors: “Talk to your doctor about Fentiva,” “Look for more information about Fentiva,” and “Take Fentiva if prescribed” (1 = not at all likely, 4 = extremely likely; Cronbach’s α = .82 in both designs).
We measured demographic variables including age, gender, race, ethnicity, and educational level. We also asked participants whether they were currently taking prescription medication for heartburn or acid reflux. We measured numeracy with three fill-in-the-blank math questions (24) and summed the number of correct responses.
Statistical Analysis
All analyses were conducted separately for the benefit and risk designs. We conducted 2 (benefit [risk] level) × 4 (information type) ANOVAs for continuous dependent variables and logistic regressions with forward selection for categorical dependent variables. For logistic regressions involving empty cells we added a delta (0.5) to all cells. We tested whether effects were moderated by numeracy. Significance was defined as p < .05 for main effects and interactions. We conducted pairwise comparisons to determine whether information types (including the no-information and qualitative-only conditions) significantly differed, using a Bonferroni-adjusted p < .003. We conducted analyses again with covariates (demographics and heartburn medication status) included. The pattern of results did not change; therefore, we present the results of the bivariate analyses.
Results
How did the no-information condition differ from the other information type conditions?
Benefit and risk designs.
Supporting past research, participants in the full condition were more likely to accurately answer all six of the quantitative knowledge questions than were participants in the no-information condition, ps < .001 (Table 2). Extending past research, participants in the numeric and numeric + qualitative conditions also were more likely to accurately answer all six of the quantitative knowledge questions than were participants in the no-information condition, ps < .001. Compared with participants in the no-information condition, participants in the absolute difference + qualitative condition were only more likely to accurately answer the comparative benefit and risk questions, and participants in the qualitative-only condition were only more likely to accurately answer the comparative benefit question, and in the risk design only, the drug benefit question, ps < .001.
Table 2.
Percentage of Participants who Gave the Correct Response to the Quantitative Knowledge Questions, by Information Type in Each Design
| Information Type | ||||||
|---|---|---|---|---|---|---|
| Quantitative Knowledge | No-information | Qualitative only | Numeric | Numeric + qualitative | Absolute difference + qualitative | Full |
| Benefit Design | ||||||
| Drug benefit | 0.4 | 2.0 | 85.8*♦^ | 87.5*♦^ | 0.6 | 81.0*♦^ |
| Placebo benefit | 0.0 | 0.0 | 75.7*♦^ | 76.8*♦^ | 0.0 | 73.9*♦^ |
| Comparative benefit | 23.4 | 39.9* | 77.9*♦ | 76.0*♦ | 83.3*♦ | 82.0*♦ |
| Drug risk | 0.8 | 0.4 | 78.7*♦^ | 77.6*♦^ | 0.2 | 72.5*♦^ |
| Placebo risk | 2.0 | 1.6 | 81.7*♦^ | 80.6*♦^ | 4.5 | 77.3*♦^ |
| Comparative risk | 10.9 | 9.1 | 77.5*♦ | 73.1*♦^ | 82.4*♦ | 79.4*♦ |
| Risk Design | ||||||
| Drug benefit | 3.2 | 17.8* | 89.3*♦^† | 86.8*♦^ | 3.1♦ | 82.7*♦^ |
| Placebo benefit | 0.0 | 0.0 | 81.6*♦^ | 81.5*♦^ | 1.0 | 80.1*♦^ |
| Comparative benefit | 9.1 | 16.6 | 78.8*♦ | 78.4*♦ | 84.7*♦ | 83.5*♦ |
| Drug risk | 4.0 | 4.9 | 80.0*♦^ | 78.0*♦^ | 1.0 | 76.4*♦^ |
| Placebo risk | 4.7 | 1.6 | 75.2*♦^ | 74.1*♦^ | 2.8 | 73.6*♦^ |
| Comparative risk | 23.3 | 47.8* | 86.3*♦ | 85.3*♦ | 91.2*♦ | 87.2*♦ |
Note.
Significantly different from the no-information conditions.
Significantly different from the qualitative-only conditions
Significantly different from the absolute difference + qualitative conditions.
Significantly different from the full conditions. Significance was defined as p < .003.
In the benefit design, participants in the no-information condition had significantly higher perceived benefit and higher intentions compared with all low-benefit conditions: numeric, numeric + qualitative, absolute difference + qualitative and full, ps < .001, Cohen’s fs (effect size) = .10–.23 (Table 3). Participants in the no-information condition also had significantly lower perceived risk than participants in the qualitative-only condition, p < .001, f = .12.
Table 3.
Mean (Standard Deviation) Perceptions and Intentions by Information Type and Benefit or Risk Level in Each Design
| Numeric | Numeric + Qualitative | Absolute Difference + Qualitative | Full | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No-information | Qualitative- Only | Low | High | Low | High | Low | High | Low | High | |
| Benefit Design | ||||||||||
| N | 256 | 253 | 255 | 252 | 250 | 255 | 255 | 255 | 256 | 250 |
| Perceived benefit | 5.43 (1.11) | 5.12 (1.23) | 4.32*♦ (1.51) | 5.54♦ (1.18) | 4.71*♦ (1.34) | 5.61♦ (1.05) | 4.91* (1.27) | 5.26 (1.12) | 4.47*♦ (1.58) | 5.56♦ (0.91) |
| Perceived risk | 4.13 (1.23) | 4.67* (1.30) | 4.42 (1.29) | 3.83♦ (1.50) | 4.34 (1.36) | 3.91♦ (1.39) | 4.29♦ (1.32) | 4.00♦ (1.40) | 4.25♦ (1.41) | 3.83♦ (1.35) |
| Intention | 2.32 (0.88) | 2.12 (0.90) | 1.77*♦ (0.80) | 2.33 (0.86) | 1.92* (0.84) | 2.26 (0.78) | 2.02* (0.92) | 2.17 (0.81) | 2.03* (0.92) | 2.35 (0.84) |
| Risk Design | ||||||||||
| N | 253 | 247 | 255 | 250 | 254 | 255 | 255 | 254 | 255 | 253 |
| Perceived benefit | 5.16 (1.25) | 4.94 (1.31) | 5.20 (1.11) | 5.28♦ (1.06) | 5.10 (1.17) | 5.27♦ (1.11) | 5.07 (1.21) | 5.08 (1.13) | 5.19 (1.10) | 5.10 (1.04) |
| Perceived risk | 3.71 (1.26) | 4.41* (1.35) | 3.37♦ (1.38) | 4.01♦ (1.28) | 3.61♦ (1.33) | 3.89♦ (1.25) | 3.55♦ (1.48) | 4.00♦ (1.32) | 3.42♦ (1.28) | 3.96♦ (1.39) |
| Intention | 2.46 (0.92) | 2.16* (0.85) | 2.44♦ (0.82) | 2.11* (0.79) | 2.32 (0.80) | 2.30 (0.84) | 2.29 (0.83) | 2.22* (0.82) | 2.31 (0.85) | 2.24* (0.86) |
Note. Low = low benefit or low risk. High = high benefit or high risk. The following scales were used: perceived benefit (1 = very unlikely/would eliminate very little of my heartburn, 7 = very likely/would eliminate all of my heartburn), perceived risk (1 = very unlikely/not at all serious, 7 = very likely/very serious), and intention (1 = not at all likely, 4 = extremely likely).
Significantly different from no-information conditions.
Significantly different from qualitative-only conditions
Significantly different from absolute difference + qualitative conditions.
Significantly different from full conditions. Significance was defined as p < .003.
In the risk design, participants in the no-information condition had higher intentions than participants in the qualitative-only condition, p < .001, f = .10, and most of the high-risk conditions: numeric, absolute difference + qualitative, and full, p ≤ .001, fs = .08–.12.
How did the qualitative-only condition differ from the quantitative information conditions?
Benefit and risk designs.
Participants in the numeric, numeric + qualitative, and full conditions were more likely to accurately answer all six of the quantitative knowledge questions than were participants in the qualitative-only condition, ps < .001 (Table 2). Compared with participants in the qualitative-only condition, participants in the absolute difference + qualitative condition were only more likely to accurately answer the comparative benefit and risk questions, and in the risk design only, were less likely to accurately answer the drug benefit question, ps < .001.
In the benefit design, participants in the qualitative-only condition had significantly higher perceived benefit compared with participants in most low-benefit conditions: numeric, numeric + qualitative, and full, ps < .001, fs = .09–.17. In contrast, they had significantly lower perceived benefit compared with most of the high-benefit conditions: numeric, numeric + qualitative, and full, ps < .001, fs = .11–.13. Participants in the qualitative-only condition had significantly higher perceived risk than participants in all high-benefit conditions: numeric, numeric + qualitative, absolute difference + qualitative, and full, ps < .001, fs = .14–.18. They also had significantly higher perceived risk than participants in two low-benefit conditions: absolute difference + qualitative and full, ps ≤ .001, fs = .08–.09. Finally, they had higher intentions than participants in the low-benefit/numeric condition, p < .001, f = .12.
In the risk design, participants in the qualitative-only condition had higher perceived risk than participants in all other conditions, p < .001, fs = .08–.22. In addition, they had lower perceived benefit than those in the high risk/numeric and high risk/numeric + qualitative conditions, ps = .001 fs = .03–.08, and lower intentions than participants in the low risk/numeric condition, p = .001, f = .10.
How did drug facts boxes with quantitative information differ from one another?
Benefit and risk designs.
Participants in the numeric, numeric + qualitative, and full conditions were more likely to accurately answer four of the quantitative knowledge questions (drug benefit, placebo benefit, drug risk, placebo risk) than were participants in the absolute difference + qualitative conditions, ps < .001 (Table 2).
In the benefit design, participants in the numeric + qualitative conditions were less likely to accurately answer the comparative risk question than were participants in the absolute difference + qualitative conditions, p < .001. In the risk design, participants in the numeric conditions were more likely to accurately answer the drug benefit question than were participants in the full conditions, p = .003.
Did participants distinguish between levels of benefit and risk?
Benefit design.
As expected, participants were able to use the quantitative information about drug benefits to form perceptions and intentions. There was a main effect for benefit level when predicting perceived benefit, p < .001, f = .35 and intention, p < .001, f = .20. Both of these main effects were qualified by an interaction with information type, p < .001, f = .45 for perceived benefit and p = .002, f = .08 for intention: the difference between the high- and low-benefit conditions was statistically significant for all quantitative conditions except the absolute difference + qualitative conditions, ps < .001, fs = .10–.17. In addition, participants in the high-benefit conditions had lower perceived risk than participants in the low-benefit conditions, p < .001, f = .56.
Unexpectedly, benefit level also influenced two of the quantitative knowledge measures. More participants in the low-benefit conditions (60.4%) correctly answered the placebo benefit knowledge question, compared with the high-benefit conditions (52.6%), p < .001. There was a significant effect of benefit level on comparative benefit knowledge, p = .01, which was qualified by an interaction with information type: for participants in the high-benefit conditions only, participants in the absolute difference + qualitative and full conditions were more likely to give the correct answer, compared with those in the numeric and numeric + qualitative conditions, p < .001.
Risk design.
As expected, participants were able to use the quantitative information about risk to form perceptions and intentions. Participants in the high-risk conditions had higher perceived risk than participants in the low-risk conditions, p < .001, f = 18. There was also a main effect of risk level when predicting intention, p < .001, f = .08, that was qualified by an interaction with information type, p = .01, f = .07: the difference between low-risk and high-risk conditions was only significantly different in the numeric condition, p < .001, f = .10.
Unexpectedly, risk level also influenced one of the quantitative knowledge measures. Participants in the low-risk conditions (90.1%) were more likely to correctly answer the comparative-risk knowledge question, compared with participants in the high-risk conditions (84.9%), p < .001.
What effect did numeracy have?
Benefit design.
As numeracy increased, the likelihood of correctly answering all the quantitative knowledge questions increased, p < .05, perceived risk decreased, p = .002, f = .09, and intentions decreased, p < .001, f = .10. There were also a few significant interactions. The relation between numeracy and correctly answering the placebo benefit knowledge question was not as strong in the high-benefit conditions, compared with the low-benefit conditions, p = .001. When predicting perceived benefit, the interaction between numeracy and benefit level was significant, p < .001, f = .10: the difference in perceived benefit between the low- and high-benefit conditions was greater for high numeracy participants (those who answered two or three of the three numeracy questions correct), fs = .10–.11, compared with low numeracy participants (those who answered none or one of the three numeracy questions correct), fs = .23–.24. The interaction between numeracy and information type was also significant for perceived benefit, p = .004, f = .11: in the absolute difference + qualitative conditions only, participants high in numeracy had lower perceived benefit than did participants low in numeracy, p = .002, f = .06.
Risk design.
As numeracy increased, the likelihood of correctly answering all the quantitative knowledge questions increased, p < .001, perceived risk decreased, p < .001, f = .13, and intentions decreased, p < .001, f = .12. There were also a few significant interactions. The relation between quantitative knowledge and numeracy was not as strong in the absolute difference + qualitative condition when compared with the numeric conditions (numeric, numeric + qualitative, full) for the comparative benefit knowledge question, p = .03, and the placebo risk-knowledge question, p < .001. When predicting perceived risk, the interaction between numeracy and risk level was significant, p < .001, f = .11: only high-numeracy participants (those who answered two or three of the three numeracy questions correct) had significantly higher perceived risk in the high-risk, compared with the low-risk conditions, ps < .001, fs = .10–.17.
Discussion
Past research showed that providing qualitative and quantitative information about benefits and risks in a drug facts box caused people to more accurately report information about a drug than when that information was not provided (24). In our study, the majority of participants who viewed quantitative information accurately reported it. Thus, including simple quantitative information about drug benefits and risks in DTC advertising may improve individuals’ decisions about prescription drugs. We extended the previous research by demonstrating that people who viewed a prescription drug ad for a fictitious heartburn drug were better able to accurately report quantitative benefit and risk information when they were provided with numerical information in the form of absolute frequencies and percentages, regardless of whether they also saw a qualitative label or an absolute difference. This finding is consistent with research suggesting that consumers process simple presentations of information successfully, and has implications for reducing the amount of information needed to create drug facts boxes. It also suggests that drug facts boxes can be simplified to avoid clutter and reduce word counts.
Although participants who viewed absolute frequencies and percentages were able to report both absolute frequencies (i.e., the drug and placebo knowledge questions) and the absolute difference (i.e., the comparative knowledge questions), those who saw the absolute difference without the absolute frequencies were not able to report absolute frequencies. This is similar to previous research that found participants given relative frequencies could report the relative frequencies but not the absolute frequencies (15). This suggests that absolute frequency and percentage information is the key to providing useful quantitative information. This is further highlighted by the finding that adding any combination of absolute difference or qualitative label did not increase or decrease the effect of absolute frequency and percentage information.
Our numeric condition included both percentage and absolute frequency information. Previous research (25) suggested that percentages alone are sufficient, but other research (15) found that there may be advantages to including both absolute frequencies and percentages. Future research should examine whether both absolute frequencies and percentages are necessary.
In general, participants were not only able to accurately report the quantitative information, but also used the information to form perceptions and intentions. For instance, adding absolute frequencies and percentages reduced perceived benefit when benefit was low and increased it when benefit was high, reflecting a greater understanding of the information. This finding was muted when only absolute differences were presented, indicating that absolute differences alone were not as effective. These findings suggest that including quantitative information in DTC print ads may improve individuals’ decision-making regarding prescription drugs.
Low-numeracy participants were less able to use the quantitative information, although the inclusion of quantitative information did not appear to negatively affect them. This suggests that adding information that may benefit higher-numeracy individuals will not harm lower-numeracy individuals. However, finding ways to communicate important information about prescription drugs to lower-numeracy individuals (such as including graphics [30]) should be a priority for future research.
Limitations
This study was limited to relatively simple quantitative information, but many prescription drugs contain complicated risk/benefit profiles with multiple nuances. For instance, drugs can be approved for multiple indications and approval can be based on multiple outcomes or multiple clinical trials. Clinical trial outcomes are often more complicated than simple frequencies. We did not examine these more complicated risk/benefit drug profiles so we cannot reach judgment about them. It is also important to note that this study did not address the feasibility of creating drug facts boxes. In addition, a more thorough analysis would examine all levels of benefit at all levels of risk (and vice versa) and would examine each element separately (e.g., presenting absolute difference information alone).
Finally, the sample was not nationally representative. We recruited individuals who suffered from heartburn to ensure that participants would have some interest in our fictitious ad. Future research should examine these issues with other prescription drugs, including those with more serious risks and more complicated benefits.
Conclusion
This study adds to a growing literature suggesting that medical benefits and risks are better understood when presented quantitatively rather than qualitatively (17–19). Extending previous research (24), we demonstrated what types of quantitative information are useful for patients, namely numerical information in the form of absolute frequencies and percentages. Adding absolute difference information and qualitative labels did not provide additional benefit. Thus, a simpler presentation of quantitative drug information may be useful for individuals making decisions about prescription drugs.
Footnotes
The study presented in this manuscript was provided an exemption from FDA’s Research Involving Human Subjects Committee.
References
- 1.Prescription Drug Advertisements, 21 C.F.R. Sect. 202.1 (2012).
- 2.Aikin KJ, O’Donoghue AC, Swasy JL, Sullivan HW. Randomized trial of risk information formats in direct-to-consumer prescription drug advertisements. Med Decis Making. 2011;31(6):e23–e33. [DOI] [PubMed] [Google Scholar]
- 3.Menon AM, Deshpande AD, Perri III M, Zinkan GM. Consumers’ attention to the brief summary in print direct-to-consumer advertisements: Perceived usefulness in patient-physician discussions. J Public Policy Mark. 2003;22(2):181–91. [Google Scholar]
- 4.Schwartz LM, Wolshin S. The drug facts box: Improving the communication of prescription drug information. Proc Natl Acad Sci. 2013;110(3):14069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woloshin S, Schwartz LM. Getting to better prescription drug information. J Gen Intern Med. 2012;27(12):1582–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhutada NS, Deshpande AD, Menon AM, Perri III M. Consumers’ evaluations of brief summary formats of print direct-to-consumer advertisements. Int J Pharm Health Care Mark. 2013;7(3):296–312. [Google Scholar]
- 7.Davis JJ. Riskier than we think? The relationship between risk statement completeness and perceptions of direct to consumer advertised prescription drugs. J Health Comm. 2000;5:349–69. [DOI] [PubMed] [Google Scholar]
- 8.Davis JJ. Consumers’ preference for the communication of risk information in drug advertising. Health Affair. 2007;26(3):863–70. [DOI] [PubMed] [Google Scholar]
- 9.Over-the-counter human drugs; labeling requirements. Federal Register. 1999;64:13254–303. Codified at 21 C.F.R. Section 201.66. [PubMed] [Google Scholar]
- 10.Vigilante WJ, Wogalter MS. The preferred order of over-the-counter (OTC) pharmaceutical label components. Drug Inf J. 1997;31:973–88. [Google Scholar]
- 11.Levy AS, Fein SB, Schucker RE. More effective nutrition label formats are not necessarily more preferred. J Am Diet Assoc. 1992;92:1230–4. [PubMed] [Google Scholar]
- 12.Lorch R, Lorch E. Effects of organizational signals on free recall of expository text. J Educ Psychol. 1996;88:38–48. [Google Scholar]
- 13.Fagerlin A, Ubel PA, Smith DM, Zikmund-Fisher BJ. Making numbers matter: Present and future research in risk communication. Am J Health Behav. 2007;31:S47–56. [DOI] [PubMed] [Google Scholar]
- 14.Lipkus I. Numeric, verbal, and visual formats of conveying health risks: Suggested best practices and future recommendations. Med Decis Making. 2007;27:697–713. [DOI] [PubMed] [Google Scholar]
- 15.O’Donoghue AC, Sullivan HW, Aikin KJ, Chowdhury D, Moultrie RR, Rupert DJ. Presenting efficacy information in direct-to-consumer prescription drug advertisements. Patient Educ Couns. 2014;95(2):271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woloshin S, Schwartz LM, Welch HG. The value of benefit data in direct-to-consumer drug ads. Health Aff. 2004;W4(Suppl):234–45. [DOI] [PubMed] [Google Scholar]
- 17.Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011;10:CD001431. [DOI] [PubMed] [Google Scholar]
- 18.Trevena LJ, Zikmund-Fisher BJ, Edwards A, et al. Presenting quantitative information about decision outcomes: A risk communication primer for patient decision aid developers. BMC Med Inform Decis Mak. 2013;13(Suppl 2):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West SL, Squiers LB, McCormack L, et al. Communicating quantitative risks and benefits in promotional prescription drug labeling or print advertising. Pharmacoepidemiol Drug Saf. 2013;22(5):447–58. [DOI] [PubMed] [Google Scholar]
- 20.Berry D, Raynor T, Knapp P, Bersellini E. Over the counter medicines and the need for immediate action: a further evaluation of European Commission recommended wordings for communicating risk. Patient Educ Couns. 2004;53:129–134. [DOI] [PubMed] [Google Scholar]
- 21.Theil M The role of translations of verbal into numerical probability expressions in risk management: A meta-analysis. J Risk Res. 2002;5:177–86. [Google Scholar]
- 22.Visschers VH, Meertens RM, Passchier WW, De Vries NN. Probability information in risk communication: a review of the research literature. Risk Anal. 2009;29:267–87. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz LM, Woloshin S, Welch HG. The drug facts box: Providing consumers with simple tabular data on drug benefit and harm. Med Decis Making. 2007;27:655–62. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz LM, Woloshin S, Welch HG. Using a drug facts box to communicate drug benefits and harms: Two randomized trials. Ann Intern Med. 2009;150:516–27. [DOI] [PubMed] [Google Scholar]
- 25.Woloshin S, Schwartz LM. Communicating data about the benefits and harms of treatment: A randomized trial. Ann Intern Med. 2011;155:87–96. [DOI] [PubMed] [Google Scholar]
- 26.Peters E Beyond comprehension: The role of numeracy in judgments and decision making. Curr Dir Pyschol Sci. 2012;21:31–5. [Google Scholar]
- 27.Wilson EAH, Wolf MS. Working memory and the design of health materials: A cognitive factors perspective. Patient Educ Couns. 2009;74:318–22. [DOI] [PubMed] [Google Scholar]
- 28.National Center for Health Statistics. Data File Documentation, National Health Interview Survey, 2007 (machine readable data file and documentation). Hyattsville, MD: National Center for Health Statistics, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 29.Schwartz L, Woloshin S, Black W, Welch HG. The role of numeracy in understanding the benefit of screening mammography. Ann Intern Med. 1997;127(11):966–72. [DOI] [PubMed] [Google Scholar]
- 30.Hawley ST, Zikmund-Fisher B, Ubel P, Jancovic A, Lucas T, Fagerlin A. The impact of the format of graphical presentation on health-related knowledge and treatment choices. Patient Educ Couns. 2008;73:448–55. [DOI] [PubMed] [Google Scholar]


