Abstract
Purpose of review
In multiple sclerosis (MS), currently approved disease-modifying treatments are effective in modulating peripheral immunity and, coherently, in reducing clinical/radiological relapses, but still, they perform poorly in preventing disease progression and overall disability accrual. This review provides an up-to-date overview of the neuropathology of progressive MS, including a summary of the main mechanisms of disease progression.
Recent findings
Clinical progression in MS is likely related to the accumulation of neuro-axonal loss in a lifelong inflammatory CNS environment (both adaptive and innate) and relative un-balance between damage, repair and brain functional reserve. A critical driver appears to be the T and B-cell mediated compartmentalized inflammation within the leptomeninges and within the parenchyma. Recent perspective highlighted also the role of the glial response to such lifelong inflammatory injury as the critical player for both pathological and clinical outcomes.
Summary
The neuropathological and biological understanding of disease progression in MS have progressed in the last few years. As a consequence, new therapeutic approaches are emerging outside the modulation of T-cell activity and/or the depletion of B-cells.
Introduction
Multiple sclerosis is a chronic inflammatory and neurodegenerative disease, which leads to focal lesions in the brain and spinal cord, characterized by primary demyelination with partial preservation of axons and glial scar formation.1 In most of the patients the disease starts with a relapsing course (RRMS), which transforms at later stages into progressive disease (SPMS). A minority of patients develop progressive disease from the onset (PPMS). Currently approved disease-modifying treatments are effective in modulating peripheral immunity and, coherently, in reducing clinical/radiological relapses, but still, they perform poorly in preventing disease progression and overall disability accrual. In this review, up-to-date main neuropathological features of progressive MS and mechanisms of disease progression are discussed.
Neuropathology of progressive MS
Due to the abundance of myelin in the white matter, demyelinated lesions are easily depicted in the white matter, but more recent studies have provided ample evidence that also the grey matter is affected,2, 3 and cortical lesions are a major substrate of clinical disability in particular in patients with progressive MS.4–6 At the initial stage of the disease, seen in patients with fulminant acute MS or in brain biopsies taken for diagnostic reasons, the pathology is dominated by active inflammatory demyelinating lesions arising with new waves of T- and B-lymphocytes, which enter the central nervous system (CNS) in association with profound leakage of the blood brain barrier7, 8 and these lesions are depicted in magnetic resonance imaging (MRI) by gadolinium-contrast enhancement.9, 10 Such new active lesions are most frequently seen in the white matter, but they can also be found in lower incidence in the grey matter, such as the cerebral cortex or the deep grey matter nuclei.3, 11, 12 With increasing age and disease duration the pathology gradually changes.
In progressive MS, new focal lesions with profound blood brain barrier damage become rare, while the majority of active lesions show a radial expansion with dense rims of microglia/macrophages and ongoing demyelination at the lesion edges (chronic active lesions) or a thin rim of activated microglia with only few myelin containing macrophages (smoldering or slowly expanding lesions, Figures 1–2).7, 8 Since activated microglia at the smoldering lesion edge ingest iron, such lesions can be depicted using susceptibility-based MRI sequences by an iron ring.13 Longitudinal MRI studies showed that the expansion of such lesions is a very slow process and it takes months to years to see fusion of adjacent iron ring lesions or their significant volume increase (Figure 2).14–16 Besides these chronic active lesions, the brain and spinal cord in patients with progressive MS contain numerous chronic inactive lesions and their number increases, but their size decreases with disease duration.15, 17 Remyelinated shadow plaques are seen on average in similar incidence in the brains of patients with relapsing and progressive disease7, 8 and their incidence varies profoundly between different cases.18, 19
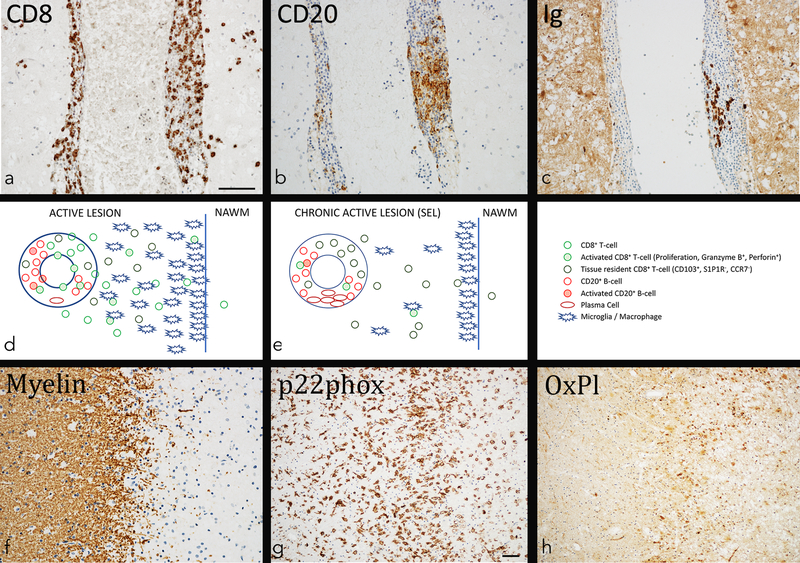
Figure 1. Inflammation and tissue injury in multiple sclerosis lesions.
a-c: Chronic active lesion in a 41-year old male patient with MS contains prominent inflammation, consistent of CD8+ T-cells (a), CD20+ B-cells (b) and immunoglobulin containing plasma cells (c). While CD8+ T-cells are present in the perivascular space and also diffusely infiltrate the lesion, infiltration by B-cells and plasma cells is largely restricted to the perivascular space.
d: Distribution and phenotype of inflammatory cells in newly formed active lesions. In newly formed lesions mainly seen in acute and relapsing MS new waves of T-cells and B-cells enter the brain and spinal cord and this is associated with profound blood brain barrier leakage. Many of the T- and B-cells are activated, but some of them already differentiate into tissue resident memory T-cells and plasma cells. Activated microglia and macrophages are seen throughout the entire lesion, but most numerous at the expanding edge.
e: Distribution and phenotype of inflammatory cells in chronic active (slowly expanding) lesions. Such lesions are mainly seen in the (late) relapsing and progressive stage of the disease. The inflammatory reaction is compartmentalized within the brain behind a (partly) closed or repaired blood brain barrier. The CD8+ T-cells dominate. Most of them are tissue resident memory cells in an inactive stage, but these cells show focally restricted activation. Activated T-cells are seen in close contact with B-cells and macrophages/microglia. Besides CD8+ T-cells many B-cells are present and a substantial number of them has differentiated into immunoglobulin containing plasma cells.
f-h: Zone of active demyelination at the edge in the same lesion, shown in figures a-c. Immunohistochemistry for proteolipid protein (myelin) reveals a sharp edge of demyelination with some granular myelin degradation products (f). The zone of active demyelination is heavily infiltrated by activated microglia cells and macrophages, expressing NADPH oxidase (p22phox) as a potential source for oxygen radicals (g). The zone of active demyelination shows precipitation of oxidized phospholipids in cells and cell processes as well as in the cytoplasmic granules of macrophages (h).
Magnification bar: 100 mm.
Figure 2. Longitudinal brain MRI features in progressive MS.
The case is a 60-year old man with primary progressive MS, EDSS 7.0 and 26 years of disease duration (treated with IFNß-1a between ages 52–57). Longitudinal MRI scans (proton density sequence) clearly show the expansion of supratentorial chronic white matter lesions (red arrows) as well as the progressive brain atrophy with lateral ventricle enlargement. The profiles of the ventricles at age 45 (red dashed line) and age 60 (blue dashed line) are superimposed to the last MRI scan to facilitate the comparison.
Another characteristic feature of the pathology of progressive MS is the abundance of cortical lesions and in particular of band like subpial lesions spanning over several adjacent gyri and sulci (Figures 3–4). In contrast to cortical lesions, in acute and early MS, T- and B-cell infiltrates are rare within active cortical lesions in progressive MS, but ongoing demyelinating activity is associated with meningeal inflammation.20 Active demyelination in the cortex occurs at sites of microglia activation. The extent of cortical demyelination varies between patients. On average it affects between 20 and 30% of the cortex,3 but it may reach up to 90% in extreme cases. Subpial cortical demyelination develops independently from white matter lesions and this may give rise to a “cortico-spinal” variant of MS, where in the brain extensive cortical demyelination is present with none or only some small focal white matter plaques.21
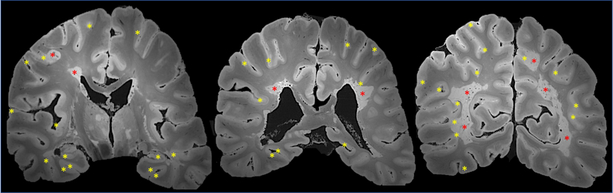
Figure 3. Demyelinated brain lesion distribution on postmortem MRI in progressive MS.
The case is a 59-year old man with progressive MS, EDSS 6.5 and 21 years of disease duration (treated with IFNß-1a between ages 49–51). Postmortem MRI scans (gradient echo sequence) of the formalin-fixed brain shows extensive cortical demyelination (yellow asterisks), especially in the depth of the sulci and the hippocampi, and confluent chronic active/slowly expanding lesions (periventricular and leukocortical; red asterisks). Some demyelinated lesions are seen also within the thalamus.
Figure 4.
Hemispheric section from the brain of a chronic progressive MS patient stained for myelin PLP. The absence of staining depicts demyelination and includes white matter lesions (arrows), subpial lesions (arrowheads) and leukocortical lesions (subcortical white matter and cortex, asterisks).
Finally, diffuse damage and atrophy of the normal appearing white and grey matter is extensive in the brain of patients with progressive disease. This diffuse white matter damage is in part due to secondary Wallerian degeneration as a result of axonal and neuronal damage in focal white and grey matter lesions. In addition, neuronal loss occurs in cortical lesions and in the normal appearing cortex22 and appears to contribute more to diffuse white matter degeneration compared to white matter lesions.3 In addition, however, factors independent from axonal and neuronal injury in focal lesions such as, for instance, meningeal inflammation may contribute to diffuse white matter injury.23
Pathological changes in the brain and spinal cord are very similar in patients with primary and secondary progressive disease.24 In particular, no significant differences are seen in the number and size of slowly expanding lesions or cortical lesions. In addition, the typical pathological changes of progressive MS are already present, although to a lower extent, in patients with relapsing disease. Thus, relapsing and progressive disease are unlikely to be driven by fundamentally different pathogenic mechanisms.
Mechanisms of progression
Clinical progression in MS is likely related to the accumulation of neuro-axonal loss in a lifelong inflammatory CNS environment (both adaptive and innate) and relative un-balance between damage, repair and brain functional reserve. A critical driver appears to be the compartmentalized inflammation within the leptomeninges and within the parenchyma. In the following paragraphs, a brief summary of the main mechanisms of progression are reported especially highlighting the most recent research trends and publications.
Compartmentalized persistent inflammation
Inflammation in progressive MS is more common than previously expected and compartmentalized within the CNS. The systematic pathological analysis of large cohorts of MS patients at different disease stages in several independent studies show that chronic active and/or smoldering/slowly expanding lesions are common in patients with progressive disease,7, 8 an observation which is also supported by recent MRI studies.14–16 In addition, inflammatory infiltrates, which are composed of CD8+ T-cells and B-cells, are mainly located in the leptomeninges and may form complex aggregates resembling features of tertiary lymph follicles.22, 25–27
This compartmentalized inflammatory process can in part be explained by the nature of the inflammatory response. CD8+ T-cells reveal a phenotype of tissue resident memory cells. Although detected in low numbers in the brain of controls without neurological disease,28 these cells are present in much higher numbers in active or chronic active MS lesions and show focally restricted activation (Figure 1).29, 30 These cells did not recognize the autoantigens, responsible for the induction of experimental autoimmune encephalomyelitis, but a fraction of them were found specific for Epstein Barr virus (EBV).29, 31 Whether they locally interact with EBV infected B-lymphocytes is still controversial.29, 32 Another recent study suggests that the antigen recognized by activated CD8 cells in MS lesions is mainly presented by mononuclear phagocytes.33 The other major lymphocyte population in the CNS of MS patients are B-cells.25 Within active or chronic active lesions they mainly express CD20, and show focal activation and proliferation (Figure 1), while in inactive lesions and at later disease stages a high numbers of plasma cells are found.30 These plasma cells are apparently the source for oligoclonal bands in the cerebrospinal fluid (CSF).
It is worth to note that active demyelination and neurodegeneration can occur at a distance from the T- and B-cell infiltrates. This is best exemplified in active subpial cortical lesions, where the lymphocytes are located in the meninges, while active demyelination takes place in the depth of the cortex and is associated with microglia activation. This might suggest that tissue damage is driven by a soluble factor, produced by T- and/or B-cells, which diffuses into the cortex and induces damage either directly or indirectly through microglia activation.20 Pro-inflammatory cytokines such as TNF-α and γ-interferon might play a critical role in this process, but subpial demyelination is specific for MS (and anti-MOG demyelinating disease), but not present in other chronic inflammatory diseases.34, 35 For this reason, the existence an MS specific soluble demyelinating factor, produced by B-cells has been suggested,36, 37 but the nature of this factor is so far unknown. The importance of soluble immune mediators is also highlighted by the significant association between high cortical lesion load and pro-inflammatory cytokines, fibrin, coagulation factors or iron related proteins in the CSF.38, 39
Although the inflammatory process in progressive MS is not associated with overt blood brain barrier damage, there is a moderate permeability increase in chronic active40 as well as inactive lesions and this leads to a perivascular accumulation of fibrin.41 Fibrinogen deposition may contribute to progressive neurodegeneration, since it may further activate microglia, induce demyelination, neurodegeneration and impair remyelination.42, 43 Inhibition of fibrinogen binding motif to CD11b (microglia) is under consideration as potential therapeutic target.43
Unbalance between damage, repair and functional reserve
Clinical progression in MS is also related to reduced repair potential with age and in a lifelong oxidative stress environment (due to the persistent inflammatory drive as described above, Figure 1). The failure in maintaining the tissue homeostasis is partly due to the reduction in neuronal expression of nuclear-encoded mitochondrial genes44 and the progressive accumulation of nuclear and/or mitochondrial DNA mutations and damage45 leading to cell death or to the upregulation of cellular senescence pathways in distinct CNS cell populations.46
In this context, it is critical the exhaustion of myelination capacity by both oligodendrocytes progenitor cells (OPCs) and residual oligodendrocytes. In recent human single-nucleus RNAseq MS studies, oligodendrocytes have been shown to be especially sensitive to oxidative stress with upregulation of genes associated with heat-shock and cellular stress responses and downregulation of genes involve in oligodendrocytes homeostasis and myelin synthesis.47, 48 In progressive MS, senescent OPCs have been found unable to fully differentiate into mature oligodendrocytes through HMGB1-mediated senescence transcriptomic changes.49 Using 14C methods to date and estimate the dynamics of oligodendrocytes generation in MS brain tissue, remyelination failure has been suggested to be due in part to the exhaustion of capacity of residual/survived oligodendrocytes to myelinate naked axons rather than to the OPCs differentiation impairment.50
The role of microglia-astrocyte axis in modulating inflammatory responses and in limiting the return to tissue homeostasis is gaining more and more attention. Part of the clinical heterogeneity seen in this disease might be related to specific gene variants involved in microglia/astrocytes functions and their specific responses to the autoimmune attack initiated by peripheral immune cells. To support this hypothesis, a recent human GWAS MS study identified disease susceptibility gene variants not only in genes involved in peripheral innate and adaptive immune response, but also in microglia activity, including C3 and CLECL1.51 Similarly, several risk genes of other neurodegenerative diseases, such as APOE-e4 in Alzheimer’s disease, are expressed by microglial cells.
In MS brain tissue, microglia downregulates homeostatic marker P2YR12 within active/chronic active lesions, but also in the normal-appearing white matter more frequently than in control brains.52 Similarly to other neurodegenerative diseases, under TREM2-APOE signaling, microglia acquires a neurodegenerative phenotype that is characterized by enhanced phagocytosis, antigen presentation, and oxidative damage.53 In this context, it has been hypothesized that microglia-specific checkpoint dysregulation might lead in MS to excessive microglia activation and reduced ability of microglia to return to homeostasis after inflammatory CNS insults propagating the tissue damage.54 Microglia can also modulate astrocytes functions toward a neurotoxic phenotype via secretion of VEFG-B.55 Although activated microglia upregulate the phagocytosis machinery, several evidences point toward an overall inefficient clearance with age of extracellular proteomes as well as of cellular and myelin debris.56, 57
Finally, potential dysregulation of cell death such as RIPK1-mediated necroptosis, a recently described type of inflammatory apoptosis induced by TNF-α, can further drive microglia activation as well as oligodendrocytes and axonal degeneration in MS.58 RIPK1 inhibition might contribute to limit cell death and neuroinflammation. For this reason, it is under consideration as potential therapeutic target in several neurodegenerative diseases,59 including amyotrophic lateral sclerosis60 and Alzheimer’s disease.61
Final pathways of neuro-axonal dysfunction and death
Neuro-axonal dysfunction and death are final pathways of tissue injury leading to a disconnection syndrome, that is clinically eloquent especially in the spinal cord.
1. Axonal dysfunction and degeneration of naked/chronically demyelinated axons
There are several interconnected explanations for the relentless dysfunction and degeneration of chronically demyelinated axons over the years after the acute wave of inflammatory demyelination at lesion onset. Overall, demyelinated axons are vulnerable due to the loss of the trophic support provided by the myelin sheath and oligodendrocytes. Demyelination increases the energy demand of nerve conduction. The demyelinated axon initially compensates for the increased need for ATP by increasing the volume of axonal mitochondria.62 With time, however, the function of axonal mitochondria are compromised by reductions in mitochondrial gene expression in neurons,44 mtDNA mutations 63 and oxidative stress (nitric oxide and free radicals released from activated microglia and iron accumulation).62
Myelination decreases the energy demands of nerve conduction by concentrating voltage gated Na+ channels in the nodal axolemma. Upon axonal firing, Na+ enters the axon at the node and is rapidly exchanged for extracellular K+ via the Na+K+-ATPase in an energy-dependent manner. This re-polarization of the axons is an essential aspect of neuronal function as rapid and repetitive axonal firing is often required for neural network function. Myelin, therefore, facilitates rapid nerve conduction and conserves energy. In demyelinated axons, Na+ channels are distributed along the entire axolemma. When Na influx is continuous along the axon, the need for Na+/K+ exchange and axoplasmic ATP is dramatically increased. Demyelinated axons become vulnerable when they cannot generate sufficient ATP to exchange axoplasmic Na+ for extracellular K+. This results in increased axoplasmic Na+ which is exchanged for extracellular Ca+ by the Na+/Ca+ exchanger in an energy-independent manner. Increased axoplasmic Ca+ is thought to play a pivotal role in axonal dysfunction and degeneration as it activates a cascade of proteolytic enzymes.
2. Synaptic pathology, neuronal dysfunction and death
Although cortical demyelination is a prominent feature in progressive MS cases, neuronal pathology is far more widespread than focal cortical demyelination, supporting the hypothesis that neuronal dysfunction might occur independently from inflammatory demyelination per se.64
The recent report of myelocortical MS provides further support to this hypothesis.21 Myelocortical MS patients have spinal cord and subpial cortical demyelination, but few if any cerebral white matter lesions. Despite the paucity of cerebral white matter lesions, cortical neuronal loss is significant.21 No correlation was found between cortical neuronal loss and cerebral white matter or cortical demyelination in these patients.21 These data support the concept that neurodegeneration and demyelination can be independent events in MS.
Other causes of neuronal injury/loss include retrograde neurodegeneration due to multifocal axonal injury and transection in the white matter, 64 as well as neuronal oxidative stress and mitochondrial dysfunction likely triggered by the compartmentalized leptomeningeal inflammation and related intracortical diffusion of inflammatory cytokines and toxic metabolites.38, 39 Oxidative stress can affect neurons directly or through neurodegenerative microglia and astrocytes’ inflammatory and cytotoxic mediators.64 In this context, excitatory CUX2 upper cortical neurons have been shown to be especially susceptible to meningeal-driven oxidative stress and death in comparison to adjacent neuronal subtypes.47
Recent studies trigger attention also on the possibility that neuronal connectivity in MS (as well as in other neurodegenerative diseases)65 can be dramatically impaired by enhanced and aberrant complement-mediated synaptic pruning by microglia and astrocytes.66–70 Resembling normal CNS development, less active synapses are tagged by early complement molecules, such as C1q and C3, and promptly removed by microglia. Of note, in a recent genome-wide association study, C3 gene variants have been associated to accelerated retinal thinning in MS.71 Therapeutic inhibition of the complement system is under consideration in several neurodegenerative diseases.72
Conclusions
Although the compartmentalized T and B-cell mediated inflammation likely remains the fundamental driver of clinical progression in MS, recent perspective highlighted the role of the CNS tissue response to such lifelong inflammatory injury as the critical player for both pathological and clinical outcomes. As a consequence, new therapeutic approaches are emerging outside the modulation of T-cell activity and/or the depletion of B-cells, and some of them include immunotherapy against fibrinogen, complement system and RIPK1 downstream effects.
Of relevance, some of the discussed mechanisms of progression are already active in the relapsing phase of the disease. Hence, the opportunity to apply new neuroprotective strategies before the progression is clinically evident should be considered.
Key bullet points.
Inflammation in progressive MS is more common than previously expected and compartmentalized within the CNS (within the leptomeninges and within parenchymal lesions).
Clinical progression is likely related to the accumulation of neuro-axonal loss in a lifelong inflammatory CNS environment and relative un-balance between damage, repair and brain functional reserve.
Recent perspective highlighted also the role of the glial response to such lifelong inflammatory injury as the critical player for both pathological and clinical outcomes.
Acknowledgments
We thank Dr. Daniel S. Reich and Prof. Peter Calabresi for the fruitful discussion with M.A. about some of the topics of this review. MR images have been collected at the Translational Neuroradiology Section (Dr. Reich’s lab), NINDS, NIH.
Financial support
Dr. Martina Absinta is currently supported by the Conrad N. Hilton Foundation (grant#17313). Dr. Trapp is supported by the National Institutes of Health (R35 099588 NINDS).
Footnotes
Conflicts of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Lassmann H, Bruck W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol 2007;17:210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peterson JW, Bo L, Mork S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol 2001;50:389–400. [DOI] [PubMed] [Google Scholar]
- 3.Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 2005;128:2705–2712. [DOI] [PubMed] [Google Scholar]
- 4.Calabrese M, Poretto V, Favaretto A, et al. Cortical lesion load associates with progression of disability in multiple sclerosis. Brain 2012;135:2952–2961. [DOI] [PubMed] [Google Scholar]
- 5.Harrison DM, Roy S, Oh J, et al. Association of Cortical Lesion Burden on 7-T Magnetic Resonance Imaging With Cognition and Disability in Multiple Sclerosis. JAMA Neurol 2015;72:1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magliozzi R, Reynolds R, Calabrese M. MRI of cortical lesions and its use in studying their role in MS pathogenesis and disease course. Brain Pathol 2018;28:735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frischer JM, Weigand SD, Guo Y, et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann Neurol 2015;78:710–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luchetti S, Fransen NL, van Eden CG, Ramaglia V, Mason M, Huitinga I. Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: a retrospective autopsy cohort analysis. Acta Neuropathol 2018;135:511–528.* This is a recent large neuropathological study of progressive MS cases highlighting the associations among clinical data and pathological lesion stage distribution. Chronic active/smoldering lesions are much more common in progressive MS than previously thought.
- 9.Frank JA, Stone LA, Smith ME, Albert PS, Maloni H, McFarland HF. Serial contrast-enhanced magnetic resonance imaging in patients with early relapsing-remitting multiple sclerosis: implications for treatment trials. Ann Neurol 1994;36 Suppl:S86–90. [DOI] [PubMed] [Google Scholar]
- 10.Gaitan MI, Shea CD, Evangelou IE, et al. Evolution of the blood-brain barrier in newly forming multiple sclerosis lesions. Ann Neurol 2011;70:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucchinetti CF, Popescu BF, Bunyan RF, et al. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med 2011;365:2188–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bevan RJ, Evans R, Griffiths L, et al. Meningeal inflammation and cortical demyelination in acute multiple sclerosis. Ann Neurol 2018;84:829–842. [DOI] [PubMed] [Google Scholar]
- 13.Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest 2016;126:2597–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol 2017;133:25–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Absinta M, Sati P, Masuzzo F, et al. Association of Chronic Active Multiple Sclerosis Lesions With Disability In Vivo. JAMA Neurol 2019.** This study shows that patients forming several chronic active lesions, seen on in vivo and postmortem MRI, are more likely to reach disability at younger age. Chronic active lesions are stable or expanding over 10 years due to smoldering inflammatory demyelination and axpnal degeneration at their edge, while inactive lesions overall shrink.
- 16.Elliott C, Belachew S, Wolinsky JS, et al. Chronic white matter lesion activity predicts clinical progression in primary progressive multiple sclerosis. Brain 2019;142:2787–2799.** In this re-analysis of the phase III trial, randomized, placebo-controlled, double-blind ORATORIO study conducted in patients with primary progressive MS, longitudinal MRI evaluation of slowly expanding lesions (volume and T1-intensity), but not brain atrophy were able to predict disability worsening at week 120.
- 17.Sethi V, Nair G, Absinta M, et al. Slowly eroding lesions in multiple sclerosis. Mult Scler 2017;23:464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patrikios P, Stadelmann C, Kutzelnigg A, et al. Remyelination is extensive in a subset of multiple sclerosis patients. Brain 2006;129:3165–3172. [DOI] [PubMed] [Google Scholar]
- 19.Patani R, Balaratnam M, Vora A, Reynolds R. Remyelination can be extensive in multiple sclerosis despite a long disease course. Neuropathol Appl Neurobiol 2007;33:277–287. [DOI] [PubMed] [Google Scholar]
- 20.Magliozzi R, Howell O, Vora A, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 2007;130:1089–1104. [DOI] [PubMed] [Google Scholar]
- 21.Trapp BD, Vignos M, Dudman J, et al. Cortical neuronal densities and cerebral white matter demyelination in multiple sclerosis: a retrospective study. Lancet Neurol 2018;17:870–884.** This pathological study describes a “myelocortical” variant of MS, characterized by extensive cortical and spinal cord demyelination with none or only few small focal white matter brain plaques. This suggests that cortical demyelination and neuronal loss can develop independently from white matter lesions demyelination.
- 22.Magliozzi R, Howell OW, Reeves C, et al. A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol 2010;68:477–493. [DOI] [PubMed] [Google Scholar]
- 23.Androdias G, Reynolds R, Chanal M, Ritleng C, Confavreux C, Nataf S. Meningeal T cells associate with diffuse axonal loss in multiple sclerosis spinal cords. Ann Neurol 2010;68:465–476. [DOI] [PubMed] [Google Scholar]
- 24.Lassmann H Pathogenic Mechanisms Associated With Different Clinical Courses of Multiple Sclerosis. Front Immunol 2018;9:3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol 2004;14:164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howell OW, Reeves CA, Nicholas R, et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain 2011;134:2755–2771. [DOI] [PubMed] [Google Scholar]
- 27.Choi SR, Howell OW, Carassiti D, et al. Meningeal inflammation plays a role in the pathology of primary progressive multiple sclerosis. Brain 2012;135:2925–2937. [DOI] [PubMed] [Google Scholar]
- 28.Smolders J, Heutinck KM, Fransen NL, et al. Tissue-resident memory T cells populate the human brain. Nat Commun 2018;9:4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Nierop GP, van Luijn MM, Michels SS, et al. Phenotypic and functional characterization of T cells in white matter lesions of multiple sclerosis patients. Acta Neuropathol 2017;134:383–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Machado-Santos J, Saji E, Troscher AR, et al. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain 2018;141:2066–2082.** In this pathological study, the compartmentalized lymphocytic inflammatory response within autopsy MS lesions is analyzed. The Authors found that it is dominated by CD20 B-cells (some of them plasmacells) and CD8 T-cells with features of cytotoxic and tissue-resident effector memory cells. The key property of these cells is that they remain at sites of previous inflammation and get re-activated, when they again encounter their antigen.
- 31.Veroni C, Serafini B, Rosicarelli B, Fagnani C, Aloisi F. Transcriptional profile and Epstein-Barr virus infection status of laser-cut immune infiltrates from the brain of patients with progressive multiple sclerosis. J Neuroinflammation 2018;15:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serafini B, Rosicarelli B, Veroni C, Mazzola GA, Aloisi F. Epstein-Barr virus-specific CD8 T cells selectively infiltrate the multiple sclerosis brain and interact locally with virus infected cells: clue for a virus-driven immunopathological mechanism. J Virol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konjevic Sabolek M, Held K, Beltran E, et al. Communication of CD8(+) T cells with mononuclear phagocytes in multiple sclerosis. Ann Clin Transl Neurol 2019;6:1151–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moll NM, Rietsch AM, Ransohoff AJ, et al. Cortical demyelination in PML and MS: Similarities and differences. Neurology 2008;70:336–343. [DOI] [PubMed] [Google Scholar]
- 35.Fischer MT, Wimmer I, Hoftberger R, et al. Disease-specific molecular events in cortical multiple sclerosis lesions. Brain 2013;136:1799–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lisak RP, Benjamins JA, Nedelkoska L, et al. Secretory products of multiple sclerosis B cells are cytotoxic to oligodendroglia in vitro. J Neuroimmunol 2012;246:85–95. [DOI] [PubMed] [Google Scholar]
- 37.Lisak RP, Nedelkoska L, Benjamins JA, et al. B cells from patients with multiple sclerosis induce cell death via apoptosis in neurons in vitro. J Neuroimmunol 2017;309:88–99. [DOI] [PubMed] [Google Scholar]
- 38.Magliozzi R, Howell OW, Nicholas R, et al. Inflammatory intrathecal profiles and cortical damage in multiple sclerosis. Ann Neurol 2018;83:739–755. [DOI] [PubMed] [Google Scholar]
- 39.Magliozzi R, Hametner S, Facchiano F, et al. Iron homeostasis, complement, and coagulation cascade as CSF signature of cortical lesions in early multiple sclerosis. Ann Clin Transl Neurol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee NJ, Ha SK, Sati P, et al. Spatiotemporal distribution of fibrinogen in marmoset and human inflammatory demyelination. Brain 2018;141:1637–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yates RL, Esiri MM, Palace J, Jacobs B, Perera R, DeLuca GC. Fibrin(ogen) and neurodegeneration in the progressive multiple sclerosis cortex. Ann Neurol 2017;82:259–270. [DOI] [PubMed] [Google Scholar]
- 42.Petersen MA, Ryu JK, Chang KJ, et al. Fibrinogen Activates BMP Signaling in Oligodendrocyte Progenitor Cells and Inhibits Remyelination after Vascular Damage. Neuron 2017;96:1003–1012 e1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryu JK, Rafalski VA, Meyer-Franke A, et al. Fibrin-targeting immunotherapy protects against neuroinflammation and neurodegeneration. Nat Immunol 2018;19:1212–1223.** This research groups did extensive work on the pathological role of fibrin/fibrinogen deposition in inflammatory and neurodegenerative CNS diseases over the years. In this paper, a monoclonal antibody against the fibrin binding motif to CD11b (microglia) is shown to effectively block fibrin-induced CNS inflammation without interfering with coagulation, potentially opening the avenue for testing fibrin-targeting immunotheraphy in human neurological diseases.
- 44.Dutta R, McDonough J, Yin X, et al. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol 2006;59:478–489. [DOI] [PubMed] [Google Scholar]
- 45.Mahad D, Ziabreva I, Lassmann H, Turnbull D. Mitochondrial defects in acute multiple sclerosis lesions. Brain 2008;131:1722–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker DJ, Petersen RC. Cellular senescence in brain aging and neurodegenerative diseases: evidence and perspectives. J Clin Invest 2018;128:1208–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schirmer L, Velmeshev D, Holmqvist S, et al. Neuronal vulnerability and multilineage diversity in multiple sclerosis. Nature 2019;573:75–82.* Using a single-nucleus RNA-seq approach, the transcriptome of different cell populations was simultaneously explored in autopsy MS brain tissue and compared to non-neurological control brains. The Authors found that the excitatory CUX2 upper cortical neurons are especially susceptible to meningeal-driven inflammation and oxidative stress.
- 48.Jakel S, Agirre E, Mendanha Falcao A, et al. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 2019;566:543–547.* Using a single-nucleus RNA-seq approach, in MS autopsy tissue, the Authors identified several clusters of oligodendrocytes, suggesting population heterogeneity and different functional states not only within MS lesions, but also in the MS normal-appearing white matter.
- 49.Nicaise AM, Wagstaff LJ, Willis CM, et al. Cellular senescence in progenitor cells contributes to diminished remyelination potential in progressive multiple sclerosis. Proc Natl Acad Sci U S A 2019;116:9030–9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yeung MSY, Djelloul M, Steiner E, et al. Dynamics of oligodendrocyte generation in multiple sclerosis. Nature 2019;566:538–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.International Multiple Sclerosis Genetics C. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science 2019;365.** This is the most recent human GWAS MS study conducted in 47,000 MS cases and 68,000 controls. It identifies more than 200 disease susceptibility gene variants not only in genes involved in peripheral innate and adaptive immune response, but also in the activity of brain-resident immune cells, such as microglia.
- 52.Zrzavy T, Hametner S, Wimmer I, Butovsky O, Weiner HL, Lassmann H. Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain 2017;140:1900–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krasemann S, Madore C, Cialic R, et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 2017;47:566–581 e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deczkowska A, Amit I, Schwartz M. Microglial immune checkpoint mechanisms. Nat Neurosci 2018;21:779–786. [DOI] [PubMed] [Google Scholar]
- 55.Rothhammer V, Borucki DM, Tjon EC, et al. Microglial control of astrocytes in response to microbial metabolites. Nature 2018;557:724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lampron A, Larochelle A, Laflamme N, et al. Inefficient clearance of myelin debris by microglia impairs remyelinating processes. J Exp Med 2015;212:481–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Safaiyan S, Kannaiyan N, Snaidero N, et al. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat Neurosci 2016;19:995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ofengeim D, Ito Y, Najafov A, et al. Activation of necroptosis in multiple sclerosis. Cell Rep 2015;10:1836–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan J, Amin P, Ofengeim D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat Rev Neurosci 2019;20:19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ito Y, Ofengeim D, Najafov A, et al. RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS. Science 2016;353:603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ofengeim D, Mazzitelli S, Ito Y, et al. RIPK1 mediates a disease-associated microglial response in Alzheimer’s disease. Proc Natl Acad Sci U S A 2017;114:E8788–E8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol 2015;14:183–193. [DOI] [PubMed] [Google Scholar]
- 63.Mahad DJ, Ziabreva I, Campbell G, et al. Mitochondrial changes within axons in multiple sclerosis. Brain 2009;132:1161–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haider L, Zrzavy T, Hametner S, et al. The topograpy of demyelination and neurodegeneration in the multiple sclerosis brain. Brain 2016;139:807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hong S, Beja-Glasser VF, Nfonoyim BM, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 2016;352:712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Michailidou I, Naessens DM, Hametner S, et al. Complement C3 on microglial clusters in multiple sclerosis occur in chronic but not acute disease: Implication for disease pathogenesis. Glia 2017;65:264–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Michailidou I, Willems JG, Kooi EJ, et al. Complement C1q-C3-associated synaptic changes in multiple sclerosis hippocampus. Ann Neurol 2015;77:1007–1026. [DOI] [PubMed] [Google Scholar]
- 68.Ramaglia V, Hughes TR, Donev RM, et al. C3-dependent mechanism of microglial priming relevant to multiple sclerosis. Proc Natl Acad Sci U S A 2012;109:965–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Watkins LM, Neal JW, Loveless S, et al. Complement is activated in progressive multiple sclerosis cortical grey matter lesions. J Neuroinflammation 2016;13:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Werneburg S JJ, Kunjamma RB, Ha SK, Luciano NJ, Willis CM, Gao G, Crocker SJ, Popko B, Reich DS, Schafer DP. Targeted complement inhibition at synapses prevents microglial synaptic engulfment and synapse loss in demyelinating disease. Immunity 2019, in press.* In this study, nonhuman primate and mouse MS models have been implemented to study synapse complement C3-tagging and pruning by microglia in demyelinated and non-demyelinated brain regions. In mice, complement C3-inhibition was directly able to obstacle synapses stripping from microglia.
- 71.Fitzgerald KC, Kim K, Smith MD, et al. Early complement genes are associated with visual system degeneration in multiple sclerosis. Brain 2019;142:2722–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carpanini SM, Torvell M, Morgan BP. Therapeutic Inhibition of the Complement System in Diseases of the Central Nervous System. Front Immunol 2019;10:362. [DOI] [PMC free article] [PubMed] [Google Scholar]