Abstract
Introduction
Smokeless tobacco (ST) use significantly affects morbidity and mortality and remains disproportionally prevalent in rural and medically underserved communities. Few programs exist for rural smokeless tobacco users. Text-based interventions may increase the reach of cessation interventions; yet, none has tested them in ST users. We evaluated the feasibility, acceptability, and preliminary efficacy of a text-based Scheduled Gradual Reduction (SGR) intervention in rural and underserved ST users.
Methods
ST users were randomized in 2:1 fashion to the SGR group (N=65), a text-based reduction program plus text-based support counseling messages or text-based support messages only group (N=33). We surveyed participants at 30-days post intervention initiation to assess feasibility and acceptability and examined self-report 7-day point prevalence cessation at 30-days and 6-months post intervention initiation in the two arms.
Results
We achieved benchmarks for feasibility and acceptability. Among the SGR participants 51% (n=48) reported that intervention was useful in helping them quit, 83% (n=48) indicated that they would recommend the intervention to a friend. Over 95% (n=39) of SGR participants said that they read all alert texts. The SGR participants had a higher quit rate at 30-days compared to support messages alone (SGR=21.5%, Control= 9.1%, p=0.1627, Cohen’s d equivalent=0.56, medium effect). However, the quit rate at 6-months was 21% (p=0.9703) for both groups.
Conclusions
A text-based intervention was feasible and acceptable among underserved ST users. SGR helped promote short-term cessation. The text-based interventions both had long-term efficacy. Given that text-based interventions have the potential to increase reach in underserved ST users, further testing is warranted.
Keywords: Smokeless Tobacco, Rural, Underserved, Tobacco Cessation
1. Introduction
Smokeless tobacco (ST) use remains prevalent in rural and medically underserved populations, leading to disparities in tobacco-related chronic disease. Unlike cigarette smoking that has been steadily decreasing, ST use has consistently increased since 2000.1,2 Rates of ST use are as high as 9% in some U.S. states, triple the population norm (3%).2 Further, among males, rates are as high as 17%. 2 ST users have a higher risk of developing oral cancer than non-users. Even though ST cessation significantly decreases morbidity and mortality, few can quit in part due to lack of programs. This is especially true for those in rural and medically underserved areas who have little access to effective interventions.
The most recent Cochrane review for ST cessation reports modest support for pharmacotherapy including the nicotine lozenge and behavior interventions including telephone support.3 However the quality of this evidence is low, and results have been mixed.3 Web-based cessation interventions for adult smokeless tobacco users have been modestly effective in reducing smokeless tobacco use with intent to treat quit rates around 13%.4 However, many underserved Americans lack consistent computer access; therefore, they are less likely to access existing web-based interventions. Text-based counseling interventions may be an effective way to increase the reach of interventions for ST users and have the potential to consistently reach many more underserved ST users compared to available clinic, quit-line, and web-based interventions.4,5–7 Text-based interventions have been effective in helping cigarette smokers quit;8 however, none has tested them in ST users. Scheduled Gradual Reduction (SGR) may be an efficacious addition to text-based counseling interventions in helping ST users quit as many ST users use reduction when quitting on their own.7,9 SGR is a reduction-based cessation method that first assesses ST user patterns, then gradually reduces the number of chews/dips per day by lengthening the time interval between use ending with a definitive quit date at the end of the reduction period.7,9,10,11 Chewing on a schedule has the important function of evenly spacing nicotine to manage withdrawal symptoms and not chewing in response to environmental or internal chewing cues or chewing ritualistically (at specific events – waking, eating etc.) throughout the day. SGR has been minimally studied among ST users however the available studies suggest that it promotes cessation.7,9 We are aware of two ST cessation studies that used LifeSign, a small computer designed to assist quitters with gradual reduction of ST using methods like SGR for reduction. Short-term abstinence rates ranged from 19%−25%.7,9 To our knowledge SGR has not been delivered via text message to ST users. Therefore, the purpose of this pilot study was to evaluate the feasibility, acceptability and preliminary efficacy of an SGR intervention via text messaging plus text-based support counseling messages by comparing it to a support messages program alone in decreasing ST use.
2. Materials and Methods
2.1. Design
This study was a pilot randomized controlled trial comparing a text-based SGR intervention plus support messages (SGR) to a text-based support message only. We surveyed participants at baseline, 30-days, and 6-months post intervention initiation. Interviews were conducted with a subset of participants (N=16) to obtain feedback on the intervention. This study received Duke University Institutional Review Board approval.
2.2. Participants
We recruited 116 ST users using Duke Primarily Care Clinics and social media recruitment. We determined Rural status by a Rural Urban Continuum Code (RUCC) of 4 or greater 12 and Medical Underserved Areas as designated by an Index of Medical Underservice (IMU) of 62 or less. 13 Inclusion criteria were: (1) 18 years of age and older; (2) used ST daily and chews/dips at least 3 times a day; (3) residential address in a rural county defined by a RUCC code of 4–10 or in medically underserved area defined as having an IMU of 62 or less; 4) own a cell phone with unlimited texting, and 5) interested in quitting. Among the 116 recruited, 98 were randomized to a treatment arm and analyzed. A 2:1 randomization with permuted block sizes of three was used to assign participants to the SGR (N=65) or support message only (N=33) arm.
2.3. Treatment Arms
Control Arm: Text-based support messages alone
Participants received support text messages over the 4-week study period and set a quit date within two weeks of the start of the intervention. The support messages were based on constructs of the Health Belief Model 14 and Social Cognitive Theory.15 We pilot tested messages with five rural ST users for content and readability. Participants received two messages a day during weeks 1 and 4 and up to three a day during weeks 2 and 3 when the quit attempt would occur. Messages were sent at various times throughout the day. Text messages were delivered by Mosio (https://www.mosio.com/), a two-way texting platform. Example messages are in Table 1.
Table 1.
Text Message Examples
| Message Topic | Example Message |
|---|---|
| Benefits of Quitting | Oral cancer kills one person every hour. Many are young and otherwise healthy chewers. Now is the time to quit. |
| Motivation | Quitting saves money. Write down the things you will do with the money you spend on chew. Put it somewhere to keep you on track. |
| Temping Situations | Your body knows when to expect to chew. If you fool it by changing your routine, you may get fewer cravings. |
SGR Arm: Text-based SGR intervention plus support messages
Participants received 4 weeks of support text messages as detailed above as well as the 4-week SGR intervention. During Week 1, we asked participants to chew as usual and text “s” every time they chewed. We calculated the mean number of chews per day. Then over the next three weeks, we reduced this number by 1/3 each week down to zero. 10,11 Those with a baseline mean of 10 or over were given the option of an extra reduction week. The last day of program was considered the quit date. Participants over the reduction period were instructed not to chew unless they received a text message telling them to do so. Participants were instructed to respond to the aforementioned text with “s” if they chewed and “ns” if they did not. We did not reduce length of chew and asked that participants only keep chew in for 30 minutes when they were alerted to chew.
2.4. Study Procedure
We recruited participants from clinics and via social media. We pulled from the Electronic Medical Record all ST users seen at three Duke Primary Care Clinics in the past 12 months and sent participants a letter describing the study and giving them a number to call to opt out. Staff called patients who did not opt out and screened them for eligibility. For Facebook recruitment, we created an advertising profile for the study and linked the advertisement to a secure Qualtrics survey where participants could enter their name and contact phone number. Participants were screened, randomized, provided baseline assessment, completed the intervention and then were contacted to complete surveys at 30-days and 6-months post intervention initiation. Those participants who reported complete abstinence from ST use were asked to complete a NicAlert test using saliva and return via mail. Participants received $50 for completing all surveys ($15 for baseline, $15 for 30-days, and $20 for 6-months). Participants were asked if they wanted to participate in an interview at the 30-days follow-up assessment to provide feedback on the intervention. Participants were recruited until saturation was achieved.
2.5. Study Measures
We determined feasibility by the proportion of support messages read per participant and the proportion of return text messages sent per SGR participant upon receiving a text message to chew at 30-days follow-up. A priori, the intervention was deemed feasible if most participants (over 50%) read the entirety of messages received and if participants in the SGR group responded to over 50% of the texts to chew.
To determine acceptability at 30-days, we asked how useful the intervention was and if they would recommend the intervention to a friend. A priori, the intervention was deemed acceptable if most participants (over 50%) found the intervention very or extremely useful and would be very or extremely likely to recommend it to a friend.
To evaluate efficacy, we measured self-reported 7-day point prevalence abstinence (quit rate) at 30-days and 6-months post intervention initiation. Among those who self-reported 7-day abstinence at 30 days, tobacco use status was also biochemically confirmed using NicAlert Semiquantitative test strips with a cut off of 10 ng/mL indicating exposure. The median percent change in number of chews per day per participant during the past 7 days was assessed as a secondary outcome for those that did not report quitting at 30-days.
2.6. Data Analysis
For this preliminary study, we performed non-directional statistical tests with the significance level set at 0.05 for each test. We used Chi-Square/Fisher’s Exact tests for categorical measures and non-parametric Wilcoxon Two-Sample Tests for continuous measures due to severe skewness of the data distribution. For the assessment of abstinence at 30-days and 6-months, intent-to-treat analyses were performed (those missing cessation outcomes were considered chewers or not quit). The intent-to-treat analyses included all 98 participants in the analysis sample, regardless of treatment or study compliance or completion. For other outcomes (feasibility and acceptability) we noted sample size in table and did not statistically adjust for missing data. Percent change in number of chews per day was analyzed among non-quitters for (a) baseline to 30-days and (b) baseline to 6-months post intervention initiation. Percent change was then categorized as a 50% or greater reduction in chews per day or not. To analyze the interview data, we conducted directed content analysis, which explores existing theories and increases the understanding of the subject matter.16 This type of content analysis is important when evaluating programs because the researcher can collect a broader range of thoughts and those opinions can be compared overall and used to improve a service or program.17 Data analysis of the open-ended question was conducted by the lead author to systematically identify and organize themes. Subthemes for broad categories were then identified through a visual inspection of the data.
3. Results
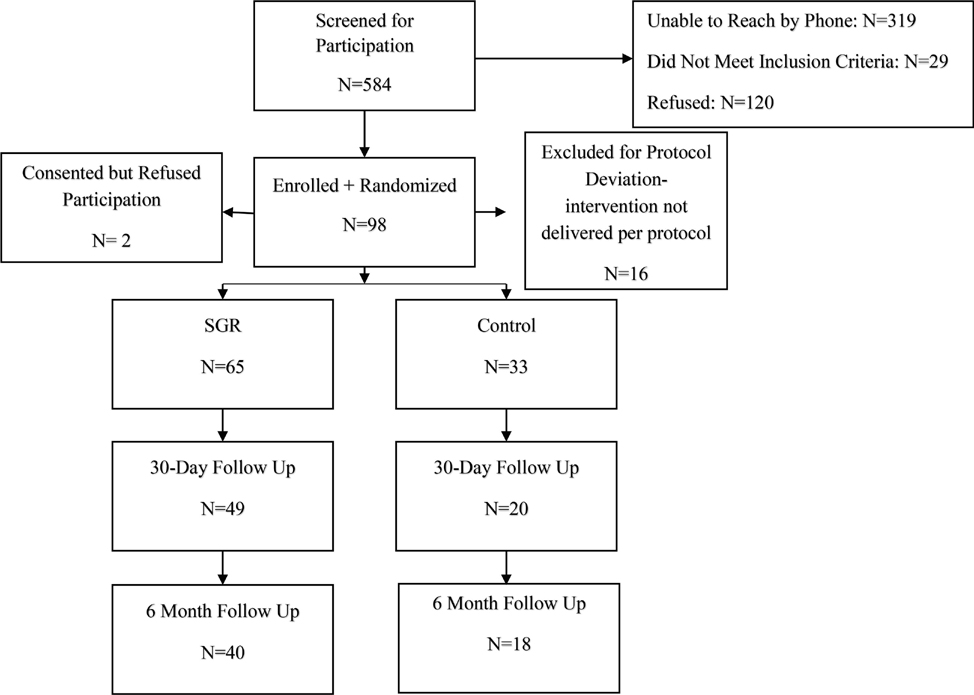
Flow of recruitment and follow-up is shown in Figure 1. We recruited 116 ST users over 19 months. Of those, 70 were recruited during the final 12-month period after we moved to a solely social media recruitment approach. In total 13% (n=13) of participants were recruited from Clinics, and the other 87% (n=85) were recruited from using online and/or social media modalities (Email, Facebook, Instagram). A total of 98 participants were randomized and analyzed (See Figure 1). Table 2 details the baseline characteristics as well as the feasibility, acceptability, and ST use outcomes for the total sample and by treatment arm. The median age of the participants was 31.0 (range: 19–60), 99% were male, 99% were White, and none of the ST users were Hispanic/Latino.
Figure 1:
CONSORT Diagram
Table 2.
Sample Demographic Characteristics and Study Outcomes
| Characteristic/Outcome | Total (N=98) | SGR (N=65) | Control (N=33) | p-value |
|---|---|---|---|---|
| Baseline sample characteristics | N=98 | N=65 | N=33 | |
| Age, in years | 31.0 (26.0, 45.0) | 30.0 (26.0, 43.0) | 33.0 (26.0, 47.0) | 0.4936 |
| Male gender | 89 (98.9%) | 58 (98.3%) | 31 (100.0%) | 1.0000 |
| Post-secondary education | 72 (75.0%) | 47 (74.6%) | 25 (75.8%) | 0.9013 |
| Caucasian | 86 (98.9%) | 55 (98.2%) | 31 (100.0% | 1.0000 |
| Household income > $1500/month | 78 (83.9%) | 51 (83.6%) | 27 (84.4%) | 0.9237 |
| Married/Living with partner | 61 (62.9%) | 39 (60.9%) | 22 (66.7%) | 0.5800 |
| Lives in a Rural County | 18 (22.5%) | 14 (26.9%) | 4 (14.2%) | 0.2657 |
| Number of chews per day | 10.0 (6.0, 12.0) | 10.0 (6.0, 12.0) | 10.0 (5.0, 14.0) | 0.8441 |
| Feasibility | N=53 | N=39 | N=14 | |
| Read support messages right away | 38 (71.7%) | 27 (69.2%) | 11 (78.6%) | 0.7316 |
| Read whole text message | 51 (96.2%) | 37 (97.4%) | 14 (93.3%) | 0.4898 |
| Acceptability: Very much/extremely | N=69 | N=48 | N=20 | |
| Recommend to a friend | 55 (80.9%) | 40 (83.3%) | 15 (75.0%) | 0.5033 |
| Usefulness of the intervention | 33 (47.8%) | 25 (51.0%) | 8 (40.0%) | 0.4057 |
| Tobacco use: Intention-to-treat | N=98 | N=65 | N=33 | |
| Quit rate at 30-days | 17 (17.4%) | 14 (21.5%) | 3 (9.1%) | 0.1627 |
| Quit rate at 6-months | 21 (21.4%) | 14 (21.5%) | 7 (21.2%) | 0.9703 |
| Tobacco use: Completers at 30 days | N=69 | N=49 | N=20 | |
| Completers’ quit rate | 17 (24.6%) | 14 (28.6%) | 3 (15.0%) | 0.3575 |
| 50% or greater reduction in daily chews among those who did not quit | 36 (78.3%) | 24 (77.4%) | 12 (80.0%) | 1.000 |
Continuous measure: Median (25th, 75th percentile) and Wilcoxon Two-Sample Test; Categorical measure: n (%) and chi-square/Fisher’s Exact Test.
We achieved feasibility. Over 96% of the participants reported that they typically read their text messages, and 72% indicated that they read their messages right away. The two groups did not differ on these measures. Among those in the SGR intervention, 95% indicated that they read all alert texts received and the median response rate per participant to alerts sent was 72% (25th, 75th percentile: 57.4, 92.7), while 64% said they either never or very few times chewed tobacco off schedule.
The acceptability assessment indicated that a higher percent of the SGR participants relative to support messages alone participants found the intervention useful in helping them quit smoking (51% versus 40%) and would recommend the intervention to a friend (83% versus 75%). The intervention groups, however, did not significantly differ on these acceptability measures (p>0.05).
The SGR intervention had a significantly higher self-reported quit rate at 30-days than the support messages alone. The intent-to treat quit rate at 30-days was 21.5% for the SGR arm compared to 9.1% for support message only arm (Fisher’s p=0.1627, Cohen’s d equivalent=0.56, medium effect). Biochemical validation proved not to be feasible in this text-based intervention as we had over 50% missing data who provided saliva samples.
Sixty-nine of the 98 participants completed the assessment at 30-days (SGR: N=49; Control: N=20). The quit rate for completers was 29% for SGR and 15% for support messages alone (Fisher’s p=0.3575). Although the support messages alone group had a higher attrition rate (39%) compared to the SGR intervention group (25%), the difference in attrition was not statistically significant (z= −1.52, p=0.1285). A sensitivity analysis indicated that completers at 30-days did not significantly differ from non-completers on demographic characteristics (age, education, income, and marital status). Among the completers who did not report a 7-day self-reported abstinence at 30-days, 77% in the SGR intervention compared to 80% in the support messages alone had a 50% or greater reduction in daily chews (Fisher’s p = 1.000).
The intention-to treat quit rate at 6-months was 21% for both intervention groups. Of the 98 randomized participants, 58 completed the 6-month assessment (SGR: N=40, Support message alone: N= 18). The attrition rate at 6-months was 39% for the SGR intervention and 46% for support messages alone (z=−0.6662, p=0.5028). Within the SGR intervention group, 75% of those who quit at 30-days also reported abstinence at 6-months.
Common themes from 16 interviews with SGR participants revealed that many thought the SGR program was too short and the short time frame made quitting completely difficult. SGR participants also commented that stopping their support messages at the same time their reduction schedule ended was like “dropping them off a cliff”. Participants suggested that extending the support messages past the end of their reduction schedule would be beneficial. SGR intervention participants stated that the SGR intervention helped them mange cravings, increase their confidence in quitting and not use smokeless tobacco when they were feeling the urge. Participants in the support messages alone group stated that the support messages that talked about health effects and money saved from quitting were most beneficial and suggested increasing the number of messages on these topics.
4. Discussion
This is the first study to establish feasibility and acceptability of a text-based SGR intervention among rural and medically underserved ST users. We found that the SGR intervention resulted in higher quit rates compared to the support messages alone intervention signaling that an SGR intervention may be an efficacious way to increase quit rates in ST users at least in the short term. Long-term outcomes did not show any difference between the SGR intervention and the support messages alone intervention. However, it is noteworthy that the quit rate stayed at 21.5% in the SGR intervention across the six months with 75% of those who quit at 30-days remaining quit at the 6-month follow-up. This suggests that the SGR intervention can sustain quit rates among those who do indeed quit. Using feedback from our qualitative interviews we can strengthen the SGR intervention for future research to increase short and long-term cessation rates. For example, the 3-week reduction period with the option of extending for a 4th week was too short. Many participants commented that the reduction schedule was too difficult and that they were just not able to quit in that short of time frame. Thus, future studies should consider increased reduction periods, especially for frequent ST users. SGR participants also commented that stopping their support messages at the same time their reduction schedule ended was to abrupt right after their quit day. Future studies should time the support messages much like traditional text-based programs (e.g. SMOKEFREE.TXT) where the support messages start two weeks before the quit date (e.g. last day of reduction) and continue for 4–6 weeks after the quit date.
It is interesting that the support messages alone group quit rate matched that of the intervention at six months. This could be due to a delayed intervention effect “as we did provide our control group with an active text-based cessation intervention” or from participants using other cessation methods. Given our small sample size, we still believe future reduction-based studies are warranted in smokeless tobacco users. Future studies should increase the number of assessments over the 6-months to determine salient cessation and/or relapse points to provide context for the current study results. This assessment could be easily captured over text-messaging.
From this feasibility study, we learned other valuable information that will inform future research. Recruitment of ST users from clinics proved difficult and was not feasible. After we moved to a social media recruitment, we improved our metrics favorably. Other large ST studies have relied on media-based recruitment with much success.3,4 We used primary care clinic EHR data to identify participants and sent them a letter about the study that was signed by their provider. This cold call form of clinic recruitment was not feasible but other modes of clinic recruitment such as having in office providers, nurses or medical assistants screen and recruit participants or using Mychart to recruit may be more successful.
Participants in the SGR group commented how the gradual reduction helped them to manage cravings and develop confidence that they could manage craving as they waited to chew based on their reduction schedule. Given SGR interventions are posited to help ST users develop coping skills to refrain from tobacco use cues, 9, 10, 11 this provides preliminary evidence that the intervention may be working as intended. Future studies should examine the mechanism of intervention effects in a larger trial.
We also saw about a 30% attrition rate of our sample at 30-days and 41% at 6-months. Moderate attrition rates have been reported in other low-touch ST use cessation trials.3,4 Comparing our completers to those who dropped out, we found no significant differences on key demographic and tobacco use behaviors. In future trials more engagement with participants over the follow-up period via texting and emails and mailed pre-incentives prior to surveys may be beneficial in retaining participants.18
Our attempt to biochemically confirm cessation was not feasible in this light-touch intervention with minimal participant contact. We attempted to have participants mail back a saliva Nic Alert test strip in a stamped return envelope, which resulted in over 50% missing. Given the current recommendation from the Society for Research on Nicotine and Tobacco (SRNT) Subcommittee to obtain biochemical verification,19 that most other ST trials rely on self-report data and given the lack of feasibility in our sample, alternate approaches to obtain biochemical confirmation of quitting should be reconsidered for future trials.
Limitations of this analysis include the small underpowered sample and high attrition rate. Our sample was predominantly male and white, limiting generalizability; however, this is consistent with both national and state prevalence rates.1,2 The reliance on self-report cessation data is also a limitation and may have inflated our actual quit rates. Further we did not objectively determine if participants chewed when they texted they did in the SGR group. We did ask participants if they chewed off schedule and 64% said they either never or only very few times chewed off schedule. Finally, we did not reduce duration of chew. Duration times vary widely from 5 minutes to 4 hours among ST users.7 This may have affected our cessation outcomes. We asked particpants to only keep chew in for 30 minutes during the SGR period but we were not able to objectively determine if they did so. Future approaches may consider texting a starting and stopping time to address this limitation.
Text-based interventions have the potential to reach many more underserved ST and decrease tobacco related morbidity and mortality in this vulnerable group of tobacco users. Our pilot work supports the feasibility and acceptability of this approach signaling the need for further testing of text-based cessation interventions for ST users in larger clinical trials with the goal of increasing access to cessation interventions in underserved communities.
Highlights.
A text-based Scheduled Gradual Reduction (SGR) smokeless tobacco (ST) cessation intervention was feasible and acceptable among rural and underserved ST users.
SGR helped promote short-term cessation and had long term efficacy, however there were no statistically significant differences in quit rates at six-month follow-up between the SGR intervention group and the support messages alone group.
This work expands the current literature on text-based approaches for smokeless tobacco cessation as the literature is limited compared to cigarette cessation interventions.
Enhancing the SGR intervention based on participant feedback is warranted to improve intervention efficacy.
Footnotes
Conflict of Interest: All authors declare no conflict of interests.
DECLERATION OF INTERESTS: None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control (CDC). Smokeless Tobacco Use in the United States. Accessed Dec. 2019 at https://www.cdc.gov/tobacco/data_statistics/fact_sheets/smokeless/use_us/index.htm https://www.cdc.gov/tobacco/data_statistics/fact_sheets/smokeless/use_us/index.htm
- 2.Centers for Disease Control (CDC). State-Specific Prevalence of Cigarette Smoking and Smokeless Tobacco Use Among Adults --- United States, 2009. Morbidity and Mortality Weekly Report (MMWR). 2010;59(43):1400–1406. [PubMed] [Google Scholar]
- 3.Ebbert JO, Elrashidi MY, Stead LF. Interventions for smokeless tobacco use cessation. Cochrane Database of Systematic Reviews. 2015(10). doi: 10.1002/14651858.CD004306.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Severson HH, Gordon JS, Danaher BG, Akers L. ChewFree.com: evaluation of a Web-based cessation program for smokeless tobacco users. Nicotine & Tobacco Research. 2008;10(2):381–391. doi. 10.1080/14622200802279872 [DOI] [PubMed] [Google Scholar]
- 5.Ebbert JO, Elrashidi MY, Stead LF. Interventions for smokeless tobacco use cessation. Cochrane Database of Systematic Reviews. 2015(10). doi: 10.1002/14651858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danaher BG, Severson HH, Crowley R, et al. Randomized controlled trial examining the adjunctive use of nicotine lozenges with MyLastDip: An eHealth smokeless tobacco cessation intervention. Internet Interventions. 2015;2(1):69–76. doi. 10.1016/j.invent.2014.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Severson HH, Akers L, Andrews JA, Lichtenstein E, Jerome A. Evaluating two self-help interventions for smokeless tobacco cessation. Addict Behav. 2000;25(3):465–470. doi. 10.1016/S0306-4603(99)00032-5 [DOI] [PubMed] [Google Scholar]
- 8.Whittaker R, McRobbie H, Bullen C, Rodgers A, Gu Y. Mobile phone-based interventions for smoking cessation. The Cochrane database of systematic reviews. 2016;4:Cd006611. doi: 10.1002/14651858.CD006611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jerome A, Fiero PL, Behar A. Computerized scheduled gradual reduction for smokeless tobacco cessation: development and preliminary evaluation of a self-help program. Computers in Human Behavior. 2000;16(5):493–505. DOI: 10.1016/S0747-5632(00)00024-8 [DOI] [Google Scholar]
- 10.Cinciripini PM, Lapitsky L, Seay S, Wallfisch A, Kitchens K, Van Vunakis H. The effects of smoking schedules on cessation outcome: can we improve on common methods of gradual and abrupt nicotine withdrawal? Journal of consulting and clinical psychology. 1995;63(3):388–399. doi. 10.1037/0022-006X.63.3.388 [DOI] [PubMed] [Google Scholar]
- 11.Cinciripini PM, Wetter DW, McClure JB. Scheduled reduced smoking: effects on smoking abstinence and potential mechanisms of action. Addict Behav. 1997;22(6):759–767. doi. 10.1016/S0306-4603(97)00061-0 [DOI] [PubMed] [Google Scholar]
- 12.U.S. Department of Agriculture, Economic Research Service. Urban Influence Codes. 2013; http://www.ers.usda.gov/data-products/urban-influence-codes.aspx
- 13.Health Resources and Service Administration (HRSA). Medically Underserved Areas and Populations. https://bhw.hrsa.gov/shortage-designation/muap Accessed January 11, 2016.
- 14.Rosenstock IM, Strecher V, Becker MH. Social learning theory and the Health Belief Model. Health Educ Q. 1988;15(2):175–183. [DOI] [PubMed] [Google Scholar]
- 15.Bandura A Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215. [DOI] [PubMed] [Google Scholar]
- 16.Hill CE, Knox S, Thompson BJ, Williams EN, Hess SA. Consensual qualitative research: An update. Journal of Counseling Psychology. 2005;52, 196–205. doi. 10.1037/0022-0167.52.2.196 [DOI] [Google Scholar]
- 17.Hsieh H, Shannon SE. Three approaches to qualitative content analysis. Qualitative Health Research. 2005;15:1277–1288. doi. 10.1177/1049732305276687 [DOI] [PubMed] [Google Scholar]
- 18.Watson NL, Mull KE, Heffner JL, McClure JB, Bricker JB. Participant Recruitment and Retention in Remote eHealth Intervention Trials: Methods and Lessons Learned from a Large Randomized Controlled Trial of Two Web-Based Smoking Interventions. J Med Internet Res. 2018;20(8):e10351. doi: 10.2196/10351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.SRNT Subcommitee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine & tobacco research. 2002;4(2):149–159. doi. 10.1080/14622200210123581 [DOI] [PubMed] [Google Scholar]