Abstract
Heparin-like substances (HLS) have been described in various clinical situations, including in settings of liver disease associated with infection, transplant, and metastasis. HLS are generally attributed to circulating glycosaminoglycans. Initial results for this patient showed coagulopathy due to liver disease without HLS. Two weeks after liver transplantation, a 10 year-old female with liver failure patient began to bleed from catheter insertion sites, mouth, and nares and HLS was suspected. The patient subsequently died and these clinical samples resulted in the isolation of a single heparan sulfate (HS) present at high concentrations in the plasma. Analysis of this HS showed it had an intermediate between heparin and HS with low antithrombin-mediated anticoagulant activity. We speculate that this 10-year old patient might have a platelet function defect influenced by this unusual HS. Endothelial defects not measurable by our methods might have also contributed to the observed bleeding complications.
Keywords: heparin-like substance, heparan sulfate, bleeding, liver failure
1. Introduction
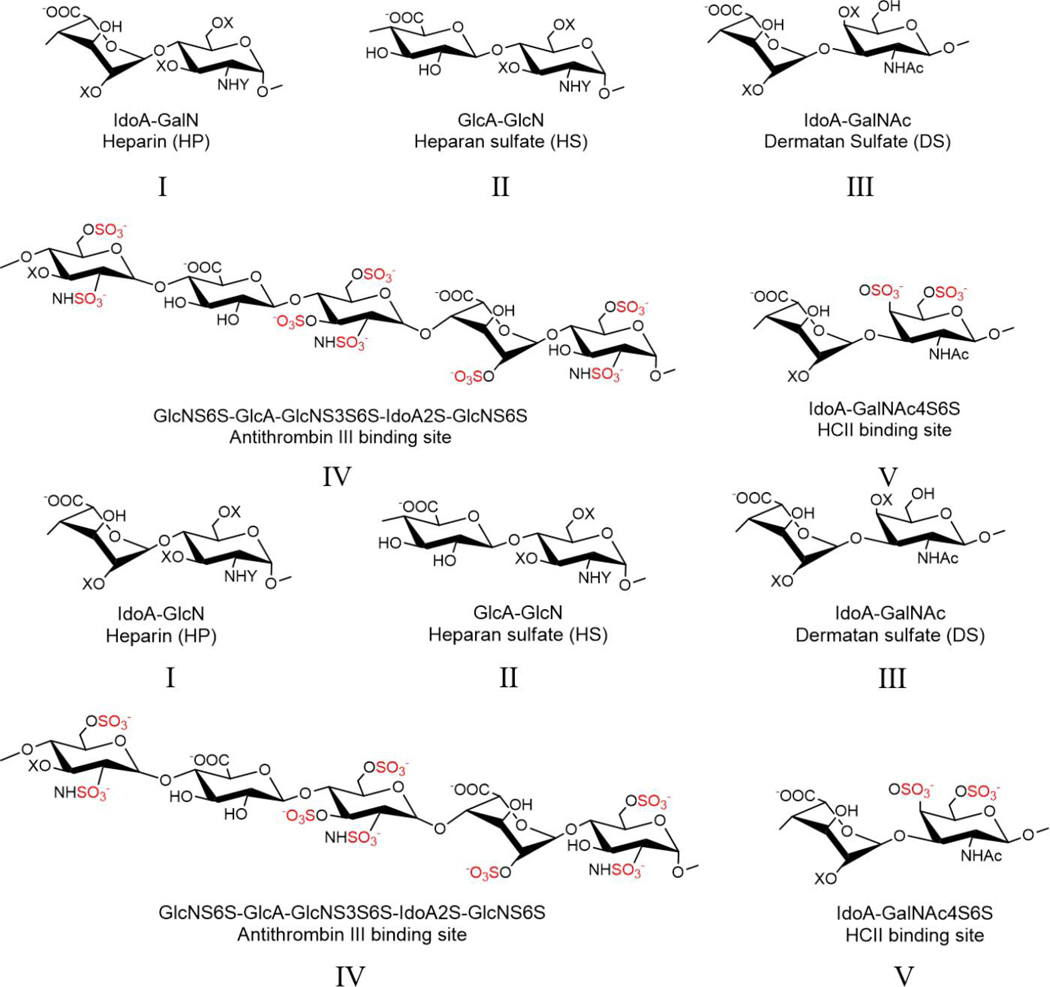
Glycosaminoglycans (GAGs) are polydisperse, microheterogeneous (with sequence variability), linear, heterocopolysaccharides synthesized in the Golgi of animal cells (DeAngelis, 2012; Zhang, Lin, Huang, & Linhardt, 2020). Dermatan sulfate (DS) (also known as chondroitin sulfate B) primarily consists of disaccharide repeating units of →4)α-L-iduronic acid (IdoA) (1→3) α-D-4-O-sulfo N-acetylgalactosamine (GalNAc4S) (1→ (Lin et al., 2020; Linhardt & Heilman, 1995; Trowbridge & Gallo, 2002). DS with additional sulfation at position-6 of the galactosamine residue (GalNAc4S6S) comprises the binding site for heparin cofactor II (HCII) (Linhardt et al., 1991; Maimone & Tollefsen, 1990), responsible for antithrombin (AT)-independent anti-factor IIa activity (Bara, Mardiguian, & Samama, 1990; Linhardt & Heilman, 1995). Heparin (HP) and heparan sulfate (HS) are closely related GAGs consisting of alternating uronic acid (IdoA or β-D-glucuronic acid (GlcA)) (1→4) glycosidically linked to glucosamine (α-D-N-sulfoglucosamine (GlcNS) or α-D-N-acetylglucosamine (GlcNAc)) disaccharide repeating units (Linhardt, 2003). The glucosamine residues are often sulfated at position-6 (GlcNS(Ac)6S) and iduronic acid is often sulfated at position-2 (IdoA2S). Additionally, one or two glucosamine residues in each HP chain can be sulfated at position-3 (GlcNS(Ac)6S3S) found in the center of small group of pentasaccharide sequences (Chang et al., 2014; Chen et al., 2017) responsible for heparins antithrombin (AT)-mediated anticoagulant activity (Onishi, St. Ange, Dordick, & Linhardt, 2016). In general, HP is more highly modified than HS in its Golgi-based biosynthesis (Esko & Selleck, 2002).
Heparin-like substances (HLS) have been described in various clinical situations, including in settings of liver disease associated with infection, transplant, and metastasis (Fahl, Poon, Badani, & Benson, 2009; Premkumar et al., 2020; Senzolo et al., 2009). HLS are generally attributed to circulating GAGs, primarily HS and DS (Figure 1). The suggested mechanisms leading to the appearance of HLS in the circulation include: 1. decreased clearance of circulating GAGs during acute liver failure; 2. neutrophil-mediated injury of the hepatocytes that can release HS; and 3. the direct release of GAGs from the endothelial surface and mast cells during an acute phase response (Sobczak, Pitt, & Stewart, 2018). The best approach to therapeutic management in the setting of HLS associated with bleeding is unclear.
Figure 1.
Structures of dermatan, heparan sulfate and heparin. The antithrombin III and heparin cofactor II binding sites are also shown. X is either the hydrogen or sulfo (SO3-) group, and Y is either the acetyl (Ac) or sulfo group. I. Heparin; II. Heparan sulfate; III. Dermatan sulfate; IV. ATIII binding site; V. HCII binding site
Herein, we describe plasma samples obtained from a 10 year-old female with liver failure post orthotopic liver transplant. Initial results for this patient showed coagulopathy due to liver disease without HLS. Two weeks after liver transplantation, the patient began to bleed from catheter insertion sites, mouth, and nares. HLS was suspected based on laboratory data (Table 1). Bolus infusion of the heparin neutralization drug, protamine sulfate (PS), resulted in only transient slowing of bleeding and little correction of coagulation values. Bleeding finally resolved after therapeutic plasma exchange (TPE). In addition, in vitro PS neutralization of thrombin time (TT), anti-Xa and anti-IIa assays were performed on normal pooled plasma spiked with heparin and patient’s plasma sample removed during TPE. Clinical data, laboratory results, and citrated plasma samples were collected with institutional review board approval. The patient subsequently died from multi organ failure, but not from bleeding, and these clinical samples were used for the detailed analysis presented in this manuscript.
Table 1.
Coagulation tests on patient’s clinical samples taken at various time points.
| Coagulation Test (Reference Range) | Bleeding Onset | After PS Infusion | After Plasma Exchange |
|---|---|---|---|
| Prothrombin Time (PT) (11.4–15.8 s) | 14.4 s | 13.5 s | 13.3 s |
| Activated Partial Thromboplastin Time (aPTT) (24.8–34.4 s) | 136.1 s | 118.4 s | 46.0 s |
| aPTT with heparinase (Hepzymed) (24.8–34.4 s) | 47.9 s | 45.4 s | 33.3 s |
| Thrombin Time (TT) (15.0–19.0 s) | >120.0 s | >120.0 s | 69.5 s |
| Anti-Xa (< 0.1 U/dL) | 0.40 U/mL | 0.37 U/mL | < 0.10 U/mL |
| Anti-IIa | 0.00 U/mL | 0.01 U/mL | - |
2. Materials and methods
2.1. Materials
Plasma samples 7/24/17 09:00 (not treated with Hepzymed); 7/25/17 18:13 (treated with Hepzymed); 7/26/17 04:23 (treated with Hepzymed); 07/31/2017 16:10 pm (not treated with Hepzymed); plasma exchange specimen (70–75% patient and 25–30% NHP, 14 mL) and normal human plasma samples: commercial normal pooled plasma NPP Cryocheck, Lot A1218 (Precision BioLogic Inc., Dartmouth, NS, Canada); normal adult donor citrated plasma were provided from Dr. Teruya’s laboratory at Texas Children’s Hospital with IRB approval. Actinase E was provided by Prof. Toshihiko Toida (Chiba University, Japan). Porcine HS and United States Pharmacopeia (USP) HP were from Celsus (Cincinnati, OH). Recombinant Flavobacterial heparin lyase I, II, III and chondroitin lyase ABC from Proteus vulgaris were expressed using Escherichia coli in our laboratory as previously described (Linhardt et al., 2006). Unsaturated disaccharide standards of HS (0SHS: ΔUA-GlcNAc; NSHS: ΔUA-GlcNS; 6SHS: ΔUA-GlcNAc6S; 2SHS: ΔUA2S-GlcNAc; 2SNSHS:ΔUA2S-GlcNS; NS6SHS: ΔUA-GlcNS6S; 2S6SHS: ΔUA2S-GlcNAc6S; TriSHS: ΔUA2S-GlcNS6S) were purchased from Iduron (Manchester, UK). AMAC and NaCNBH3 were obtained from Sigma-Aldrich (St. Louis, MO, USA). Q Sepharose fast flow resin was purchased from GE healthcare (Pittsburgh, USA). Frozen normal human pool plasma (NHP) for supplementation studies was obtained from Loyola University Medical Center (blood bank). For prothrombin time, HemosIL™ reagent was obtained from Instrumentation Laboratory (Bedford, MA).aPTT reagent was obtained from TriniCLOT aPTT reagent (Diagnostica Stago, Parsippany, NJ).
2.2. Clinical analysis
Coagulation assays: aPTT, aPTT with heparinase, thrombin time and anti-Xa activity were measured on STA-R Evolution analyzer (Diagnostica Stago, Parsippany, NJ, USA) using commercially available reagents. Anti-IIa assay (Hyphen Biomed, Neuville-sur-Oise, France) was also performed on STA-R coagulometer. Normal human plasma (NHP) was spiked with unfractionated heparin (100 USP units/mL, APP Pharmaceuticals, Schaumburg, IL, USA) at final concentration 0.4 units/mL with or without protamine sulfate (10 mg/mL, APP Pharmaceuticals, Schaumburg, IL, USA) at final concentration of 5 µg/ml, patient plasma was mixed with PS at final concentration of 5 and 20 µg/mL.
2.3. Disaccharide analysis
Disaccharide analysis was carried out by high performance liquid chromatography (HPLC)-tandem mass spectrometry (MS/MS) with 2-aminoacridone (AMAC) labeling. Plasma samples (20 µL) or recovered GAGs (5 µg) were treated with a mixture of heparin lyase I, II, III (20 mU/each) and chondroitin lyase ABC (20 mU) in 300 µL digestion buffer (50 mM ammonium acetate containing 10 mM calcium chloride, pH 7.4). The reaction was incubated at 37 °C for 12 h and terminated by centrifuging through a 3 kDa molecular weight cut-off (MWCO) spin column. The spin column was washed twice with 300 µL distilled water and the filtrate was collected and lyophilized.
The freeze-dried sample was AMAC-labeled by adding 10 µL of 0.1 M AMAC in dimethyl sulfoxide/acetic acid (17/3, v/v) incubating at room temperature for 10 min, followed by adding 10 µL of 1 M aqueous sodium cyanoborohydride (NaCNBH4) for 1 h at 45 °C. The supernatant (10 µL) was collected after centrifugation for 10 min at 10000 × g for further HPLC-MSMS analysis. A mixture containing HS/chondroitin sulfate/hyaluronan disaccharides standards were prepared at a concentration of 0.5 µg/mL using the same AMAC-labeled method as standards.
LC was performed on an Agilent 1200 LC system using an Agilent Poroshell 120 EC-C18 (2.7 µm, 3.0 × 50 mm) column. Mobile phase A was 50 mM ammonium acetate and mobile phase B was methanol. The flow rate was set at 300 µL/min. The gradient was set as the concentration of mobile phase B increased from 5% to 45% in 10 min, and then rose to 100% in the following 0.2 min, kept for 4 min at 100%. The column was connected to a triple quadrupole mass spectrometry equipped with an electrospray ionization source (Thermo Fisher Scientific, San Jose, CA). The online mass spectrometry (MS) analysis was at the Multiple reaction monitoring mode. MS parameters: negative ionization mode with a spray voltage of 3000 V, a vaporizer temperature of 300 °C, and a capillary temperature of 270 °C.
2.4. Recovery of heparan sulfate from plasma exchange specimen
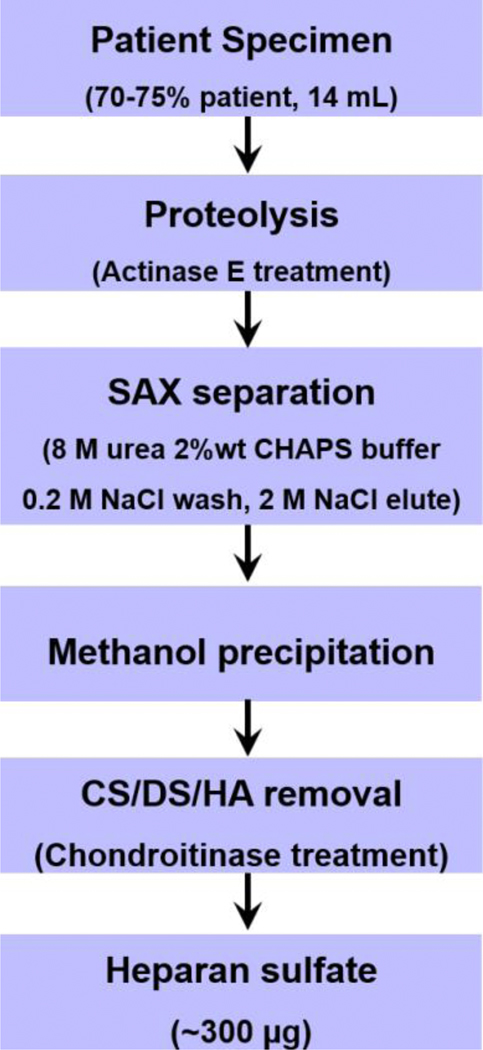
The flowchart of heparan sulfate recovery was shown in Fig 2. Briefly, plasma exchange specimen (70~75% patient, 14 mL) were lyophilized and re-dissolved in 10 mg/mL actinase E for proteolysis at 55 °C for 48 h. The reaction was stopped by boiling for 10 min and then centrifuged at 4000 × g for 10 min. The supernatant was collected and lyophilized. Dried sample was dissolved in 10 mL solution of 8 M urea solution containing 2% 3-[(3-chloramidopropyl) dimethylamminio]-1-propanesulfonate. The resulting cloudy solution was clarified by being passed through 0.2 µm membrane syringe filter and was then loaded onto a pre-equilibrated 3 mL Q Sepharose fast flow resin (GE Life Sciences, Pittsburgh, USA). The column was washed with 5 column volumes of 0.2 M sodium chloride and eluted by 3 CV of 2 M sodium chloride. Methanol was added to the eluted solution to afford a final concentration of 80 vol% and the GAGs was precipitated at 4 °C overnight. The precipitated GAGs were recovered and collected by centrifugation at 4000 × g for 20 min.
Figure 2.
Heparan sulfate recovery flow chart
The GAGs were next treated with chondroitin lyase ABC (20 U/mL, 100 µL) in digestion buffer (50 mM ammonium acetate containing 10 mM calcium chloride, pH 7.4) overnight to completely break down chondroitin sulfate and hyaluronan to disaccharides. The reaction was terminated by boiling at 100 °C for 10 min and the proteins were removed by centrifugation at 10000 × g for 10 min. The chondroitin sulfate/hyaluronan disaccharides and salts were removed by passing through a 3 kDa MWCO spin column and washed 3-times with distilled water. The retentate containing heparan sulfate was recovered from the tube and lyophilized for further use.
2.5. HPLC-GPC analysis
The molecular weight was determined by HPLC-gel permeation chromatography (GPC) using USP heparin as standard. HPLC system is consisting of Shimadzu LC-10Ai pump, a Shimadzu CBM-20A controller and a Shimadzu RID-10A refractive index detector (Shimadzu, Kyoto, Japan). A guard column TSK SWXL 6 mm × 4 cm, 7 µL diameter was used to protect two analytical columns: TSK G4000 SWXL 7.8 mm × 30 cm, 8 µm in series with TSK G3000SWXL 7.8 mm × 30 cm, 5 µm (Tosoh Corporation, Tokyo, Japan). The mobile phase was 0.1 M ammonium acetate with 0.02% (w/v) sodium azide. Columns were maintained at 30 °C using an Eppendorf column heater (Eppendorf, Hamburg, Germany). The sample injection volume was 20 µL with concentration at 5 mg/mL and flow rate set at 0.6 mL/min.
2.6. Nuclear magnetic resonance NMR spectroscopy analysis
The NMR spectra was obtained on a Bruker 800 MHz (18.8T) standard-bore NMR spectrometer equipped with a 1H/2H/13C/15N cryoprobe with z-axis gradients. Sample was dissolved in 0.4 mL of 99.96% D2O and lyophilized, and then repeated twice. 1H spectroscopy was carried out at 25 °C.
2.7. Coagulation analysis
Lyophilized HS was dissolved in sterile saline to obtain a stock concentration of 400 µg/mL. Additional working solution was prepared at 200 and 100 µg/mL to determine the anticoagulant properties 100 µg of 400 µg/mL was supplemented to 0.9 mL of plasma. A parallel saline control was used in normal human plasma (NHP). Clotting studies, to determine prothrombin time (PT) and PT were carried out in duplicate, same plasma was used for the measurement of anti-Xa and anti-IIa activity. Platelet aggregation studies were carried out by supplementing HS to obtain a final concentration of 10 µg/mL using 100 µg/ml stock solution. Results were expressed in terms of % aggregation. Saline was used as a control. Thrombin generation studies were carried out in NHP containing 40 µg/mL Results were obtained in terms of peak thrombin, lag time and area under the curve (AUC).
Anti-factor Xa and anti-factor IIa activities were measured using kinetic amidolytic methods. Bovine factor Xa (Enzyme Research Laboratories, South Bend, IN) ) was diluted in 50 nM Tris buffer (pH = 8.4) to a concentration of 1.25 IU/ml and factor Xa substrate (BioMedica Diagnostics, CT, U.S.A) reconstituted in sterile water at 2.5 µM were used in this assay. Human thrombin (Enzyme Research Laboratories, South Bend, IN) diluted in 50 nM Tris buffer (pH = 8.4) to a concentration of 1.25 IU/ml and factor IIa substrate (BioMedica Diagnostics, CT, U.S.A) reconstituted in sterile water to a concentration1 µM were used in this assay. Both the clotting and amidolytic studies were carried out on the ACL-Elite instrument. Platelet aggregation studies were performed on platelet rich plasma (PRP) obtained from freshly drawn normal human citrated blood upon centrifugation at 800 rpm. Commercially available agonist such as epinephrine, adenosine diphosphate (ADP), collagen, thrombin, were used for platelet aggregation studies. All of the platelet aggregation studies were performed on a PAP-8 aggregometer (Biodata Corporation, Horsham, Pennsylvania) and the results are recorded in terms of percent aggregation. Inhibition of thrombin generation was measured by using a Fluoroskan Ascent fluorimeter, calibrated automated thrombogram (CAT), (Diagnostica Stago, Parsippany, NJ). Reagents used in this assay included the fluo-substrate, fluo-buffer, tissue factor high reagent (mixture of tissue factor and phospholipids) and a thrombin calibrator.
3. Results and Discussion
3.1. Clinical data
The presence of HLS in the liver failure patient’s blood was suspected based on laboratory data (Table 1). The aPTT coagulation assays of patient plasma showed a 2.5- to 3-times higher value for untreated plasma compared to heparinase-treated plasma (Hepzymed process (Keller et al., 1998)) despite the absence of exogenously administered HP, suggesting the presence of endogenous plasma HP or HS. The patient repeatedly had measurable anti-Xa activity and a thrombin time (TT) of >120 sec with a reference range of 15–18 sec. Another recently reported case described an infant with HLS induced bleeding that developed after 2 weeks post liver transplantation (Nacoti et al., 2018). While this infant patient’s PT and aPTT were only slightly prolonged, HLS was detected by thromboelastography (TEG). Similar to case of our 10-yr old patient, these coagulation parameters were partially corrected by treating the infant’s plasma with heparinase (Hepzymed process). In addition, the in vitro PS neutralization of TT, anti-Xa and anti-IIa assays were performed on normal pooled plasma spiked with heparin and our 10-year old patient’s plasma sample removed during TPE. However, PS infusion had no reported effect on bleeding. Another report of HLS in a patient with multiple myeloma, however, reported resolution of bleeding after threw bolus infusions of PS (Torjemane et al., 2007). Our 10-year old patient showed only a mild clinical improvement after PS bolus. However this was short-lived, likely due to the short half-life of PS (Pai & Crowther, 2012). In addition, our in vitro PS neutralization suggests PS is not as effective in neutralizing HLS as it is for heparin. (Table S1.)
Clotting times measured by rotational thromboelastography (ROTEM) relying on ellagic acid to initiate clotting through the intrinsic pathway (INTEM) and using tissue factor to initiate the extrinsic clotting cascade (EXTEM) were prolonged with only partial correction on using heparinase (Hepzymed process) in addition to ellagic acid (HEPTEM) (Ponschab, Voelckel, Pavelka, Schlimp, & Schöchl, 2015). (Figure S1 and Table S2.) These assays again suggest the presence of a HLS in the plasma of our 10-year old patient. Specimen was carefully collected in order to avoid heparin contamination when the specimen was collected from an arterial line which is locked with a small amount of heparin.
3.2. Analysis of HS/HP content
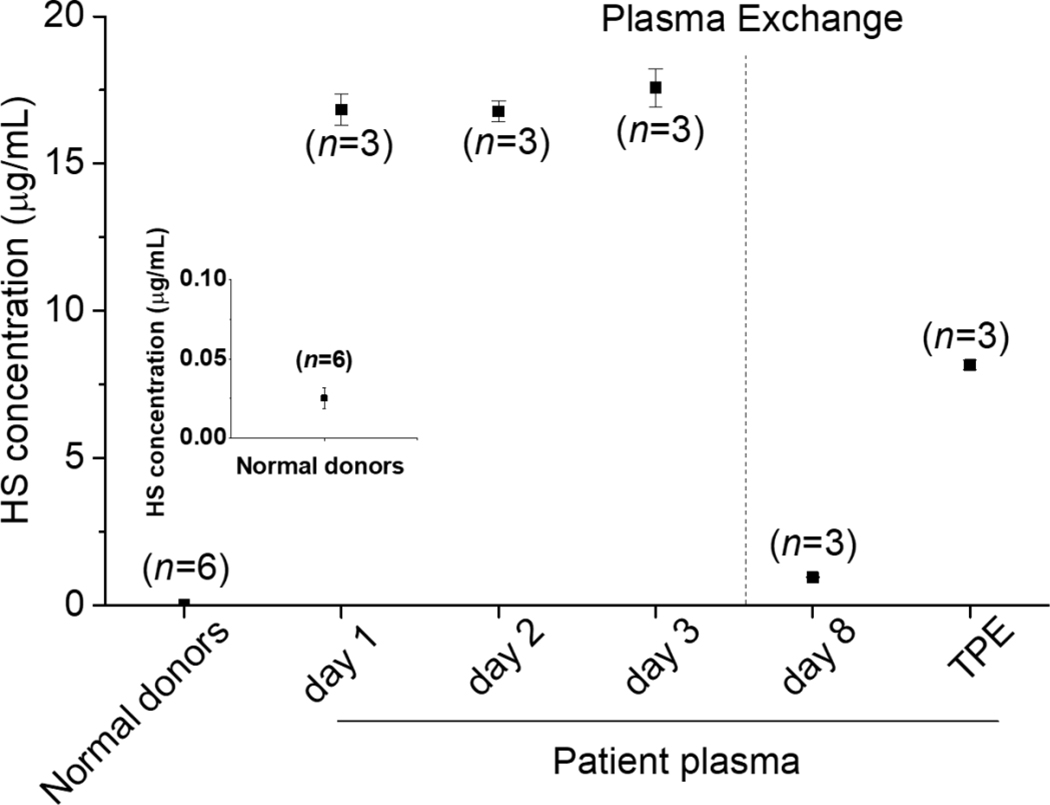
HS and HP are polysaccharides having a continuum of sizes with closely related structures and thus in mixtures are difficult to separate and identify. Such structural complexity is further complicated by their different levels of production in organisms of different ages (Yu et al., 2017) and within different tissues and organs (Andrade, et al., 2013; Shi & Zaia, 2009; Warda et al., 2006). Disaccharide analysis represents a useful tool for assessing the structure and concentration of these GAGs (Okamoto, Higashi, Linhardt, & Toida, 2018). For example, HP has a much higher content of trisulfated disaccharide repeating units ([→4) IdoA2S (1→4) GlcNS6S(1→]n) and HS has a much higher content of unsulfated disaccharide repeating units ([→4) GlcA (1→4) GlcNAc (1→]n) (Fu, Suflita, & Linhardt, 2016; Linhardt, 2003). Thus, we performed HPLC-MS dependent heparan sulfate content analysis in plasma to determine whether endogenous heparin-like substance was released into the plasma after liver transplantation in our 10-yr old patient. This patient had an abnormally HS/HP high concentration of 16.83 µg/mL in plasma collected the first day after bleeding (without Hepzymed process) (Figure 3). In contrast, the HS concentration in pooled normal human plasma (NHP) was 0.025 µg/mL consistent with other reports (Gatto et al., 2016; Schmidt et al., 2014). Thus, HS/HP in our 10-yr old patient was 672-times higher than normal human plasma. The plasma collected at the second day and third day after hepzymed process were also greatly elevated at 16.77 µg/mL and 17.58 µg/mL, respectively. However, the bleeding did not stop at this period even after administration of PS. Bleeding was finally resolved after TPE. The plasma after TPE at day 8, collected and retained for subsequent analysis consisted of 25% NHP and 70–75% plasma of the 10-yr old patient. The HS content in day 8 TPE plasma decreased greatly to 0.94 µg/mL, although, this HS level is still 37.6- fold higher than the HS of NHP. The final TPE patient specimen, containing 8.16 µg/mL HS, was used to isolate the larger quantities of HS required for more complete structural characterization.
Figure 3.
Analysis of HS/HP content. Normal donors result was zoomed and inserted in the figure. The patient was therapeutic plasma exchange (TPE), day 1 was not hepzymed, day 2 and day 3 were hepzymed, day 8 was after TPE and it was not hepzemed, TPE was patient specimen after plasma exchange and contains 70–75% patient plasma.
3.3. HS compositional analysis
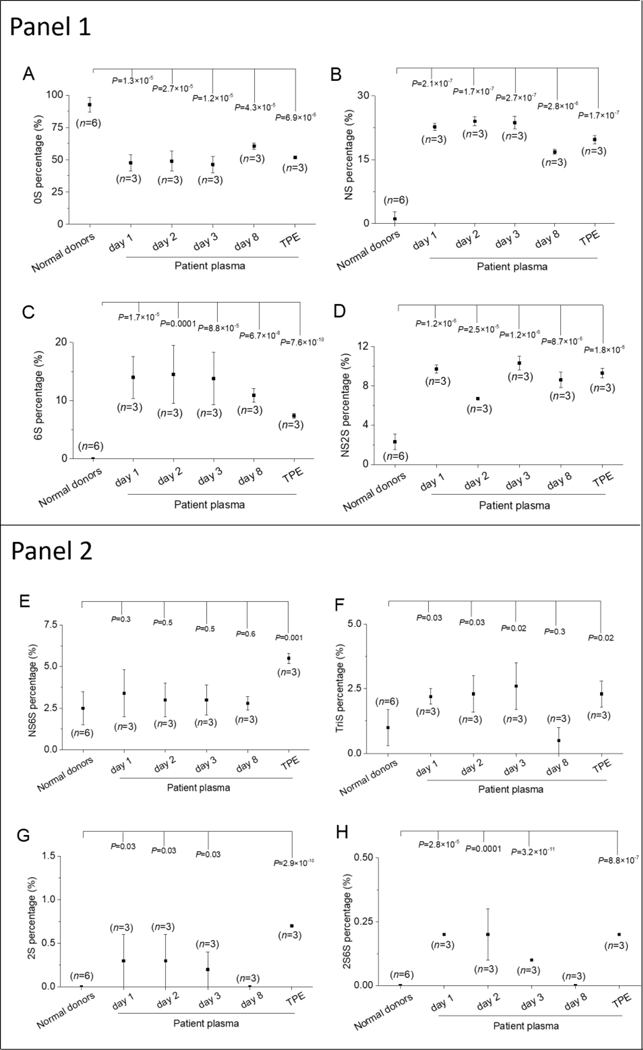
Disaccharides compositional analysis provides important structural information and is an effective method for distinguishing between HS and HP. HS/HP contains different disaccharide sequences, which on exhaustive treatment with heparin lyases, give rise to eight disaccharides, corresponding to ΔUA-GlcNAc (0S), ΔUA2S-GlcNAc (2S), ΔUA-GlcNS (NS), ΔUA-GlcNAc6S (6S), ΔUA2S-GlcNAc6S (2S6S), ΔUA2S-GlcNS (2SNS), ΔUA-GlcNS6S (6SNS), ΔUA2S-GlcNS6S (TriS) (where ΔUA corresponds to 4-deoxy-α-L-threo-hex-4-enopyranosyluronic acid). We separated these eight major disaccharides into two groups, group A containing the major disaccharides found in HS including 0S, NS, 6S and 2SNS and the second group B containing the major disaccharides found in HS, including 2S, 2S6S, NS6S and TriS. The major disaccharides in group A were significantly different in NHP and the plasma of the 10-yr old patient (Figure 4). While the major HS disaccharide in both was 0S, in NHP we observed 92.5% 0S was significantly different (P<0.001) from the percentage of 0S in the patient’s during the first three days after bleeding, were 47.6%, 48.9% and 46.3%, respectively (Figure 4A). On day 8, after TPE, the 0S increased to 60.6%, slightly higher than the first 3 days, but still significantly different (P<0.001) from that observed in normal human plasma.
Figure 4.
HS compositional analysis in normal and patient plasma samples. Panel 1. major disaccharides of patient showing significant differences compared to normal human, Panel 2. minor disaccharides showing less significant differences. A. Heparan sulfate disaccharides without sulfation (0S), B. NS, C. 6S, D. NS2S, E. NS6S, F. TriS, G.2S, H. 2S6S.
3.4. Flow chart for recovery of HS from plasma
The isolation of HS from the patient’s plasma exchange specimen (Figure 3, final TPE sample) was next undertaken using the method shown in Figure 2. Plasma proteins were completely digested using a non-specific protease, actinase E, and the GAG components were recovered from the digestion mixture by strong anion exchange (SAX) (Volpi & Linhardt, 2010). Highly acidic polysaccharides bind tightly to SAX resin, while the digested peptides and other plasma impurities can be washed from the SAX resin with 0.2 M NaCl. The GAGs were eluted by 16% NaCl and then precipitated using 80% (v/v) methanol. The recovered GAGs were dissolved in 100 mM ammonium acetate containing 10 mM calcium chloride and exhaustively digested with 2 U chondroitinase ABC to remove chondroitin and dermatan sulfates (Derby and Pintar., 1978; Li et al., 2015). HS (300 µg) was recovered and used for further structural characterization and to perform coagulation assays.
3.5. GPC analysis of HS isolated from plasma
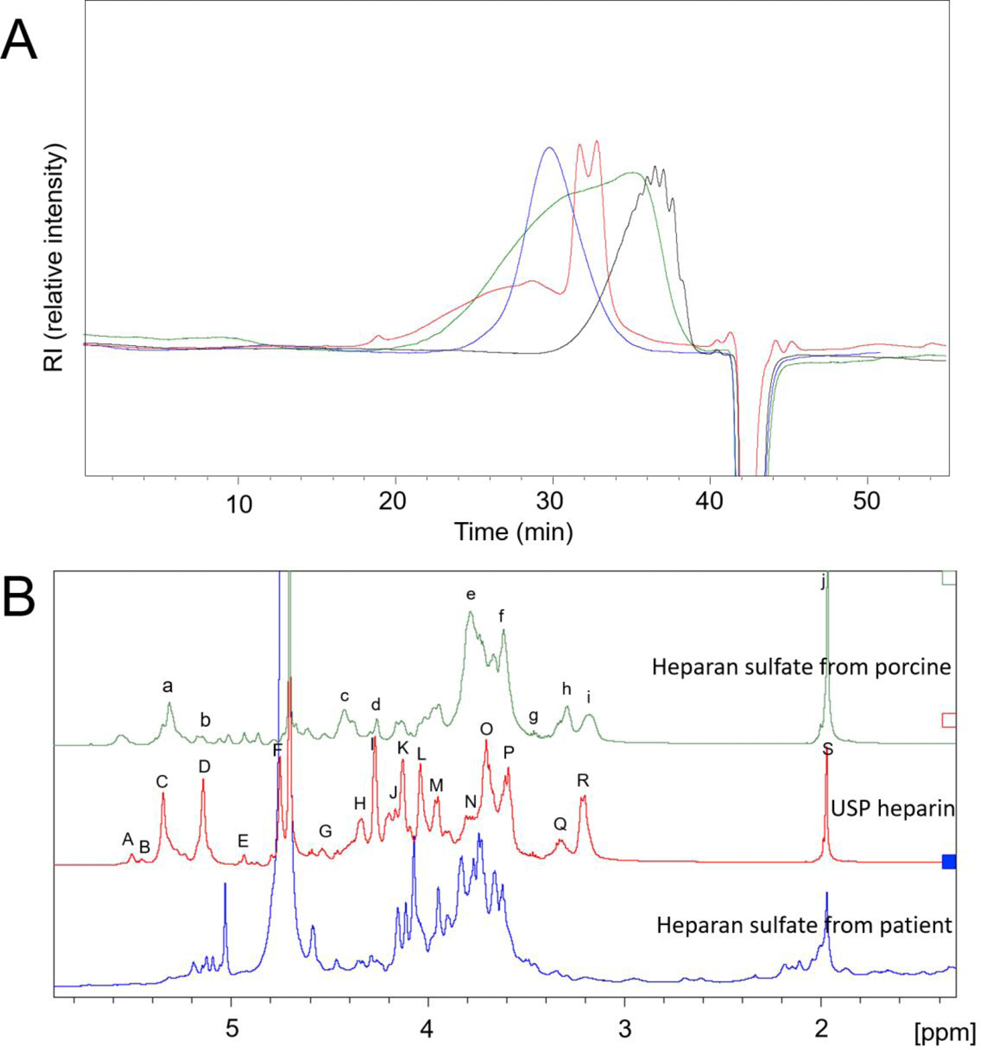
The molecular weight of the resulting HS was analyzed by HPLC-GPC (USP monograph, 2014) (Figure 5A). USP HP, a low molecular weight HP, enoxaparin, and commercial HS, were all obtained from porcine intestine and used as molecular weight standards. The HP and enoxaparin have average molecular weight (MWavg) of 16,000 and 4,000 Da, respectively. Porcine intestinal HS showed a wide molecular weight distribution ranging from 1,000 to 40,000 Da with an MWavg of 11,700 Da. The HS recovered from the patient’s TPE specimen had a similarly wide molecular weight distribution but showed two major peaks at 7,000 to 11,000 Da.
Figure 5.
GPC and NMR analysis of heparan sulfate substance from patient. A. GPC analysis, red, patient HS, green, HS standard from Celsus sourced from porcine intestine mucosa, blue, heparin standard, black, Enoxaparin. B. 1H NMR analysis of heparan sulfate from patient. The signals in porcine heparan sulfate were correspond to a. GlcN (Ac or S), H1 (5.3 ppm); b. IdoA, H1 (5.1 ppm); c. GlcA, H1 (4.4 ppm); d. IdoA2S, H2 (4.3 ppm); e. GlcNAc, H2 (3.8 ppm); f. GlcA, H4&H5 (3.6 ppm); g. GlcNS/GlcA, H3 (3.5 ppm); h. GlcA, H2 (3.3 ppm); i, GlcNS, H2 (3.2 ppm); j. N-acetyl (CH3) GlcNAc (2.0 ppm). The signals in heparin were correspond to A. GlcNS6S, H1 (5.5 ppm); B. GlcNS3S, H1 (5.4 ppm); C. GlcNY6X (where Y = Ac or S and X = H or S), H1 (5.3 ppm); D. IdoA2S, H1 (5.2 ppm); E. IdoA2SGlcNS, H1 (4.9 ppm); F. IdoA2S, H5 (4.7 ppm); G. GlcA, H1 (4.5 ppm); H. GlcNS, H6 (4.3 ppm); I. IdoA2S, H2 (4.3 ppm); J. GlcNS6S, H6 (4.2 ppm); K. IdoA2S, H3 (4.1 ppm); L. IdoA2S, H4 (4.0 ppm); M. GlcNS6S, H5 (3.9 ppm); N. GlcNS, H6 (3.8 ppm); O. GlcNS6S, H4 (3.7 ppm); P. GlcA, H3 (3.6 ppm); Q. GlcA/GlcNS3S, H2 (3.3 ppm); R. GlcNS, H2 (3.2 ppm); S. N-acetyl (CH3) GlcNAc (2.0 ppm).
3.6. NMR analysis of HS isolated from plasma
The 1H NMR spectra of HP, porcine HS and HS recovered from the patient’s TPE specimen are presented in Figure 5B. The major signals in HP and porcine HS could be assigned based on literature values (Fu et al., 2013; Warda et al., 2006). The HS recovered from the patient’s TPE specimen showed an NMR spectrum that was clearly similar to but distinctly different from both the HP and porcine HS spectra. These data are consistent with the disaccharide compositional analysis (Figure 4) showing that the HS had a structure that was intermediate between HP and porcine HS. The HS sample used in the non-destructive NMR studies was recovered and next used to perform anticoagulant activity studies.
3.7. Activity analysis of HS isolated from plasma
Anticoagulant activity studies were next performed on the HS isolated from this patient’s plasma. Preliminary studies using amidolytic anti-Xa and anti-IIa assays were undertaken on this purified HS by dissolving it in NHP at a concentration of 40 µg/mL. Platelet aggregation that was carried out at 10 μg/mL. The data obtained, while generally consistent with those obtained on the clinical plasma samples (Table 1) were very interesting. The HS sample clearly showed anti-Xa and anti-IIa activities by amidolytic assay at values of ~10 to 15% of those of USP HP. While these values were considerably lower than those of USP HP, they were much higher than those of porcine HS. Porcine HS anticoagulant is less than 5 U/mg, less than 2.5% of USP HP (Griffin et al., 1995).
A more detailed analysis of the anticoagulant activity of 200 µg of this purified HS isolated from the patient’s TPE specimen was undertaken to better understand the reason for the bleeding observed clinically. Lyophilized HS sample was diluted in 0.05 mL of sterile saline providing a concentration of 400 µg/mL. Aliquots (100 µL) of this solution were supplemented to normal human plasma and platelet rich plasma to study clotting, anti-protease, thrombin generation and inflated aggregation profiles. Saline supplemented plasma and platelet rich plasma were used as controls. The results (Table 2 and Table S3) showed that this HS had only a weak strong anticoagulant activity at the concentrations studied by PT (extrinsic) and aPTT (intrinsic) pathways. However, mild antithrombin activity was measurable in a diluted TT assay. Similarly in the anti-Xa and anti-IIa assay for patient HS supplemented samples showed only very small increases in anti-protease activity compared to controls. In the thrombin generation inhibition assay no differences were noted in the HS supplemented and control plasma. In agonist induced platelet aggregation assays none of the agonists induced aggregation was compromised except for thrombin. This control was 91% whereas the HS supplemented PRP was 72%.
Table 2.
Coagulation studies on NHP supplemented with purified HS recovered from patient’s TPE samples
| Parameter | Control (s) | HS supplemented plasma (s) |
|---|---|---|
| PT | 11.8 | 11.6 |
| aPTT | 31.4 | 33.7 |
| TT | 21.6 | 27.8 |
| Anti-Xa | 5 | 7 |
| Anti-IIa | 4 | 9 |
Based on these results we carried out some control studies in liver disease patients from the Hepatology clinic at Loyola University Medical Center and found that those patients with severe liver disease have impaired liver function and their plasma inhibited platelet activation by various agonists. Thus, we speculate that this 10-year old patient might have a platelet function defect. Lastly, it is important to underscore that endothelial defects not measurable by our methods might also contribute to the bleeding complications in liver disease patients.
4. Conclusion
Plasma samples were obtained from a 10 year-old female with liver failure requiring transplant that contained HLS. We detected very high levels of HS in this patient’s plasma. From the patient’s TPE specimen and isolated sufficient human HS (300 μg) to perform proton NMR spectroscopy as well as a number of coagulation assays. This patient’s HS looked more like heparin than the other HS samples that we have studied in our laboratory (Li et al., 2015; Warda et al., 2006) based on both disaccharide analysis relying on LC-MS and on NMR analysis. The plasma of this patient contained high levels of this structurally unusual HS that we identified as the HLS affecting the patient’s coagulation. The isolated HS showed only a low level of the classical heparin-like anticoagulant activity with respect to the soluble coagulation cascade. We speculate that a platelet function defect or an endothelial defect not measurable by the methods used might also contribute to the bleeding complications observed in this 10-yr old liver failure patient.
Supplementary Material
Highlights.
Heparin-like substance (HLS) was recovered from patient’s plasma
This HLS has a disaccharide composition between HP and HS
This HLS has an unusual structure when compared with other HS samples
This HLS shows low antithrombin-mediated anticoagulant activity in vitro assay
Acknowledgements
The authors thank the National Institutes of Health (CA231074 and DK111958) for supporting this research.
Footnotes
Declaration of competing interest.
The authors declare no conflicts of interests.
Appendix A. Supplementary data.
Tables S1-S3 and Figure S1.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrade GPV, Lima MA, de Souza Junior AA, Fareed J, Hoppensteadt DA, Santos EA, et al. (2013). A heparin-like compound isolated from a marine crab rich in glycuronic acid 2-O-sulfate presents low anticoagulant activity. Carbohydrate Polymers, 94(1), 647–654. [DOI] [PubMed] [Google Scholar]
- Bara L, Mardiguian J, & Samama M (1990). In vitro effect on heptest® of low molecular weight heparin fractions and preparations with various anti-IIa and anti-Xa activities. Thrombosis Research, 57(4), 585–592. [DOI] [PubMed] [Google Scholar]
- Chang C, Lico LS, Huang T, Lin S, Chang C, Arco SD, et al. (2014). Synthesis of the heparin-based anticoagulant drug fondaparinux. Angewandte Chemie International Edition, 53, 9876–9879. [DOI] [PubMed] [Google Scholar]
- Chen Y, Lin L, Agyekum I, Zhang X, St. Ange K, Yu Y, et al. (2017). Structural analysis of heparin-derived 3-O-sulfated tetrasaccharides: antithrombin binding site variants. Journal of Pharmaceutical Sciences, 106, 973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAngelis PL (2012). Glycosaminoglycan polysaccharide biosynthesis and production: today and tomorrow. Applied Microbiology and Biotechnology, 94, 295–305. [DOI] [PubMed] [Google Scholar]
- Derby MA, & Pintar JE (1978). The histochemical specificity of Streptomyces hyaluronidase and chondroitinase ABC. The Histochemical Journal, 10, 529–547. [DOI] [PubMed] [Google Scholar]
- Esko JD, & Selleck SB (2002). Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annual Review of Biochemistry, 71, 435–471. [DOI] [PubMed] [Google Scholar]
- Fahl KN, Poon SA, Badani KK, & Benson MC (2009). Paraneoplastic production of heparin-like anticoagulant in a patient with metastatic transitional cell carcinoma. Canadian Urological Association Journal, 3(5), E61–E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Li G, Yang B, Onishi A, Li L, Sun P, et al. (2013). Structural characterization of pharmaceutical heparins prepared from different animal tissue. Journal of Pharmaceutical Sciences, 102(5), 1447–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Suflita M, & Linhardt RJ (2016). Bioengineered heparins and heparan sulfates. Advanced Drug Delivery Reviews, 97(1), 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto F, Volpi N, Nilsson H, Nookaew I, Maruzzo M, Roma A, et al. (2016). Glycosaminoglycan profiling in patients’ plasma and urine predicts the occurrence of metastatic clear cell renal cell carcinoma. Cell Report, 15, 1822–1836. [DOI] [PubMed] [Google Scholar]
- Griffin CC, Linhardt RJ, Van Gorp CL, Toida T, Hileman RE, Schubert II RL, et al. (1995). Isolation and characterization of heparan sulfate from crude porcine intestinal mucosal peptidoglycan heparin. Carbohydrate Research, 276, 183–197. [DOI] [PubMed] [Google Scholar]
- Keller FG, DeFazio J, Jencks F, Steiner M, Rogers J, & Ritchey AK (1998). The use of heparinase to neutralize residual heparin in blood samples drawn through pediatric indwelling central venous catheters. Journal of Pediatrics, 132(1), 165–167. [DOI] [PubMed] [Google Scholar]
- Li G, Tian F, Zhang L, Xue C, Li L, & Linhardt RJ (2015). Glycosaminoglycanomics of cultured cells using a rapid and sensitive LC-MS/MS approach. ACS Chemical Biology, 10, 1303–1310. [DOI] [PubMed] [Google Scholar]
- Lin TS, Hsieh CH, Kuo C, Juang YP, Hsieh YSY, Chiang H, et al. (2020). Sulfation pattern of chondroitin sulfate in human osteoarthritis cartilages reveals a lower level of chondroitin-4-sulfate. Carbohydrate Polymers, 229, 115496. [DOI] [PubMed] [Google Scholar]
- Linhardt RJ (2003). Heparin: Structure and activity. Journal of Medicinal Chemistry, 46, 2551–2554. [DOI] [PubMed] [Google Scholar]
- Linhardt RJ, Al-Hakim A, Liu J, Hoppensteadt D, Mascellani G, Bianchini P, et al. (1991). Structural features of dermatan sulfates and their relationship to anticoagulant and antithrombotic activities. Biochemical Pharmacology, 42, 1609–1619. [DOI] [PubMed] [Google Scholar]
- Linhardt RJ, & Hileman RE (1995). Dermatan sulfate as a potential therapeutic agent. General Pharmacology, 26, 443–451. [DOI] [PubMed] [Google Scholar]
- Maimone M, & Tollefsen DM (1990). Structure of a dermatan sulfate hexasaccharide that binds to heparin cofactor II with high affinity. Journal of Biological Chemistry, 265, 18263–19271. [PubMed] [Google Scholar]
- Nacoti M, Cantù D, Bonacina D, Lussana F, Bonanomi E, Marchetti M, et al. (2018). Heparin-like effect resistant to protamine in a child with hemorrhagic shock. Do we need heparinase? Blood Transfus, 16, 394–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y, Higashi K, Linhardt RJ, & Toida T (2018). Comprehensive analysis of glycosaminoglycans from the edible shellfish. Carbohydrate Polymers, 184, 269–276. [DOI] [PubMed] [Google Scholar]
- Onishi A, St. Ange K, Dordick JS, & Linhardt RJ (2016). Heparin and anticoagulation. Frontiers in Bioscience, 21, 1372–1392. [DOI] [PubMed] [Google Scholar]
- Pai M, & Crowther MA (2012). Neutralization of heparin activity. Handbook of Experimental Pharmacology, 207, 265–277. [DOI] [PubMed] [Google Scholar]
- Ponschab M, Voelckel W, Pavelka M, Schlimp CJ, & Schöchl H (2015). Effect of coagulation factor concentrate administration on ROTEM® parameters in major trauma. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine, 23, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar M, Bihari C, Saxena P, Devurgowda DR, Vyas T, Mirza R, et al. (2020). Heparin-like effect associated with risk of bleeding, sepsis, and death in patients with severe alcohol-associated hepatitis. Clinical Gastroenterology and Hepatology, 18(2), 486–495. [DOI] [PubMed] [Google Scholar]
- Schmidt EP, Li G, Li L, Fu L, Yang Y, Overdier KH, et al. (2014). The circulating glycosaminoglycan signature of respiratory failure in critically III adults. Journal of Biological Chemistry, 289, 8194–8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senzolo M, Agarwal S, Zappoli P, Vibhakorn S, Mallett S, & Burroughs AK (2009). Heparin-like effect contributes to the coagulopathy in patients with acute liver failure undergoing liver transplantation. Liver International, 29(5), 754–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, & Zaia J (2009). Organ-specific heparan sulfate structural phenotypes. Journal of Biological Chemistry, 284, 11806–11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobczak AIS, Pitt SJ, & Stewart AJ (2018). Glycosaminoglycan neutralization in coagulation control. Arteriosclerosis Thrombosis Vascular Biology, 38, 1258–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torjemane L, Guermazi S, Ladeb S, Ben Romdhane N, Lakhal A, Abdelkefi A, et al. (2007). Heparin-like anticoagulant associated with multiple myeloma and neutralized with protamine sulfate. Blood Coagul Fibrinolysis, 18(3), 279–281. [DOI] [PubMed] [Google Scholar]
- Trowbridge JM, & Gallo RL (2002). Dermatan sulfate: new functions from an old glycosaminoglycan. Glycobiology, 12(9), 117–125. [DOI] [PubMed] [Google Scholar]
- United States Pharmacopeial Convention, USP37. (2014). Official monography, heparin sodium, D In: molecular weight determinaations. Rockville, MD, United States Pharmacopeial Convention, 3224. [Google Scholar]
- Volpi N, & Linhardt RJ (2010). High-performance liquid chromatography-mass spectrometry for mapping and sequencing glycosaminoglycan-derived oligosaccharide. Nature Protocols, 5(6), 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warda M, Toida T, Zhang F, Sun P, Munoz E, Xie J, et al. (2006). Isolation and characterization of heparan sulfate from various murine tissues. Glycoconjugate Journal, 23, 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Chen Y, Mikael P, Zhang F, Stalcup AM, German R, et al. (2017). Surprising absence of heparin in the intestinal mucosa of baby pigs. Glycobiology, 1(1), 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Lin L, Huang H, & Linhardt RJ (2020). Chemoenzymatic synthesis of glycosaminoglycans. Accounts of Chemical Research, 53, 335–346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.