Abstract
Macrophages not only regulate intestinal homeostasis by recognizing pathogens to control enteric infections, but also employ negative feedback mechanisms to prevent chronic inflammation. Hence, macrophages are intriguing targets for immune-mediated therapies, especially when barrier function in the gut is compromised to trigger aberrant inflammatory responses, most notably during inflammatory bowel diseases. Recently, there has been considerable progress in our understanding of human macrophage biology in different tissues, including the intestines. In this review, we discuss some new findings on the properties of distinct populations of intestinal macrophages, how resolution of inflammation and tissue repair by macrophages could be promoted by type-2 cytokines as well as other therapeutic interventions, and highlight some challenges for translating these findings into the future for this exciting area of immunology research.
Introduction
The human gastrointestinal tract is persistently exposed to a high antigenic load of microbial and dietary nature, and as a result, necessitates a delicate coupling of immune resistance to infectious organisms and tolerance to tissue damage and inflammation. While failure to mount a robust protective response against pathogens can result in susceptibility to recurrent infection, an inadequate ability to dampen the inflammatory response and limit disease severity may contribute to excessive inflammation and irreversible tissue damage. Macrophages are a critical component of this immunological balancing act. These mononuclear phagocytes play an indispensable role in distinguishing commensal luminal antigens and potentially life-threatening pathogens, as well as in resolving inflammatory responses. Therefore, understanding the regulatory mechanisms of macrophages in states of health and intestinal disease holds promise for the optimization of therapeutics aimed at resolving inflammation and resisting pathogens. In this article, we will briefly discuss the mechanisms by which macrophages function under normal physiological conditions in the steady state (e.g. regulation of enteric neurons), and their training by the local intestinal milieu. Finally, we will discuss how macrophage-established homeostasis is perturbed and subsequently restored in the resolution of intestinal inflammation, before offering our perspective on the role of type 2 immune responses and helminth infections in this process.
Features and functions of intestinal macrophages
As the largest pool of mononuclear phagocytes in the body, macrophages are ubiquitous throughout the gastrointestinal tract, with major populations found in the stomach as well as along the length of the small and large intestines. (1). However, there are marked differences in luminal content dependent on regional specialization throughout the gastrointestinal tract and thus, the presentation of distinct challenges to the immune system. The role of the duodenum and jejunum of the upper small intestine in brush border enzyme-dependent digestion and nutrition allows for a relatively sterile and largely food antigen-derived microenvironment compared to the ileum and commensal-harboring colon which are selected for in inflammatory bowel diseases (2). In line with the role of resident macrophages found in other tissues, intestinal macrophages are highly phagocytic, expressing genes such as Mrc1, CD36, Timd4, and Mertk in the mouse (3, 4), and are important for clearing apoptotic cells through a process called efferocytosis (5, 6). Intestinal macrophages express CD64 (7) and Mertk (8), as well as CD11c, MHCII and F4/80. CD64 is also expressed on human macrophages (9) rendering it a useful marker across species. There is considerable confusion and controversy with respect to distinguishing between intestinal macrophages and dendritic cells (10), but we will not address this in further detail. The combination of CD14, HLA-DR, CD11c and CD11b is able to distinguish among four distinct macrophage populations in the human small intestine (9), which are highly phagocytic but less responsive to toll-like receptor (TLR) ligand stimulation. Importantly, while macrophages express MHCII and antigen presentation machinery, they are inferior to dendritic cells in activating naïve T cells in the lymph nodes (11, 12), but likely affect effector T cell function in the tissue (see below.)
The privileged position of macrophages in the lamina propria (LP) in close proximity to the epithelial layer and gut lumen facilitates mucosal barrier protection and the elimination of transgressing bacteria. LP macrophages play a key role in immune sampling of luminal bacteria (Figure 1). By extension of transepithelial dendrites (TEDs), macrophages expressing the fractalkine receptor CX3CR1 can penetrate the epithelial barrier with minimal disruption to integrity (10, 13–15). The ability of LP macrophages to sample gut antigens may be altered by affecting intestinal epithelial cell differentiation as a result of disrupted crypts and reduction of Lgr5+ stem cells by CSF1R blockade (16). Furthermore, CSF1R-dependent macrophages affect the differentiation of additional cell types important for immune defense within the gut including Paneth cells, goblet cells, and M cells (16). Despite possessing bactericidal properties when encountering pathogens, intestinal macrophages do not elicit an overt inflammatory response to commensals (10, 17). Constitutive IL-10 signaling is essential in maintaining the appropriate regulation of intestinal macrophages and the prevention of aberrant inflammation. Deletion of the IL-10 receptor on CX3CR1-expressing macrophages in the intestine leads to spontaneous severe colitis in mice (18) and can lead to the development of severe early onset inflammatory bowel disease (IBD) in pediatric patients (19, 20). In addition to bacterial sampling, intestinal macrophages are important influences on T cell function in the gut. The activity and function of Tregs (via production of IL-10 (21)) and Th17 cells (as a source of IL-1β (22)) in particular are likely to be influenced by the activation status of intestinal macrophages (see below.) Hence, macrophages facilitate the maintenance of homeostasis in the gut through a combination of phagocytic and anti-bacterial functions and immune regulation.
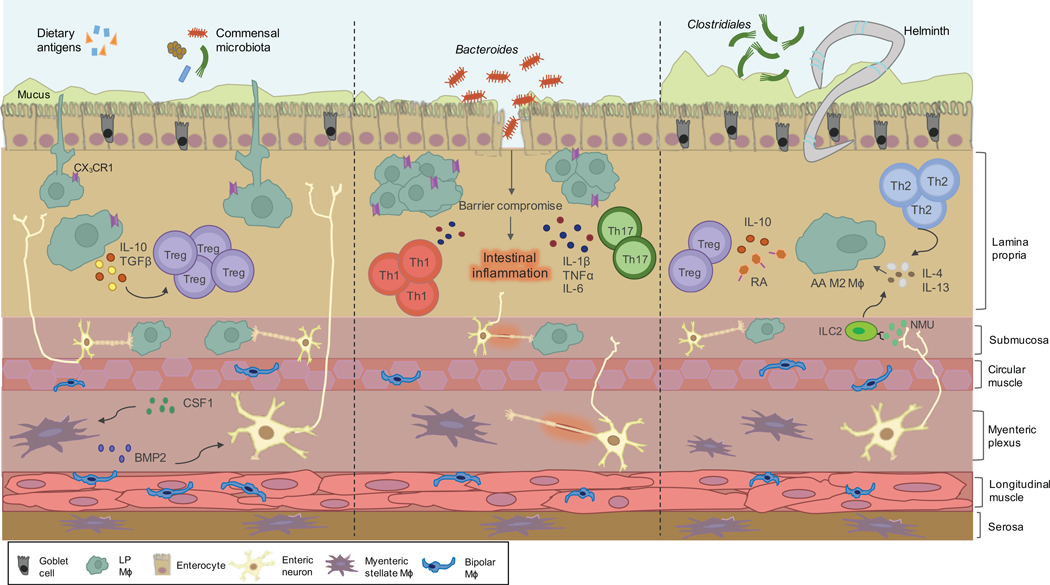
Figure 1.
Orchestration of intestinal macrophages in homeostasis, inflammation and helminth infection. Macrophages residing in the lamina propria (LP) are strategically positioned in close proximity to the epithelial layer. CX3CR1+ LP macrophages participate in sampling of luminal contents via extension of transepithelial dendrites and phagocytose transgressing pathogens. Through the secretion of immunoregulatory cytokines IL-10 and TGF𝛃, LP macrophages support the expansion of regulatory T cells (Tregs). Within the deeper layers of the gut wall, the submucosa and muscularis externa, macrophages engage in a reciprocal crosstalk with a network of enteric neurons. This molecular dialog is mediated by the secretion of bone morphogenic protein 2 (BMP2) by macrophages. In response, enteric neurons secrete CSF1 which further promotes BMP2 secretion. In the setting of intestinal inflammation and breach of the epithelial layer, proinflammatory macrophages are recruited and elicit a protective immune response mediated by the secretion of inflammatory cytokines IL-1𝛃, TNFα and IL-6. Helminth infection induces a type 2 immune response mediated by an induction of Tregs and Th2 cells. Enteric neurons may sense infection by helminths and produce the neuropeptide neuromedin U (NMU) that stimulates innate lymphoid type 2 cells (ILC2s) to produce type 2 cytokines that can alternatively activate intestinal macrophages.
Tissue-resident and monocyte-derived intestinal macrophages
It is now clear that a significant proportion of tissue-resident macrophages are maintained in situ by self-proliferation from progenitors that are already seeded in the tissues during embryonic development (23, 24), whereas pro-inflammatory macrophages differentiate from infiltrating monocytes of hematopoietic origin during inflammatory responses. Depending on the particular tissue in which tissue-resident macrophages inhabit, the macrophage pool can be self-maintained throughout adulthood or rely on constant replenishment from infiltrating monocytes in the blood (25). In the case of the intestines, incoming monocytes were thought to continuously replace tissue-resident intestinal macrophages (26–28), in stark contrast to other tissue that contain macrophage populations of both embryonic and monocyte-derived origin (29). However, long lived macrophages defined by CD4 and Tim-4 expression were recently described for the mouse intestine (3). Fate-mapping experiments and bone marrow chimeric mice indicate that these macrophages can persist for 8 months in the gut (3), self-renew from local maintenance, and consist of embryonic as well as hematopoietic cells (3). This transcriptionally distinct minority of self-maintaining gut macrophages with enrichment of gene transcripts such as Nova1, Chrm2, and Efr3b involved in tissue protective and supportive processes may support the maintenance of submucosal vascular architecture and positively regulate neuronal differentiation (4). Why the gut mucosa is comprised of macrophages predominantly from monocytes, whereas other tissues have additional macrophage subsets of varying origin remains unclear. However, niche accessibility is thought to play an active role in this molecular decision (23, 30). The gut mucosa represents a vast amount of surface area available for contact with the external environment and is freely accessible throughout life. Therefore, recruitment of monocytes to the intestines could be explained by the baseline inflammatory tone of the gut due to persistent antigen exposure.
Environmental conditioning of intestinal macrophages
The molecular cues responsible for the orchestration of the differentiation of monocytes into intestinal macrophages are not entirely understood. The local gastrointestinal microenvironment must provide macrophages with a repertoire of instructive signals. CSF1 is an important growth factor for myeloid cells as well as other populations within the gut (16, 31, 32). Transcriptional profiling of human monocyte-derived macrophages grown in CSF1 and exposed to lipopolysaccharide (LPS) identified enriched promoters for CSF1-dependent monocyte-macrophage transition in IBD susceptibility loci, suggesting that maladaptation of monocytes to the gut mucosa is a key trigger for developing IBD (33).TGFβ signaling, perhaps acting through Runt-related transcription factor 3 (RUNX3), may especially be important for terminal differentiation (34). Notch signaling is another developmental signal that is likely required for intestinal differentiation (35). A vital role of the gut microbiota and microbe-derived molecules has also been suggested in the influence of macrophage population dynamics (36). Furthermore, depletion of the gut microbiota by broad spectrum antibiotics greatly reduces macrophage turnover (26). Mature intestinal macrophages upregulate genes encoding monocyte chemoattractants such as Ccl7 and Ccl8, suggesting developmental hardwiring of a self-replacement program (37). The phagocytosis of apoptotic intestinal epithelial cells by intestinal macrophages may also lead to suppression of pro-inflammatory genes (38). Hence, the combination of microbiota signals and normal apoptotic epithelial cells (which could be linked via unknown mechanisms) may lead to the conditioning of intestinal macrophages that are less responsive to stimulation by microbial antigens. Notably, as macrophages in the intestine mature and become CX3CR1hi, they are conditioned towards adopting this tolerogenic phenotype (39), which is a process that clearly requires the IL-10 axis (Figure 1).
Neuro-immune interactions mediated by macrophages
The immune and nervous systems are charged with the task of sensation within a landscape of fluctuating conditions typically found at barrier surfaces like that of the gastrointestinal tract. Although ultimately employing structurally distinct processes, i.e. immune recognition of pathogens versus nociception of potentially harmful stimuli, both systems rely on direct cell-cell communication mediated by series of soluble signaling molecules, including cytokines and neurotransmitters, and possess memory capacity in anticipation of re-challenge. The adult gastrointestinal tract harbors not only the largest immune cell compartment in the body, but also a prolific neuronal network which rivals that of the spinal cord (40, 41). While many research efforts have been directed towards macrophages in the lamina propria, macrophages that reside in the deeper layers of the gut wall, namely the submucosa, the muscularis externa which comprises the circular and longitudinal smooth muscle layers, and the serosa, have largely been neglected until recent advancements in imaging techniques and transcriptional profiling (42). These macrophage subsets are functionally and morphologically distinct. In contrast to proinflammatory LP macrophages that likely undergo training by luminal cues, muscularis macrophages (MM) adopt a tissue-protective phenotype, upregulating M2 genes such as Arg1 and Cd163 (42). Anatomic positioning of CX3CR1+ macrophages in the muscularis externa may influence morphology (43), with the adoption of either a stellate or bipolar morphology (42). There is now evidence to suggest that gut macrophages are intimately associated with the enteric nervous system and engage in a reciprocal crosstalk (44). Gastrointestinal motility can be regulated by bone morphogenic protein 2 (BMP2) secreted by muscularis macrophages (31). In a positive feedback mechanism, BMP2 from MM acts on its receptor BMPR on the surface of enteric neurons to induce CSF1 secretion, which in turn stimulates further BMP2 expression (31, 42). However, recent findings of an expected number and distribution of phenotypically intact MM in Ret −/− mice and aganglionic colons of Hirschsprung disease patients undermine this signaling circuit and suggest a role for environmental influences (45). Notably, antibiotic treatment reduces BMP2 expression on MM, indicating that the gut microbiota may play a role in regulating this process (31), although the mechanism of this interaction is still not clear. These interactions could then regulate peristalsis, or contraction of smooth muscle layers, which propels an ingested bolus through the gastrointestinal tract for eventual elimination. This mechanism is further illustrated by the grossly disturbed gastrointestinal functional motility upon treatment of mice with broad spectrum antibiotics (46). Clinically, postoperative ileus (POI) may occur when this system is transiently dysfunctional and may prolong hospitalization in patients who have undergone major abdominal surgical procedures. In the presentation of POI, a subtle proinflammatory response by resident macrophages in the muscularis externa could increase neutrophil recruitment and upregulate adhesion molecules to inhibit muscle contractility, resulting in delayed intestinal transit (43, 47, 48). Anti-inflammatory molecules that counteract inflammation such as polyunsaturated fatty acid-derived pro-resolving lipid mediators may rescue gut motility (49), however the role of IL-10 in this process remains controversial (50, 51). While macrophages can influence neuronal function in this context, neuronal sensing of helminth infections may also lead to activation of immune cells in the gut (52), which may lead to the appropriate activation of intestinal macrophages (Figure 1). Hence, there are intriguing bidirectional communication mechanisms between neuronal cells and immune cells (including macrophages) in the gut that are just beginning to be elucidated.
Macrophages in the setting of gut inflammation and infection
During intestinal inflammation, there is a rapid and abundant accumulation of inflammatory Ly6Chi monocytes in the gut. These monocytes differentiate into macrophages, but unlike their counterparts present under homeostatic conditions, they are highly responsive to stimulation by microbial antigens. They will upregulate pro-inflammatory cytokines (e.g. IL-1β, IL-6, TNF) and produce reactive oxygen species in response to TLR agonist stimulation. One possibility is that the infiltrating monocytes and differentiating macrophages have not been appropriately conditioned to the gut environment yet to become anti-inflammatory. The resident macrophages that are CX3CR1hi still retain their anti-inflammatory signature during inflammation. Since expression of CX3CR1 is a final step in the maturation of mature macrophages from Ly6Chi monocytes in the monocyte differentiation “waterfall”, it seems likely that the pro-inflammatory nature of infiltrating monocytes is slowly deconditioned in the normal gut, but under inflammatory settings they become persistently activated without becoming fully differentiated into resident immunoregulatory intestinal macrophages. The resident macrophages that already express high levels of CX3CR1 may thus be particularly important in immune regulation during inflammation. The presence of a normal microbiota may also be important in conditioning CX3CR1+ macrophages to adopt these immune regulatory properties (53), whereas in the presence of pathogens these macrophages may adopt dendritic cell-like properties by trafficking back to the lymph nodes via CCR7 to induce T cell and B cell responses (54).
While data on macrophage function in inflammation of the human gut is sparse and correlative compared to findings obtained from mouse studies, the underlying principles likely remain similar. The breakdown of mucosal immune homeostasis established and maintained by tissue resident macrophages may result in acute and chronic inflammatory states of the gut. This manifests most notably in inflammatory bowel disease, comprising Crohn’s disease and ulcerative colitis, which is a chronic inflammatory disorder of the gastrointestinal tract requiring lifelong management as a consequence of exacerbated immune responses in genetically predisposed individuals. In response to inflammatory signals from the intestinal epithelial layer, usually the initiating step of intestinal inflammation, newly recruited CD14hiCD11chi monocytes and immature macrophages challenge the existing immunosuppressive population of resident macrophages (CD64+HLA-DRhiCD14lo) and differentiate into proinflammatory macrophages with a signature expression of TNFα, IL-1β, and IL-6 (55, 56). Notably, the majority of IBD susceptibility loci are expressed in monocytes and have been identified by genome-wide association studies as well as functional studies (33, 57–59). Recruited proinflammatory macrophages in this context express TREM1 (60) and could further disrupt integrity and introduce a leakage of luminal content through increased pro-inflammatory responses. CCR2+CX3CR1+ macrophage subsets have also been demonstrated to undergo expansion in the mucosa of patients with active IBD (61). Furthermore, in the human inflamed gut, accumulated CD14hi macrophages may provide an indication of IBD severity (62). This population is similar to the murine equivalent Ly6Chi population that dominates under conditions of infection (63). The parallels between macrophages observed in patient endoscopic biopsy material and murine models may be key to identifying possible therapeutic targets through translational studies.
Alterations to macrophage function during resolution of inflammation
While macrophages are critical for a protective inflammatory response against pathogens, they are also of equal importance in the resolution phase of inflammation (64). Clearance of accumulated neutrophils that responded to inflammatory stimuli in the gut occurs mainly through efferocytosis by macrophages as they become apoptotic. While both tissue-resident macrophages and monocyte-derived inflammatory macrophages can clear apoptotic cells, how they respond to this process may be distinct (65). For example, expression of Tim-4 can distinguish between tissue-resident and inflammatory macrophages. Tim-4 is a PtdSer recognition receptor and macrophages lacking Tim-4 have reduced apoptotic cell engulfment (66). Probably, a combination of intrinsic signals (e.g. triggered by phagocytosis) and extrinsic signals (e.g. cytokines such as IL-4, IL-13 and IL-10) enable the intestinal macrophages to adopt a wound healing anti-inflammatory response to counteract tissue damage and restore homeostasis. Efferocytosis can induce TGF-β (67), which promotes the differentiation of Treg cells. Retinoic acid produced by IL-4 activated macrophages (68) as well as intestinal dendritic cells (69), acts synergistically with TGF-β to promote Treg differentiation. Hence, intestinal macrophages represent a key link between the combination of immune regulatory responses (e.g. Tregs and IL-10) as well as type-2 responses in the process of resolving tissue inflammation and promoting mucosal healing. ILC2s responding to the breach in barrier signals, including IL-25 and IL-33, are an important source of IL-13 alongside CD4+ TH2 cells. This type-2 cytokine environment then promotes the accumulation of alternatively activated M2 phenotype macrophages.
The traditional concept of activated pro-inflammatory M1 macrophages and anti-inflammatory M2 macrophages being important for antibacterial responses and inflammation resolution respectively (70), is becoming increasingly outdated in light of the remarkable heterogeneity of macrophage populations as revealed by single-cell RNA sequencing studies and other transcriptomic analyses (71–73). While polarization of macrophages partially contributes to this heterogeneity, it nonetheless does not provide a complete explanation. However, there are clearly macrophage populations that are important for wound healing and tissue repair that are dependent on type-2 cytokines (70, 74), and are further enhanced by phagocytosis of apoptotic cells (75).
Mucosal healing is a widely recognized treatment goal in the management of complex IBD patients. However, remission is achieved in only a proportion of patients, many of whom lose response over time and require surgical management (76–78). In patients who demonstrate a clinical response to infliximab, a distinct subset of macrophages expressing CD206 is induced and expanded compared to patients who failed to respond to the biologic (79). Furthermore, tofacitinib, a small molecule JAK inhibitor developed for the treatment of IBD as well as other immune-mediated disorders, affects macrophage polarization and function (80, 81). Upon treatment with tofacitinib, both murine and human macrophages increase transcription of alternatively-activated macrophage markers and increase levels of IL-10 secretion while inhibiting IFNγ signaling, which may help ameliorate weight loss and disease activity, and further highlight its therapeutic potential (81). Hence, there is some indirect evidence that macrophage activation pathways associated with tissue repair and type 2 cytokine responses could be associated with mucosal healing in IBD patients, or with the activity of successful therapeutic agents used to treat IBD patients.
Helminth infection and intestinal macrophages
In addition to the commensal gut bacteria, the gut is home to soil-transmitted helminths, especially intestinal nematodes. These worms can cause considerable tissue damage, which requires the activation of a type 2 cytokine mediated wound healing response (82). While the type 2 response is important for protecting the gut from intestinal injury during colonization, it is also important for expulsion of the parasites and for reducing worm burden in infected individuals. Notably, infection with helminths has also been proposed to promote resolution of inflammation in IBD (83, 84). The type 2 immune response induced by helminth infections could promote goblet cell activity and restoration of the mucus layer (85), but also induce alternatively-activated M2 macrophages in the gut to promote tissue repair and resolution of inflammation (86). While for some helminths (e.g. Heligomosoides polygyrus) this macrophage response is necessary for the expulsion of the parasites (87), in other settings (e.g. Nippostrongylus brasiliensis) macrophages do not play a role in expulsion, but may instead be important for tissue repair (88, 89). In mouse studies whereby helminth infection can improve symptoms of colitis, macrophages have been credited as the cell type important for the suppression of intestinal inflammation (90, 91). Macrophages stimulated in vitro with antigens from the tapeworm Hymenolepis diminuta, or interleukin-4 can improve colitis when adoptively transferred into mice (91, 92) via a mechanism that requires intact IL-10 (93). This could be mediated via induction of regulatory T cells (94), perhaps through retinoic acid (68, 95, 96). The source of IL-4 or IL-13 for alternatively activated macrophages during helminth infections is CD4+ T cells as well as innate lymphoid type 2 cells (ILC2s). Recently, production of neuromedin U (NMU) by neurons was shown to stimulate ILC2s to produce IL-5 and IL-13 (52, 97, 98) which can occur in the intestine during helminth infections. Hence, neuronal sensing of helminth infection may lead to the production of cytokines by ILC2s necessary for macrophage alternative activation. Alternatively-activated M2 macrophages may also play a role in shifting the balance of the gut microbiota towards an increasingly anti-inflammatory microbial community while inhibiting pro-inflammatory bacteria (99). If type 2 cytokines could be specifically targeted to the gut, this could be a potential therapeutic strategy to promote the resolution of intestinal inflammation by alternatively-activated M2 macrophages, while concomitantly activating goblet cells to restore the mucus barrier. However, one concern may be that this approach would increase susceptibility to certain intestinal bacterial infections (100, 101) or colorectal cancer (102, 103).
Conclusions
Significant progress has been made in recent years in advancing our understanding of both mouse and human intestinal macrophage immunobiology in the context of inflammation. What are the key unanswered questions for the future? First, our understanding of the heterogeneity of macrophage subtypes in the gut is still at an early stage. With the advent of single cell sequencing technologies, the next few years will surely lead to the identification of additional subtypes and different activation states of intestinal macrophages, which may arise from different cellular lineages, and perform distinct functions under both homeostatic conditions as well as inflammatory conditions. The concept of M1 and M2 activated macrophages will become outdated, as we are already beginning to appreciate that the complexity of macrophage subsets far exceeds such simple classification. What common nomenclature will we then use to describe this heterogeneity? Because of the plasticity of macrophages and extensive conditioning by the local microenvironment, each unique tissue location within the gut will possess specific markers and differentiation cues for the macrophages that reside within. Defining a common nomenclature, presumably based on both cell surface receptors and transcriptional profiles, to describe macrophage types will become increasingly challenging for the purposes of comparing different studies. Secondly, greater effort has to be made to characterize human intestinal macrophages and to relate the function of human macrophage subsets with mouse data. While mechanistic causality is always difficult to demonstrate in human studies, ex vivo experiments that can demonstrate similar immunological properties between specific populations with a mouse macrophage counterpart (where in vivo roles can be established experimentally) is a key component missing from current studies. Finally, we have a poor understanding of between-individual variation in macrophage populations. Whereas inbred strains of mice are relatively homogenous as they are genetically identical and are exposed to similar laboratory conditions, genetic and environmental variation will affect human intestinal macrophage populations and functions. Most immunological studies are conducted only on C57BL/6 mice and different genetic backgrounds may provide new insight to some key questions. The identification of various bacterial and parasitic commensals that may vary between animal facilities can affect intestinal immunity and could explain different experimental outcomes from the intestinal system. Given the integral role of macrophages in immune-mediated and functional gastrointestinal disorders and their potential as an attractive therapeutic target, it is critical to understand monocyte/macrophage compartment control mechanisms that are responsible for tipping the balance from homeostasis to disease. This may improve the feasibility of treating various gastrointestinal pathologies including IBD with interventions designed to exploit the ability of macrophages to fine tune immunological responses and influence clinical outcomes.
Acknowledgments
PL is supported by grants from the NIH (HL084312, AI133977, AI130945) and the Department of Defense (W81XWH-16–1-0255).
Footnotes
Disclosures
P.L. consults for and has equity in Toilabs.
References
- 1.Bain CC, and Mowat AM. 2014. Macrophages in intestinal homeostasis and inflammation. Immunological reviews 260: 102–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mowat AM, and Agace WW. 2014. Regional specialization within the intestinal immune system. Nature reviews. Immunology 14: 667–685. [DOI] [PubMed] [Google Scholar]
- 3.Shaw TN, Houston SA, Wemyss K, Bridgeman HM, Barbera TA, Zangerle-Murray T, Strangward P, Ridley AJL, Wang P, Tamoutounour S, Allen JE, Konkel JE, and Grainger JR. 2018. Tissue-resident macrophages in the intestine are long lived and defined by Tim-4 and CD4 expression. J Exp Med 215: 1507–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Schepper S, Verheijden S, Aguilera-Lizarraga J, Viola MF, Boesmans W, Stakenborg N, Voytyuk I, Schmidt I, Boeckx B, Dierckx de Casterle I, Baekelandt V, Gonzalez Dominguez E, Mack M, Depoortere I, De Strooper B, Sprangers B, Himmelreich U, Soenen S, Guilliams M, Vanden Berghe P, Jones E, Lambrechts D, and Boeckxstaens G. 2018. Self-Maintaining Gut Macrophages Are Essential for Intestinal Homeostasis. Cell 175: 400–415.e413. [DOI] [PubMed] [Google Scholar]
- 5.N, A. G., Quintana JA, Garcia-Silva S, Mazariegos M, Gonzalez de la Aleja A, Nicolas-Avila JA, Walter W, Adrover JM, Crainiciuc G, Kuchroo VK, Rothlin CV, Peinado H, Castrillo A, Ricote M, and Hidalgo A. 2017. Phagocytosis imprints heterogeneity in tissue-resident macrophages. J Exp Med 214: 1281–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin CJ, Peters KN, and Behar SM. 2014. Macrophages clean up: efferocytosis and microbial control. Curr Opin Microbiol 17: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamoutounour S, Henri S, Lelouard H, de Bovis B, de Haar C, van der Woude CJ, Woltman AM, Reyal Y, Bonnet D, Sichien D, Bain CC, Mowat AM, Reis e Sousa C, Poulin LF, Malissen B, and Guilliams M. 2012. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. European journal of immunology 42: 3150–3166. [DOI] [PubMed] [Google Scholar]
- 8.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma’ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, and Randolph GJ. 2012. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol 13: 1118–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bujko A, Atlasy N, Landsverk OJB, Richter L, Yaqub S, Horneland R, Oyen O, Aandahl EM, Aabakken L, Stunnenberg HG, Baekkevold ES, and Jahnsen FL. 2018. Transcriptional and functional profiling defines human small intestinal macrophage subsets. J Exp Med 215: 441–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bain CC, and Schridde A. 2018. Origin, Differentiation, and Function of Intestinal Macrophages. Front Immunol 9: 2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Forster R, and Agace WW. 2005. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med 202: 1063–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, and Powrie F. 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 204: 1757–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, and Reinecker HC. 2005. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science (New York, N.Y.) 307: 254–258. [DOI] [PubMed] [Google Scholar]
- 14.Chieppa M, Rescigno M, Huang AY, and Germain RN. 2006. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med 203: 2841–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Man AL, Gicheva N, Regoli M, Rowley G, De Cunto G, Wellner N, Bassity E, Gulisano M, Bertelli E, and Nicoletti C. 2017. CX3CR1+ Cell-Mediated Salmonella Exclusion Protects the Intestinal Mucosa during the Initial Stage of Infection. Journal of immunology (Baltimore, Md. : 1950) 198: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sehgal A, Donaldson DS, Pridans C, Sauter KA, Hume DA, and Mabbott NA. 2018. The role of CSF1R-dependent macrophages in control of the intestinal stem-cell niche. Nat Commun 9: 1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, Orenstein JM, and Smith PD. 2005. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. Journal of Clinical Investigation 115: 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zigmond E, Bernshtein B, Friedlander G, Walker CR, Yona S, Kim KW, Brenner O, Krauthgamer R, Varol C, Muller W, and Jung S. 2014. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity 40: 720–733. [DOI] [PubMed] [Google Scholar]
- 19.Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D, Hatscher N, Pfeifer D, Sykora KW, Sauer M, Kreipe H, Lacher M, Nustede R, Woellner C, Baumann U, Salzer U, Koletzko S, Shah N, Segal AW, Sauerbrey A, Buderus S, Snapper SB, Grimbacher B, and Klein C. 2009. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. The New England journal of medicine 361: 2033–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shouval DS, Biswas A, Kang YH, Griffith AE, Konnikova L, Mascanfroni ID, Redhu NS, Frei SM, Field M, Doty AL, Goldsmith JD, Bhan AK, Loizides A, Weiss B, Yerushalmi B, Yanagi T, Lui X, Quintana FJ, Muise AM, Klein C, Horwitz BH, Glover SC, Bousvaros A, and Snapper SB. 2016. Interleukin 1beta Mediates Intestinal Inflammation in Mice and Patients With Interleukin 10 Receptor Deficiency. Gastroenterology 151: 1100–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, Muller W, Sparwasser T, Forster R, and Pabst O. 2011. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity 34: 237–246. [DOI] [PubMed] [Google Scholar]
- 22.Shaw MH, Kamada N, Kim YG, and Nunez G. 2012. Microbiota-induced IL-1beta, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. J Exp Med 209: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hume DA, Irvine KM, and Pridans C. 2019. The Mononuclear Phagocyte System: The Relationship between Monocytes and Macrophages. Trends in immunology 40: 98–112. [DOI] [PubMed] [Google Scholar]
- 24.Gentek R, Molawi K, and Sieweke MH. 2014. Tissue macrophage identity and self-renewal. Immunological reviews 262: 56–73. [DOI] [PubMed] [Google Scholar]
- 25.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, and Jung S. 2013. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38: 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bain CC, Bravo-Blas A, Scott CL, Perdiguero EG, Geissmann F, Henri S, Malissen B, Osborne LC, Artis D, and Mowat AM. 2014. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol 15: 929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ginhoux F, and Jung S. 2014. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nature reviews. Immunology 14: 392–404. [DOI] [PubMed] [Google Scholar]
- 28.Zigmond E, Varol C, Farache J, Elmaliah E, Satpathy AT, Friedlander G, Mack M, Shpigel N, Boneca IG, Murphy KM, Shakhar G, Halpern Z, and Jung S. 2012. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity 37: 1076–1090. [DOI] [PubMed] [Google Scholar]
- 29.Zigmond E, and Jung S. 2013. Intestinal macrophages: well educated exceptions from the rule. Trends in immunology 34: 162–168. [DOI] [PubMed] [Google Scholar]
- 30.Guilliams M, and Scott CL. 2017. Does niche competition determine the origin of tissue-resident macrophages? Nature reviews. Immunology 17: 451–460. [DOI] [PubMed] [Google Scholar]
- 31.Muller PA, Koscso B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D, Stanley ER, Dahan S, Margolis KG, Gershon MD, Merad M, and Bogunovic M. 2014. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158: 300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacDonald KP, Palmer JS, Cronau S, Seppanen E, Olver S, Raffelt NC, Kuns R, Pettit AR, Clouston A, Wainwright B, Branstetter D, Smith J, Paxton RJ, Cerretti DP, Bonham L, Hill GR, and Hume DA. 2010. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue-and tumor-associated macrophages but does not inhibit inflammation. Blood 116: 3955–3963. [DOI] [PubMed] [Google Scholar]
- 33.Baillie JK, Arner E, Daub C, De Hoon M, Itoh M, Kawaji H, Lassmann T, Carninci P, Forrest AR, Hayashizaki Y, Consortium F, Faulkner GJ, Wells CA, Rehli M, Pavli P, Summers KM, and Hume DA. 2017. Analysis of the human monocyte-derived macrophage transcriptome and response to lipopolysaccharide provides new insights into genetic aetiology of inflammatory bowel disease. PLoS Genet 13: e1006641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, and Amit I. 2014. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159: 1312–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishifune C, Maruyama S, Sasaki Y, Yagita H, Hozumi K, Tomita T, Kishihara K, and Yasutomo K. 2014. Differentiation of CD11c+ CX3CR1+ cells in the small intestine requires Notch signaling. Proc Natl Acad Sci U S A 111: 5986–5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Danne C, Ryzhakov G, Martinez-Lopez M, Ilott NE, Franchini F, Cuskin F, Lowe EC, Bullers SJ, Arthur JSC, and Powrie F. 2017. A Large Polysaccharide Produced by Helicobacter hepaticus Induces an Anti-inflammatory Gene Signature in Macrophages. Cell Host Microbe 22: 733–745 e735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schridde A, Bain CC, Mayer JU, Montgomery J, Pollet E, Denecke B, Milling SWF, Jenkins SJ, Dalod M, Henri S, Malissen B, Pabst O, and McL Mowat A. 2017. Tissue-specific differentiation of colonic macrophages requires TGFbeta receptor-mediated signaling. Mucosal Immunol 10: 1387–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cummings RJ, Barbet G, Bongers G, Hartmann BM, Gettler K, Muniz L, Furtado GC, Cho J, Lira SA, and Blander JM. 2016. Different tissue phagocytes sample apoptotic cells to direct distinct homeostasis programs. Nature 539: 565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Regoli M, Bertelli E, Gulisano M, and Nicoletti C. 2017. The Multifaceted Personality of Intestinal CX3CR1(+) Macrophages. Trends in immunology 38: 879–887. [DOI] [PubMed] [Google Scholar]
- 40.Veiga-Fernandes H, and Mucida D. 2016. Neuro-Immune Interactions at Barrier Surfaces. Cell 165: 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gershon MD 2010. Developmental determinants of the independence and complexity of the enteric nervous system. Trends Neurosci 33: 446–456. [DOI] [PubMed] [Google Scholar]
- 42.Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, and Mucida D. 2016. Neuro-immune Interactions Drive Tissue Programming in Intestinal Macrophages. Cell 164: 378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Schepper S, Stakenborg N, Matteoli G, Verheijden S, and Boeckxstaens GE. 2018. Muscularis macrophages: Key players in intestinal homeostasis and disease. Cell Immunol 330: 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veiga-Fernandes H, and Artis D. 2018. Neuronal-immune system cross-talk in homeostasis. Science (New York, N.Y.) 359: 1465–1466. [DOI] [PubMed] [Google Scholar]
- 45.Avetisyan M, Rood JE, Huerta Lopez S, Sengupta R, Wright-Jin E, Dougherty JD, Behrens EM, and Heuckeroth RO. 2018. Muscularis macrophage development in the absence of an enteric nervous system. Proc Natl Acad Sci U S A 115: 4696–4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ge X, Ding C, Zhao W, Xu L, Tian H, Gong J, Zhu M, Li J, and Li N. 2017. Antibiotics-induced depletion of mice microbiota induces changes in host serotonin biosynthesis and intestinal motility. J Transl Med 15: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farro G, Stakenborg M, Gomez-Pinilla PJ, Labeeuw E, Goverse G, Di Giovangiulio M, Stakenborg N, Meroni E, D’Errico F, Elkrim Y, Laoui D, Lisowski ZM, Sauter KA, Hume DA, Van Ginderachter JA, Boeckxstaens GE, and Matteoli G. 2017. CCR2-dependent monocyte-derived macrophages resolve inflammation and restore gut motility in postoperative ileus. Gut 66: 2098–2109. [DOI] [PubMed] [Google Scholar]
- 48.Wehner S, Behrendt FF, Lyutenski BN, Lysson M, Bauer AJ, Hirner A, and Kalff JC. 2007. Inhibition of macrophage function prevents intestinal inflammation and postoperative ileus in rodents. Gut 56: 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stein K, Stoffels M, Lysson M, Schneiker B, Dewald O, Kronke G, Kalff JC, and Wehner S. 2016. A role for 12/15-lipoxygenase-derived proresolving mediators in postoperative ileus: protectin DX-regulated neutrophil extravasation. J Leukoc Biol 99: 231–239. [DOI] [PubMed] [Google Scholar]
- 50.Stein K, Lysson M, Schumak B, Vilz T, Specht S, Heesemann J, Roers A, Kalff JC, and Wehner S. 2018. Leukocyte-Derived Interleukin-10 Aggravates Postoperative Ileus. Front Immunol 9: 2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stoffels B, Schmidt J, Nakao A, Nazir A, Chanthaphavong RS, and Bauer AJ. 2009. Role of interleukin 10 in murine postoperative ileus. Gut 58: 648–660. [DOI] [PubMed] [Google Scholar]
- 52.Cardoso V, Chesne J, Ribeiro H, Garcia-Cassani B, Carvalho T, Bouchery T, Shah K, Barbosa-Morais NL, Harris N, and Veiga-Fernandes H. 2017. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 549: 277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim M, Galan C, Hill AA, Wu WJ, Fehlner-Peach H, Song HW, Schady D, Bettini ML, Simpson KW, Longman RS, Littman DR, and Diehl GE. 2018. Critical Role for the Microbiota in CX3CR1(+) Intestinal Mononuclear Phagocyte Regulation of Intestinal T Cell Responses. Immunity 49: 151–163 e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diehl GE, Longman RS, Zhang JX, Breart B, Galan C, Cuesta A, Schwab SR, and Littman DR. 2013. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature 494: 116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lissner D, Schumann M, Batra A, Kredel LI, Kuhl AA, Erben U, May C, Schulzke JD, and Siegmund B. 2015. Monocyte and M1 Macrophage-induced Barrier Defect Contributes to Chronic Intestinal Inflammation in IBD. Inflamm Bowel Dis 21: 1297–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith PD, Smythies LE, Shen R, Greenwell-Wild T, Gliozzi M, and Wahl SM. 2011. Intestinal macrophages and response to microbial encroachment. Mucosal Immunol 4: 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Richard AC, Peters JE, Savinykh N, Lee JC, Hawley ET, Meylan F, Siegel RM, Lyons PA, and Smith KGC. 2018. Reduced monocyte and macrophage TNFSF15/TL1A expression is associated with susceptibility to inflammatory bowel disease. PLoS Genet 14: e1007458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mokry M, Middendorp S, Wiegerinck CL, Witte M, Teunissen H, Meddens CA, Cuppen E, Clevers H, and Nieuwenhuis EE. 2014. Many inflammatory bowel disease risk loci include regions that regulate gene expression in immune cells and the intestinal epithelium. Gastroenterology 146: 1040–1047. [DOI] [PubMed] [Google Scholar]
- 59.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Buning C, Cohain A, Cichon S, D’Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H, International IBDGC, Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, and Cho JH. 2012. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491: 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schenk M, Bouchon A, Seibold F, and Mueller C. 2007. TREM-1--expressing intestinal macrophages crucially amplify chronic inflammation in experimental colitis and inflammatory bowel diseases. The Journal of clinical investigation 117: 3097–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bernardo D, Marin AC, Fernandez-Tome S, Montalban-Arques A, Carrasco A, Tristan E, Ortega-Moreno L, Mora-Gutierrez I, Diaz-Guerra A, Caminero-Fernandez R, Miranda P, Casals F, Caldas M, Jimenez M, Casabona S, De la Morena F, Esteve M, Santander C, Chaparro M, and Gisbert JP. 2018. Human intestinal pro-inflammatory CD11c(high)CCR2(+)CX3CR1(+) macrophages, but not their tolerogenic CD11c(−)CCR2(−)CX3CR1(−) counterparts, are expanded in inflammatory bowel disease. Mucosal Immunol 11: 1114–1126. [DOI] [PubMed] [Google Scholar]
- 62.Jones GR, Bain CC, Fenton TM, Kelly A, Brown SL, Ivens AC, Travis MA, Cook PC, and MacDonald AS. 2018. Dynamics of Colon Monocyte and Macrophage Activation During Colitis. Front Immunol 9: 2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lauvau G, Loke P, and Hohl TM. 2015. Monocyte-mediated defense against bacteria, fungi, and parasites. Seminars in immunology 27: 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schett G, and Neurath MF. 2018. Resolution of chronic inflammatory disease: universal and tissue-specific concepts. Nat Commun 9: 3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roberts AW, Lee BL, Deguine J, John S, Shlomchik MJ, and Barton GM. 2017. Tissue-Resident Macrophages Are Locally Programmed for Silent Clearance of Apoptotic Cells. Immunity 47: 913–927 e916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Albacker LA, Karisola P, Chang YJ, Umetsu SE, Zhou M, Akbari O, Kobayashi N, Baumgarth N, Freeman GJ, Umetsu DT, and DeKruyff RH. 2010. TIM-4, a receptor for phosphatidylserine, controls adaptive immunity by regulating the removal of antigen-specific T cells. Journal of immunology (Baltimore, Md. : 1950) 185: 6839–6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, and Henson PM. 1998. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. The Journal of clinical investigation 101: 890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Broadhurst MJ, Leung JM, Lim KC, Girgis NM, Gundra UM, Fallon PG, Premenko-Lanier M, McKerrow JH, McCune JM, and Loke P. 2012. Upregulation of retinal dehydrogenase 2 in alternatively activated macrophages during retinoid-dependent type-2 immunity to helminth infection in mice. PLoS pathogens 8: e1002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iwata M. 2009. Retinoic acid production by intestinal dendritic cells and its role in T-cell trafficking. Seminars in immunology 21: 8–13. [DOI] [PubMed] [Google Scholar]
- 70.Mantovani A, Biswas SK, Galdiero MR, Sica A, and Locati M. 2013. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 229: 176–185. [DOI] [PubMed] [Google Scholar]
- 71.Lin JD, Nishi H, Poles J, Niu X, McCauley C, Rahman K, Brown EJ, Yeung ST, Vozhilla N, Weinstock A, Ramsey SA, Fisher EA, and Loke P. 2019. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gordon S, Pluddemann A, and Martinez Estrada F. 2014. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunological reviews 262: 36–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martinez FO, Gordon S, Locati M, and Mantovani A. 2006. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. Journal of immunology (Baltimore, Md. : 1950) 177: 7303–7311. [DOI] [PubMed] [Google Scholar]
- 74.Minutti CM, Jackson-Jones LH, Garcia-Fojeda B, Knipper JA, Sutherland TE, Logan N, Ringqvist E, Guillamat-Prats R, Ferenbach DA, Artigas A, Stamme C, Chroneos ZC, Zaiss DM, Casals C, and Allen JE. 2017. Local amplifiers of IL-4Ralpha-mediated macrophage activation promote repair in lung and liver. Science (New York, N.Y.) 356: 1076–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bosurgi L, Cao YG, Cabeza-Cabrerizo M, Tucci A, Hughes LD, Kong Y, Weinstein JS, Licona-Limon P, Schmid ET, Pelorosso F, Gagliani N, Craft JE, Flavell RA, Ghosh S, and Rothlin CV. 2017. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science (New York, N.Y.) 356: 1072–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shmidt E, Kochhar G, Hartke J, Chilukuri P, Meserve J, Chaudrey K, Koliani-Pace JL, Hirten R, Faleck D, Barocas M, Luo M, Lasch K, Boland BS, Singh S, Vande Casteele N, Sagi SV, Fischer M, Chang S, Bohm M, Lukin D, Sultan K, Swaminath A, Hudesman D, Gupta N, Kane S, Loftus EV Jr., Sandborn WJ, Siegel CA, Sands BE, Colombel JF, Shen B, and Dulai PS. 2018. Predictors and Management of Loss of Response to Vedolizumab in Inflammatory Bowel Disease. Inflamm Bowel Dis 24: 2461–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roda G, Jharap B, Neeraj N, and Colombel JF. 2016. Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management. Clinical and translational gastroenterology 7: e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roblin X, Marotte H, Leclerc M, Del Tedesco E, Phelip JM, Peyrin-Biroulet L, and Paul S. 2015. Combination of C-reactive protein, infliximab trough levels, and stable but not transient antibodies to infliximab are associated with loss of response to infliximab in inflammatory bowel disease. J Crohns Colitis 9: 525–531. [DOI] [PubMed] [Google Scholar]
- 79.Vos AC, Wildenberg ME, Arijs I, Duijvestein M, Verhaar AP, de Hertogh G, Vermeire S, Rutgeerts P, van den Brink GR, and Hommes DW. 2012. Regulatory macrophages induced by infliximab are involved in healing in vivo and in vitro. Inflamm Bowel Dis 18: 401–408. [DOI] [PubMed] [Google Scholar]
- 80.Zhang MZ, Wang X, Wang Y, Niu A, Wang S, Zou C, and Harris RC. 2017. IL-4/IL-13-mediated polarization of renal macrophages/dendritic cells to an M2a phenotype is essential for recovery from acute kidney injury. Kidney international 91: 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Vries LCS, Duarte JM, De Krijger M, Welting O, Van Hamersveld PHP, Van Leeuwen-Hilbers FWM, Moerland PD, Jongejan A, D’Haens GR, De Jonge WJ, and Wildenberg ME. 2019. A JAK1 Selective Kinase Inhibitor and Tofacitinib Affect Macrophage Activation and Function. Inflamm Bowel Dis. [DOI] [PubMed] [Google Scholar]
- 82.Allen JE, and Wynn TA. 2011. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS pathogens 7: e1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Summers RW, Elliott DE, Urban JF Jr., Thompson R, and Weinstock JV. 2005. Trichuris suis therapy in Crohn’s disease. Gut 54: 87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Summers RW, Elliott DE, Urban JF, Thompson RA, and Weinstock JV. 2005. Trichuris suis therapy for active ulcerative colitis: A randomized controlled trial. Gastroenterology 128: 825–832. [DOI] [PubMed] [Google Scholar]
- 85.Wolff MJ, Broadhurst MJ, and Loke P. 2012. Helminthic therapy: improving mucosal barrier function. Trends Parasitol 28: 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wynn TA, and Vannella KM. 2016. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 44: 450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maizels RM, Hewitson JP, and Smith KA. 2012. Susceptibility and immunity to helminth parasites. Current opinion in immunology 24: 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, Millman A, Van Rooijen N, Urban JF Jr, Wynn TA, and Gause WC. 2012. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nature Medicine 18: 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Borthwick LA, Barron L, Hart KM, Vannella KM, Thompson RW, Oland S, Cheever A, Sciurba J, Ramalingam TR, Fisher AJ, and Wynn TA. 2016. Macrophages are critical to the maintenance of IL-13-dependent lung inflammation and fibrosis. Mucosal Immunol 9: 38–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smith P, Mangan NE, Walsh CM, Fallon RE, McKenzie AN, van Rooijen N, and Fallon PG. 2007. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. Journal of immunology (Baltimore, Md. : 1950) 178: 4557–4566. [DOI] [PubMed] [Google Scholar]
- 91.Hunter MM, Wang A, Parhar KS, Johnston MJ, Van Rooijen N, Beck PL, and McKay DM. 2010. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology 138: 1395–1405. [DOI] [PubMed] [Google Scholar]
- 92.Leung G, Petri B, Reyes JL, Wang A, Iannuzzi J, and McKay DM. 2016. Cryopreserved Interleukin-4-Treated Macrophages Attenuate Murine Colitis in an Integrin beta7-Dependent Manner. Molecular medicine (Cambridge, Mass.) 21: 924–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leung G, Wang A, Fernando M, Phan VC, and McKay DM. 2013. Bone marrow-derived alternatively activated macrophages reduce colitis without promoting fibrosis: participation of IL-10. Am J Physiol Gastrointest Liver Physiol 304: G781–792. [DOI] [PubMed] [Google Scholar]
- 94.Reyes JL, Lopes F, Leung G, Jayme TS, Matisz CE, Shute A, Burkhard R, Carneiro M, Workentine ML, Wang A, Petri B, Beck PL, Geuking MB, and McKay DM. 2019. Macrophages treated with antigen from the tapeworm Hymenolepis diminuta condition CD25(+) T cells to suppress colitis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology: fj201802160R. [DOI] [PubMed] [Google Scholar]
- 95.Gundra UM, Girgis NM, Ruckerl D, Jenkins S, Ward LN, Kurtz ZD, Wiens KE, Tang MS, Basu-Roy U, Mansukhani A, Allen JE, and Loke P. 2014. Alternatively activated macrophages derived from monocytes and tissue macrophages are phenotypically and functionally distinct. Blood 123: e110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ouimet M, Ediriweera HN, Gundra UM, Sheedy FJ, Ramkhelawon B, Hutchison SB, Rinehold K, van Solingen C, Fullerton MD, Cecchini K, Rayner KJ, Steinberg GR, Zamore PD, Fisher EA, Loke P, and Moore KJ. 2015. MicroRNA-33-dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. The Journal of clinical investigation 125: 4334–4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Klose CSN, Mahlakoiv T, Moeller JB, Rankin LC, Flamar AL, Kabata H, Monticelli LA, Moriyama S, Putzel GG, Rakhilin N, Shen X, Kostenis E, Konig GM, Senda T, Carpenter D, Farber DL, and Artis D. 2017. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 549: 282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wallrapp A, Riesenfeld SJ, Burkett PR, Abdulnour RE, Nyman J, Dionne D, Hofree M, Cuoco MS, Rodman C, Farouq D, Haas BJ, Tickle TL, Trombetta JJ, Baral P, Klose CSN, Mahlakoiv T, Artis D, Rozenblatt-Rosen O, Chiu IM, Levy BD, Kowalczyk MS, Regev A, and Kuchroo VK. 2017. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature 549: 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ramanan D, Bowcutt R, Lee SC, Tang MS, Kurtz ZD, Ding Y, Honda K, Gause WC, Blaser MJ, Bonneau RA, Lim YA, Loke P, and Cadwell K. 2016. Helminth infection promotes colonization resistance via type 2 immunity. Science (New York, N.Y.) 352: 608–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weng M, Huntley D, Huang IF, Foye-Jackson O, Wang L, Sarkissian A, Zhou Q, Walker WA, Cherayil BJ, and Shi HN. 2007. Alternatively activated macrophages in intestinal helminth infection: effects on concurrent bacterial colitis. Journal of immunology (Baltimore, Md. : 1950) 179: 4721–4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Su CW, Cao Y, Zhang M, Kaplan J, Su L, Fu Y, Walker WA, Xavier R, Cherayil BJ, and Shi HN. 2012. Helminth infection impairs autophagy-mediated killing of bacterial enteropathogens by macrophages. Journal of immunology (Baltimore, Md. : 1950) 189: 1459–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hayes KS, Cliffe LJ, Bancroft AJ, Forman SP, Thompson S, Booth C, and Grencis RK. 2017. Chronic Trichuris muris infection causes neoplastic change in the intestine and exacerbates tumour formation in APC min/+ mice. PLoS neglected tropical diseases 11: e0005708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pastille E, Frede A, McSorley HJ, Grab J, Adamczyk A, Kollenda S, Hansen W, Epple M, Buer J, Maizels RM, Klopfleisch R, and Westendorf AM. 2017. Intestinal helminth infection drives carcinogenesis in colitis-associated colon cancer. PLoS pathogens 13: e1006649. [DOI] [PMC free article] [PubMed] [Google Scholar]