Abstract
Background
Coronavirus disease 2019 (COVID-19) is associated with a high disease burden with 10% of confirmed cases progressing towards critical illness. Nevertheless, the disease course and predictors of mortality in critically ill patients are poorly understood.
Methods
Following the critical developments in ICUs in regions experiencing early inception of the pandemic, the European-based, international RIsk Stratification in COVID-19 patients in the Intensive Care Unit (RISC-19-ICU) registry was created to provide near real-time assessment of patients developing critical illness due to COVID-19.
Findings
As of April 22, 2020, 639 critically ill patients with confirmed SARS-CoV-2 infection were included in the RISC-19-ICU registry. Of these, 398 had deceased or been discharged from the ICU. ICU-mortality was 24%, median length of stay 12 (IQR, 5–21) days. ARDS was diagnosed in 74%, with a minimum P/F-ratio of 110 (IQR, 80–148). Prone positioning, ECCO2R, or ECMO were applied in 57%. Off-label therapies were prescribed in 265 (67%) patients, and 89% of all bloodstream infections were observed in this subgroup (n = 66; RR=3·2, 95% CI [1·7–6·0]). While PCT and IL-6 levels remained similar in ICU survivors and non-survivors throughout the ICU stay (p = 0·35, 0·34), CRP, creatinine, troponin, d-dimer, lactate, neutrophil count, P/F-ratio diverged within the first seven days (p<0·01). On a multivariable Cox proportional-hazard regression model at admission, creatinine, d-dimer, lactate, potassium, P/F-ratio, alveolar-arterial gradient, and ischemic heart disease were independently associated with ICU-mortality.
Interpretation
The European RISC-19-ICU cohort demonstrates a moderate mortality of 24% in critically ill patients with COVID-19. Despite high ARDS severity, mechanical ventilation incidence was low and associated with more rescue therapies. In contrast to risk factors in hospitalized patients reported in other studies, the main mortality predictors in these critically ill patients were markers of oxygenation deficit, renal and microvascular dysfunction, and coagulatory activation. Elevated risk of bloodstream infections underscores the need to exercise caution with off-label therapies.
Keywords: COVID-19, Coronavirus, Pandemic, Public health, Acute respiratory distress syndrome
Research in context.
Evidence before this study
We performed a PubMed search through April 22, 2020 with no date or language limitations using the keywords (“COVID-19″ or “SARS-CoV-2″) and “cohort” and “characteristics”. Baseline characteristics of hospitalized patients were reported in regions such as China, Northern Italy or specific areas in the United States. Two studies that applied multivariable modeling of risk factors for mortality and severe disease in hospitalized patients, respectively, were recently reported in China.
Added value of this study
We report results from a prospective European cohort of critically ill patients due to COVID-19. The data include the evaluation of clinical, physiological, and laboratory parameters collected on a daily basis, as well as intensive care unit mortality. Our findings accurately characterize severe cases of COVID-19 and identify predictors of mortality at the onset of critical illness.
Implications of all the available evidence
The in-depth characterization of critically ill COVID-19 patients and predictors of treatment outcome presented here complement data from other cohorts to provide crucial information for decision-making during this exceptional public health crisis.
Alt-text: Unlabelled box
1. Introduction
In December 2019, a cluster of atypical severe pneumonia was described in Wuhan, China, associated with the Huanan Seafood Wholesale Market [1]. The World Health Organization (WHO) named the novel virus associated with acute respiratory distress syndrome (ARDS) as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), with the associated disease coronavirus disease 2019 (COVID-19) [2]. COVID-19 is a symptomatically and asymptomatically transmissible disease, with a presumed incubation period of up to 14 days. During the first months of 2020, a rapid global increase in case numbers and deaths have made this pandemic one of the most critical global health emergencies in modern times [3]. Approximately 10% of confirmed cases progress to critical illness [4,5] with acute lung failure and, in some cases, multi-organ failure involving the heart, kidney, and gastrointestinal tract, with a high mortality rate [6]. Reported predisposing factors for severe disease include older age, chronic arterial hypertension, and established cardiovascular disease; an underlying virally-triggered endotheliitis has been postulated as a pathophysiological mechanism [4], [5], [6], [7], [8]. Nevertheless, whilst epidemiological data on critically ill patients have been well described, the understanding of disease progression and indicators for mortality in critical ill patients remains scarce.
Following the critical spread of the disease in China, Italy and Spain, on March 13, 2020 the European-based RIsk Stratification in COVID-19 patients in the ICU (RISC-19-ICU) registry was launched to allow near-real time assessment of the main clinical characteristics of critically ill patients during the emerging COVID-19 pandemic. Understanding patient characteristics associated with severe forms of COVID-19 is crucial not only for triage and therapeutic selection in these critically ill patients, but also to generate hypotheses based on the pathophysiology of the disease and to support the design of further trials.
In the present study, we report the baseline characteristics and status at ICU admission of the first 639 European patients with confirmed COVID-19 included in the RISC-19-ICU prospective cohort. Disease progression through the initial seven days of intensive care unit (ICU) stay and prognostic factors for ICU mortality are presented for the 398 patients that had completed their ICU stay as of April 22, 2020.
2. Materials and methods
This prospective observational cohort study is based on the data collected in the RISC-19-ICU registry. The registry was deemed exempt from the need for additional ethics approval and patient informed consent by the ethics committee of the University of Zurich (KEK 2020–00,322, ClinicalTrials.gov Identifier: NCT04357275). The study complies with the Declaration of Helsinki, the Guidelines on Good Clinical Practice (GCP-Directive) issued by the European Medicines Agency as well as the Swiss law and Swiss regulatory authority requirements, and has been designed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for observational studies [9]. All collaborating centres have complied with local legal and ethical requirements.
2.1. Registry structure and data collection
A standardized dataset was prospectively collected during the ongoing COVID-19 pandemic for all critically ill COVID-19 patients admitted to the collaborating centres. Inclusion criteria for the RISC-19-ICU registry were (I) a laboratory confirmed SARS-CoV-2 infection by nucleic acid amplification according to the WHO-issued testing guidelines [10], and (II) severe manifestation of COVID-19 requiring treatment in an ICU or intermediate care unit, defined as a hospital ward specialized in the care of critically ill patients with the availability of organ support therapies including invasive mechanical ventilation and/or non-invasive ventilation. The data was collected through an anonymized electronic case report form managed by the REDCap electronic data capture tool hosted on a secure server by the Swiss Society of Intensive Care Medicine [11]. The registry has been designed to support a collaborative approach to data analysis by permitting all collaborating centres to request an analysis of the full dataset after approval of a study protocol by the registry board. Additionally, code for registry-specific data transformation and statistical analysis has been made available for collaborative development [12]. As of April 22, 2020, 54 collaborating centres in 10 countries were contributing to the RISC-19-ICU registry. Data were collected on the day of ICU admission, and on days one, two, three, five and seven thereafter. Data contained in the registry included patient characteristics, treatment modalities and organ support therapies, including the use of mechanical ventilation, prone positioning, vital parameters, arterial blood gas analyses, and laboratory values such as inflammatory, coagulation, renal, liver, cardiac, and other relevant parameters. Missing values were accounted for but not imputed for the analysis (Suppl. Tables 1 and 2).
2.2. Clinical definitions
ARDS was defined according to the Berlin definition as acute, diffuse bilateral lung infiltrates of non-cardiac origin, characterized by hypoxemia with a PaO2/FiO2 ratio (P/F ratio) ≤ 300 mmHg under positive pressure respiratory support (≥5cmH2O positive end-expiratory airway pressure or continuous positive airway pressure) [13]. Acute kidney injury was diagnosed in accordance with the KDIGO criteria as either a serum creatinine increase to more than 1.5 x the baseline value, an absolute creatinine increase of ≥ 26.5 μmol/l, or a urine output of less than 0.5 ml/kg/h for 6–12 h [14]. Acute cardiac injury was defined according to the Fourth Universal Definition of Myocardial Infarction, as an elevation in high sensitivity cardiac Troponin levels above the 99th percentile, coupled to the existence of a dynamic change in said levels [15]. Bacteraemia and fungaemia were defined as positive blood cultures for a bacterial or fungal pathogen.
2.3. Statistical analysis
For longitudinal analysis of clinical and laboratory parameters, differences between time points and outcome status were tested using linear mixed effects model analysis. As independent variable fixed effects, time point and outcome status were entered into the model, respectively, with and without interaction terms, which were retained only if they were found to contribute to the model. As random effects, intercepts for subjects as well as per-subject random slopes for the effect on dependent variables were employed. P values were calculated using a likelihood ratio test of the full model with the effect in question against a “null model” without the effect in question. P values for individual fixed effects were obtained by Satterthwaite approximation in a multi-dimensional model comprising time point and outcome status. In patients that have died in the ICU or were discharged from the ICU, the prognostic value to dichotomize ICU survival according to the study variables was analysed using univariable and multivariable Cox proportional hazard models; non-normally distributed variables were logarithmically transformed. Multivariable analysis was performed by means of an iterative, step-wise, maximum likelihood optimizing algorithm initiated with the seven most significant variables in the univariable analysis, and considering all variables with p<0·1 on the univariable analysis, for the final model. Effects of sample size reduction on hazard ratios due to missing values were considered by comparison of the final model to a model excluding the respective variable. Censoring was applied to ICU survivors at the time of discharge to account for the possibility of an unfavorable outcome during the further hospitalization. Receiver operating characteristics (ROC) analysis was employed alongside minimal Euclidean distance fitting to the (0, 1) point to determine the optimal cut-off values for variables included in the final model. ICU survival functions were generated by implementing the Kaplan-Meier estimator. Comparisons of population characteristics were performed using paired Student's t-test or Wilcoxon Signed Rank Test, as appropriate, and the chi-squared test for categorical variables. Due to the observational, prospective nature of this cohort study during the ongoing health crisis, no power calculations were performed. Statistical analysis was performed through a fully scripted data management pathway using the R environment for statistical computing version 3·6 0·1 [16]. A two-sided p<0·05 was considered statistically significant. Values are given as median with interquartile ranges or counts and percentages as appropriate.
2.4. Data statement
Any intensive care unit or other center treating critically ill COVID-19 patients is invited to join the RISC-19-ICU registry at https://www.risc-19-icu.net. While the registry protocol prevents the deposition of the full registry dataset in a third-party repository, analyses on the full dataset may be requested by any collaborating center after approval of the study protocol by the registry board. Reproducibility of the results in the present study was ensured by providing code for registry-specific data transformation and statistical analysis for collaborative development on the GitHub and Zenodo repositories [12]. The registry protocol and data dictionary is publicly accessible at https://www.risc-19-icu.net.
2.4.1. Role of the funding source
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
3. Results
3.1. Population characteristics
As of April 22, 2020, a total of 639 critically ill patients with COVID-19 admitted to European collaborating centres had been included in the RISC-19-ICU registry. The patients were 63 [53–71] years old, predominantly male (75·1%), and 315 (49·3%) had one or more comorbidities (Table 1). The first symptoms of SARS-CoV-19 infection were noted 7 [4–9] days before hospital admission, and the patients were hospitalized 1 [0–3] days before admission to the ICU. At ICU admission, 317 (49·6%) patients were intubated and 326 (51·0%) met ARDS diagnosis criteria, with a P/F ratio of 136 [90–194] mmHg and inspiratory oxygen fraction of 60 [44–80]% (Table 2). As of April 22, 2020, 301 patients had been discharged from and 97 had died in the ICU, resulting in an ICU mortality of 24·3%; ICU length of stay was 12 [5–21] days (Table 3). In 24 (24·7%) of all non-survivors, death was secondary to a failure to stabilize acute organ dysfunction, while life support was withdrawn in 73 non-survivors. The mortality rate in ARDS patients was 31% and not correlated to initial disease severity. Population characteristics (Table 1), organ function, and laboratory values at ICU admission (Table 2) were stratified by ICU mortality.
Table 1.
Patient characteristics at ICU admission.
| Overall | ICU survivor | ICU non-survivor | p | |
|---|---|---|---|---|
| n = 639 | n = 301 | n = 97 | ||
| Demographics | ||||
| Age, years | 63 [53 - 71] | 62 [54 - 70] | 71 [62 - 78] | <0·001 |
| Age stratification | <0·001 | |||
| <18 | 7 (1·1) | 1 (0·3) | 0 (0·0) | |
| 18–30 | 46 (7·2) | 2 (0·7) | 1 (1·0) | |
| 31–40 | 21 (3·3) | 16 (5·3) | 1 (1·0) | |
| 41–50 | 63 (9·9) | 39 (13·0) | 3 (3·1) | |
| 51–60 | 138 (21·6) | 71 (23·6) | 16 (16·5) | |
| 61–70 | 191 (29·9) | 103 (34·2) | 27 (27·8) | |
| 71–80 | 139 (21·8) | 55 (18·3) | 37 (38·1) | |
| 81–90 | 32 (5·0) | 12 (4·0) | 12 (12·4) | |
| >90 | 1 (0·2) | 1 (0·3) | 0 (0·0) | |
| Sex, male | 447 (75·1) | 231 (76·7) | 69 (71·1) | 0·327 |
| BMI, kg m-2 | 27·7 [25·2 - 31·1] | 27·8 [25·3 - 31·2] | 27·8 [25·0 - 30·5] | 0·589 |
| Health care workers | 21 (3·6) | 14 (4·7) | 2 (2·1) | 0·424 |
| Smoking status | 0·298 | |||
| Never Smoked | 327 (67·3) | 181 (60·1) | 47 (48·5) | |
| Smoker or previous smoker | 159 (32·7) | 74 (24·6) | 28 (28·9) | |
| ICU-admission from | 0·629 | |||
| Emergency room | 148 (31·0) | 74 (24·6) | 30 (30·9) | |
| Normal ward | 182 (38·1) | 101 (33·6) | 31 (32·0) | |
| IMC | 68 (14·2) | 26 (8·6) | 7 (7·2) | |
| Other ICU | 80 (16·7) | 32 (10·6) | 14 (14·4) | |
| Time from symptoms onset to hospitalization, days | 7 [4 - 9] | 7 [4 - 9] | 5 [3 - 7] | 0·099 |
| Time from hospitalization to ICU admission, days | 1 [0 - 3] | 1 [0 - 3] | 1 [0 - 3] | 0·352 |
| Patients with comorbidities | 315 (49·3) | 143 (47·5) | 69 (71·1) | <0·001 |
| Chronic arterial hypertension | 282 (44·1) | 136 (45·2) | 57 (58·8) | 0·027 |
| Ischemic heart disease | 81 (12·7) | 34 (11·2) | 21 (21·6) | 0·016 |
| Other heart disease | 71 (11·1) | 31 (10·3) | 20 (20·6) | 0·014 |
| Diabetes mellitus | 147 (23·0) | 70 (23·3) | 31 (32·0) | 0·114 |
| Chronic pulmonary disease | 80 (12·5) | 39 (13·0) | 18 (18·6) | 0·229 |
| Immunosuppression | 73 (11·4) | 30 (10·0) | 21 (21·6) | 0·005 |
| Country | 0·001 | |||
| Switzerland | 455 (71·2) | 204 (67·8) | 58 (59·8) | |
| Spain | 64 (10·0) | 36 (12·0) | 16 (16·5) | |
| Italy | 35 (5·5) | 23 (7·6) | 1 (1·0) | |
| France | 30 (4·7) | 21 (7·0) | 8 (8·2) | |
| Germany | 25 (3·9) | 9 (3·0) | 5 (5·2) | |
| Others | 30 (4·6) | 8 (2·7) | 9 (9·3) |
Values are given as median [IQR] or count (percent) as appropriate. Health care workers include nurses and physicians who were after infection with SARS-CoV-2 admitted to an ICU as patients. BMI, body mass index; IMC, intermediate care unit; ICU, intensive care unit.
Table 2.
Organ function, vital signs, and laboratory panel at ICU admission.
| Overall | ICU survivor | ICU non-survivor | p | |
|---|---|---|---|---|
| At ICU admission | n = 639 | n = 301 | n = 97 | |
| Organ function | ||||
| APACHE II score | 16 [8 - 21] | 15 [7 - 20] | 20 [13 - 24] | <0•001 |
| SAPS II score | 53 [32 - 68] | 51 [32 - 66] | 67 [46 - 75] | <0•001 |
| SOFA score | 9 [6 - 13] | 9 [6 - 12] | 10 [7 - 13] | 0•190 |
| Need for vasopressors | 160 (25·0) | 62 (20·2) | 27 (27·8) | 0·123 |
| Norepinephrine, µg kg−1 min−1 | 0 [0 - 0·04] | 0 [0 - 0·03] | 0 [0 - 0·08] | 0·015 |
| Respiratory support | 0·002 | |||
| Nasal Cannula | 66 (10·3) | 43 (14·3) | 8 (8·2) | |
| Mask | 89 (13·9) | 54 (17·9) | 17 (17·5) | |
| High flow oxygen therapy | 25 (3·9) | 17 (5·6) | 4 (4·1) | |
| NIV | 27 (4·2) | 12 (4·0) | 9 (9·3) | |
| Mechanical ventilation | 317 (49·6) | 135 (44·9) | 58 (59·8) | |
| ARDS diagnostic criteria fulfilled | 326 (51·0) | 142 (47·2) | 64 (66·0) | 0·648 |
| Mild | 38 (11·0) | 18 (6·0) | 9 (9·3) | |
| Moderate | 179 (52·0) | 74 (24·6) | 38 (39·2) | |
| Severe | 109 (31·7) | 50 (16·6) | 17 (17·5) | |
| FiO2, % | 60 [44 - 80] | 60 [40 - 90] | 65 [50 - 80] | 0·387 |
| P/F ratio, mmHg | 136 [90 - 194] | 139 [91 - 202] | 131 [85 - 192] | 0·214 |
| A-a gradient, mmHg | 358 [246 - 514] | 360 [226 - 516] | 361 [277 - 517] | 0·407 |
| Ventilatory ratio, ml mmHg kg−1 min−1 | 1·66 [1·32 - 2·06] | 1·61 [1·31 - 2·05] | 1·64 [1·36 - 2] | 0·702 |
| ROX index | 7·06 [4·86 - 9·87] | 6·86 [4·90 - 10·01] | 6·85 [4·56 - 9·53] | 0·665 |
| Glasgow coma scale | 15 [3 - 15] | 15 [3 - 15] | 15 [3 - 15] | 0·075 |
| Estimated urine output | 0·001 | |||
| Normal | 488 (85·3) | 266 (88·4) | 74 (76·3) | |
| Oliguric | 64 (11·2) | 27 (9·0) | 15 (15·5) | |
| Anuric | 20 (3·5) | 5 (1·7) | 8 (8·2) | |
| Vitals | ||||
| Mean arterial pressure, mmHg | 81 [71 - 92] | 85 [72·75 - 94] | 75 [69 - 86] | 0·002 |
| Heart rate, min−1 | 86 [75 - 99] | 85 [74 - 97] | 87 [75 - 99] | 0·735 |
| Respiratory rate, min−1 | 23 [19 - 28] | 22 [19 - 28] | 24 [19 - 27] | 0·758 |
| Temperature, °C | 37·4 [37·0 - 38·4] | 37·3 [37·0 - 38·2] | 37·4 [36·4 - 38·0] | 0·151 |
| Laboratory panel | ||||
| Sodium, mmol/l | 137 [134 - 140] | 137 [134 - 139] | 137 [134 - 141] | 0·214 |
| Potassium, mmol/l | 3·9 [3·6 - 4·3] | 3·9 [3·5 - 4·2] | 4·1 [3·6 - 4·6] | 0·011 |
| Hematocrit, % | 38 [34 - 42] | 37 [33 - 40] | 40 [36 - 42] | 0·398 |
| Arterial pH | 7·42 [7·35 - 7·46] | 7·43 [7·36 - 7·47] | 7·40 [7·30 - 7·44] | <0·001 |
| PaO2, kPa | 9·7 [8·2 - 12·1] | 9·7 [8·3 - 12·4] | 9·9 [8·2 - 12·1] | 0·738 |
| PaCO2, kPa | 4·9 [4·2 - 5·9] | 4·8 [4·2 - 5·6] | 5·0 [4·2 - 6·1] | 0·204 |
| Arterial HCO3, mmol/l | 23·5 [21·4 - 25·9] | 23·7 [21·9 - 25·8] | 22·9 [20·6 - 24·6] | 0·007 |
| Arterial lactate, mmol/l | 1·2 [0·9 - 1·5] | 1·1 [0·8 - 1·5] | 1·3 [1·0 - 1·8] | 0·005 |
| White blood cell count, 109/l | 7·8 [5·6 - 10·7] | 7·4 [5·3 - 10·2] | 8·0 [6·1 - 11·6] | 0·024 |
| Neutrophil count, 109/l | 6·4 [4·4 - 9·3] | 6·0 [4·1 - 8·7] | 6·9 [5·1 - 9·8] | 0·020 |
| Lymphocyte count, 109/l | 0·75 [0·51 - 1·10] | 0·80 [0·56 - 1·04] | 0·70 [0·50 - 1·10] | 0·331 |
| Neutrophil/ Lymphocyte ratio | 8·1 [5·1 - 14·4] | 7·6 [4·8 - 13·3] | 9·6 [5·6 - 16·2] | 0·061 |
| Thrombocytes, 109/l | 205 [161 - 272] | 214 [167 - 282] | 191 [148 - 247] | 0·024 |
| Interleukin-6, ng/l | 142 [50 - 361] | 104 [50 - 289] | 173 [50 - 381] | 0·703 |
| CRP, mg/l | 141 [77 - 221] | 136 [76 - 217] | 143 [93 - 216] | 0·406 |
| PCT, µg/l | 0·34 [0·19 - 1·06] | 0·26 [0·16 - 0·76] | 0·45 [0·20 - 1·44] | 0·006 |
| D-Dimers, µg/l | 1329 [800 - 2813] | 1149 [720 - 2034] | 1900 [830 - 4620] | 0·016 |
| Ferritin, µg/l | 1393 [749 - 2213] | 1283 [683 - 2126] | 1377 [791 - 2253] | 0·839 |
| LDH, U/l | 488 [378 - 679] | 465 [364 - 639] | 506 [427 - 673] | 0·035 |
| Bilirubin, µmol/l | 9 [5 - 14] | 10 [5 - 14] | 9 [6 - 14] | 0·988 |
| Urea, mmol/l | 7·7 [4·7 - 19·1] | 6·5 [4·0 - 14·9] | 12·9 [6·4 - 31·7] | <0·001 |
| Creatinine, µmol/l | 84 [67 - 112] | 79 [65 - 99] | 88 [71 - 154] | 0·002 |
| CK, U/l | 152 [70 - 352] | 137 [75 - 262] | 160 [66 - 385] | 0·636 |
| Myoglobin, µg/l | 93 [45 - 317] | 115 [51 - 297] | 93 [27 - 938] | 0·925 |
| Troponin, ng/l | 18·0 [10·0 - 48·0] | 13·1 [8·0 - 28·6] | 43·1 [16·4 - 96·0] | <0·001 |
| Albumin, g/l | 28 [23 - 32] | 28 [23 - 33] | 27 [23 - 30] | 0·226 |
Values are given as median [IQR] or count (percent) as appropriate. APACHE II, Acute Physiology And Chronic Health Evaluation II; SAPS II, Simplified Acute Physiology Score II; SOFA, Sequential Organ Failure Assessment; ARDS, Acute Respiratory Distress Syndrome; NIV, non-invasive ventilation; FiO2, fraction of inspired O2; P/F ratio, PaO2/ FiO2 ratio; A-a gradient, alveolo-arterial gradient; PaO2, partial pressure of arterial O2; PaCO2, partial pressure of arterial CO2; CRP, c-reactive protein, PCT, procalcitonin; LDH, lactate dehydrogenase; CK, creatine kinase.
Table 3.
Organ support, ICU treatment and adverse events within the first seven days of the ICU stay.
| ICU Outcome Only | ICU survivor | ICU non-survivor | p | |
|---|---|---|---|---|
| n = 398 | n = 301 | n = 97 | ||
| Organ support | ||||
| Maximal respiratory support | <0·001 | |||
| Mask | 74 (19·9) | 66 (21·9) | 8 (8·2) | |
| High flow oxygen therapy | 10 (2·5) | 10 (3·3) | 0 (0·0) | |
| NIV | 12 (3·2) | 9 (3·0) | 3 (3·1) | |
| Mechanical ventilation | 274 (68·8) | 188 (62·5) | 86 (88·7) | |
| Lowest P/F ratio in initial 7 days, mmHg | 110 [80 - 148] | 113 [84 - 153] | 94 [71 - 127] | 0·001 |
| Worst ARDS classification in initial 7 days | 0·372 | |||
| Mild | 6 (1·5) | 5 (16·6) | 1 (1·0) | |
| Moderate | 131 (32·9) | 94 (31·2) | 37 (38·1) | |
| Severe | 131 (32·9) | 83 (27·6) | 48 (49·5) | |
| Need for vasopressors | 236 (68·8) | 161 (53·5) | 75 (77·3) | 0·001 |
| Highest norepinephrine levels in initial 7 days, µg kg−1 min−1 | 0·03 [0 - 0·14] | 0·02 [0 - 0·10] | 0·12 [0·02 - 0·27] | <0·001 |
| Hemodialysis | 54 (13·6) | 34 (11·3) | 20 (20·6) | 0·031 |
| Rescue therapies | ||||
| Prone positioning | 189 (47·5) | 129 (42·9) | 60 (61·9) | 0·002 |
| Extracorporeal CO2 removal | 28 (7·0) | 20 (6·6) | 8 (8·2) | 0·758 |
| ECMO | 11 (2·8) | 7 (2·3) | 4 (4·1) | 0·560 |
| Inhaled nitric oxide | 6 (1·5) | 3 (1·0) | 3 (3·1) | 0·320 |
| Adverse events | ||||
| ARDS | 293 (73·6) | 203 (67·4) | 90 (92·8) | <0·001 |
| Acute kidney injury | 114 (28·6) | 62 (20·6) | 52 (53·6) | <0·001 |
| Hemodynamic shock | 92 (23·1) | 42 (14·0) | 50 (51·5) | <0·001 |
| Acute cardiac injury | 23 (5·8) | 9 (3·0) | 14 (14·4) | <0·001 |
| Bacteraemia* | 66 (16·6) | 46 (15·3) | 20 (20·6) | 0·284 |
| Fungaemia* | 8 (2·0) | 5 (1·7) | 3 (3·1) | 0·647 |
| Off-label and compassionate use therapies | ||||
| No experimental therapies | 133 (33·4) | 102 (33·9) | 31 (32·0) | 0·726 |
| Chloroquine/ Hydroxychloroquine | 236 (59·3) | 178 (59·1) | 58 (59·8) | 1·000 |
| Lopinavir/ Ritonavir | 112 (28·1) | 85 (28·2) | 27 (27·8) | 1·000 |
| Corticosteroids | 66 (16·6) | 43 (14·3) | 23 (23·7) | 0·044 |
| Tocilizumab | 71 (17·8) | 59 (19·6) | 12 (12·4) | 0·143 |
| Remdesivir | 23 (5·8) | 18 (6·0) | 5 (5·2) | 0·958 |
| Other Antivirals | 26 (6·5) | 21 (7·0) | 5 (5·2) | 0·693 |
| Interferon therapy | 8 (2·0) | 4 (1·3) | 4 (4·1) | 0·197 |
| Extracorporeal cytokine adsorption and plasma exchange therapy | 4 (1·0) | 0 (0·0) | 4 (4·1) | 0·003 |
| Intravenous IgG | 1 (0·3) | 1 (0·3) | 0 (0·0) | 1·000 |
| Number of simultaneous experimental therapies | 2 [1 - 3] | 2 [1 - 3] | 2 [1 - 3] | 0·858 |
| Simultaneous use of off-label therapies | 0·869 | |||
| 1 off-label therapy | 105 (26·4) | 79 (26·2) | 26 (26·8) | |
| 2 off-label therapies | 77 (19·3) | 59 (19·6) | 18 (18·6) | |
| 3 off-label therapies | 49 (12·3) | 35 (11·6) | 14 (14·4) | |
| >3 off-label therapies | 34 (8·5) | 26 (8·6) | 8 (8·2) | |
| Treatment withdrawal and length of stay | ||||
| Withdrawal of life supporting therapies | 73 (18·3) | 0 (0·0) | 73 (72·3) | <0·001 |
| ICU length of stay, days | 12 [5 - 21] | 12 [5 - 21] | 12 [5 - 21] | 0·782 |
Values are given as median [IQR] or count (percent) as appropriate. NIV, non-invasive ventilation; P/F ratio, PaO2/ FiO2 ratio; ARDS, acute respiratory distress syndrome; ECMO, extracorporeal membrane oxygenation; IgG, Immunoglobulin G; ICU, intensive care unit. *59 (86·7%) and 7 (87·5%) of all bacterial and fungal bloodstream infections developed in patients with off-label therapies.
3.2. ICU management
Of the 398 patients discharged from the ICU or who died, 274 (68·8%) patients were mechanically ventilated (Table 3). The mortality rate in these patients was 32%. There was no difference in mortality between patients intubated upon ICU admission versus those intubated at a later stage (Suppl. Figure 1). ARDS was diagnosed in 293 (73·6%) patients, with 131 (32·9%) presenting severe ARDS. The lowest median P/F ratio in the cohort was 110 [80–148] mmHg during the initial seven days of ICU treatment (Table 3). Prone positioning was applied in 189 (47·5%) patients at least once during the ICU stay, further 28 (7·0%) and 11 (2·8%) patients underwent ECCO2R and ECMO therapy, respectively. Vasopressors were prescribed in 236 (68·8%) patients during their ICU stay. Acute circulatory failure occurred in 92 (23·1%) patients, resulting in death in 52% of cases. A total of 114 (28·6%) and 23 (5·8%) patients suffered acute kidney and acute cardiac injury, respectively; 54 (13·6%) required renal replacement therapy. 16 (17·4%) of the 92 patients with acute circulatory failure suffered acute cardiac injury, of which 11 died and one of them received ECMO therapy.
Regarding co-infections, 66 (16·6%) patients had positive blood cultures for bacteria and eight patients developed fungaemia. In 265 (66·6%) patients, off-label and compassionate use therapies against COVID-19 were prescribed, and 160 (60·4%) of these patients received a combination of more than one treatment, with hydroxychloroquine and ritonavir/lopinavir being the most frequent (236 (89·1%) and 112 (43·3%) patients). Notably, all but ten (89·1%) patients with bloodstream infections with bacteria or fungi were undergoing treatment with off-label therapies, representing a risk ratio (RR) of 3·2 with a 95% confidence interval (CI) of 1·7 – 6·0 (p < 0·001). Corticosteroid and tocilizumab administration was associated with bloodstream infection in 43 (56·6%; RR = 4·2, 95% CI [2·2 – 8·0], p < 0·0001), and hydroxychloroquine in 23 (30·2%; RR = 1·3, 95% CI [0·6 – 2·6], p = 0·475) cases, seven of which were fungaemias.
3.3. Disease course through the first seven ICU days
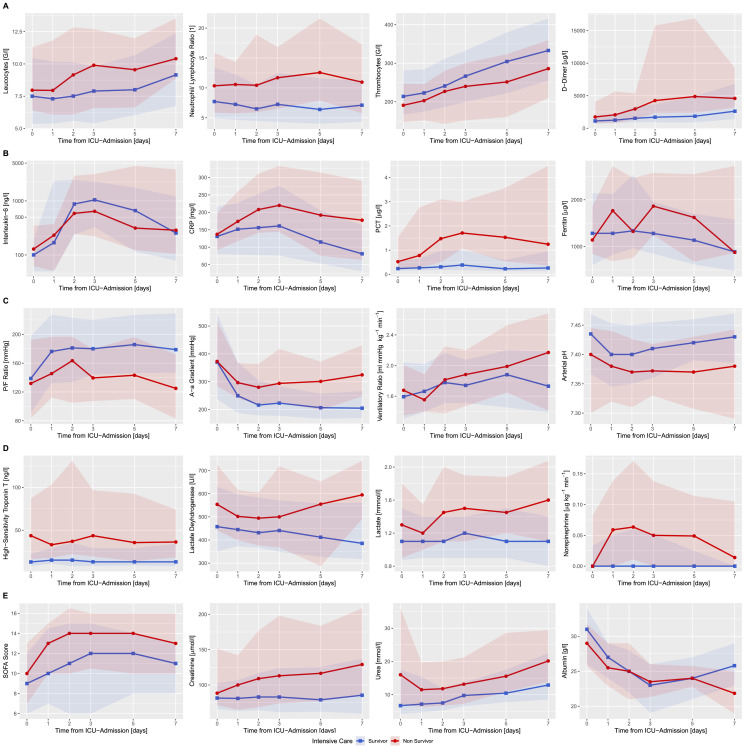
Levels of Interleukin-6 (IL-6), C-reactive protein (CRP), procalcitonin (PCT) levels and white blood cell (WBC) count increased over time, peaking between days two and three (Fig. 1A - B, Suppl. Table 3). In ICU non-survivors, the WBC count was persistently higher during the first seven days of ICU stay (p<0·01). No difference in initial IL-6 (p = 0·70) and CRP (p = 0·41) levels was observed; however, ICU non-survivors were characterized by rising CRP dynamics after ICU admission (p<0·001). The neutrophil to lymphocyte ratio was persistently higher in ICU non-survivors (p<0·001, Fig. 1A, Suppl. Table 3). Platelet count increased in all patients, with ICU survivors presenting consistently higher counts during the first seven days (p<0·001, Fig. 1A, Suppl. Table 3). d-Dimer (p = 0·01) and lactate dehydrogenase (LDH) (p<0·01) levels remained elevated in patients with unfavorable outcome (Fig. 1A, D, Suppl. Table 3). Overall organ dysfunction assessed with the Sequential Organ Failure Assessment (SOFA) score, albeit initially comparable in ICU non-survivors and survivors, diverged after day one and remained consistently worse in ICU non-survivors (p<0·0001) (Fig. 2E, Suppl. Table 3). The course of arterial lactate levels (p<0·0001) and pH (p<0·0001) further distinctly differentiated patients between non-survivors and survivors (Fig. 2D, C, Suppl. Table 3). Pulmonary function, as measured by the P/F ratio (p<0·0001) and the alveolar-arterial gradient (p<0·0001), improved within the first week in ICU survivors as opposed to non-survivors (Fig. 2C, Suppl. Table 3). Troponin T was substantially elevated in ICU non-survivors (p = 0·01). Creatinine levels remained consistently elevated (p<0·0001) and diverged between ICU survivors and non-survivors after the third day (p<0·0001, Fig. 2D, E, Suppl. Table 3).
Fig. 1.
Temporal progression of organ function, vital, and laboratory parameters over the initial seven days of ICU stay. A) Development of blood cell counts and coagulation markers, (B) inflammatory biomarkers, (C) lung function, (D) circulatory system function and (E) kidney and overall organ function, within the first seven days of ICU treatment of critically ill patients suffering from COVID-19 stratified by ICU mortality. Lines represent the median values, shaded areas the interquartile range.
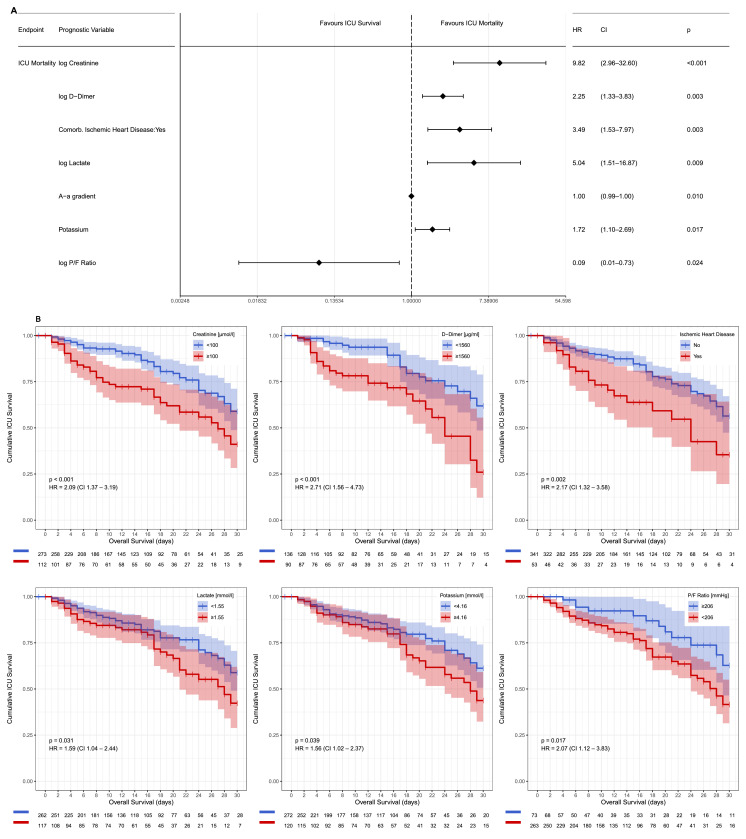
Fig. 2.
Risk factors associated with ICU mortality. Prognostication of ICU mortality in a multivariable Cox proportional hazards regression model was visualized in a Forrest plot (A). Kaplan-Meier analysis of six of the defining model components (creatinine, d-dimer, lactate and potassium levels, the P/F ratio and ischemic heart disease) demonstrate their effect on ICU mortality over time; patients discharged alive from the ICU are noted as censored (B).
3.4. Prognostic value of patient characteristics at icu admission
In a univariable Cox regression model, crude hazard ratios (HR) for 27 parameters were associated with an unfavorable ICU outcome (Suppl. Figure 2). On the multivariable Cox proportional hazard regression model the following parameters at admission were independently associated with ICU mortality: creatinine, d-dimer, lactate, and potassium levels, P/F ratio and alveolar-arterial gradient, and history of ischemic heart disease (Fig. 2A). The inclusion of d-dimer levels into the Cox proportional hazards regression model, albeit reducing final model sample size due to missing values (Suppl. Table 1), resulted in hazard ratios similar to the higher sample size model without d-dimers. Kaplan-Meier survival analysis for all seven parameters demonstrated a distinction between ICU survivors and non-survivors for all multivariable independent predictors using the cut-off values resulting from ROC analyses (Fig. 2B, Suppl. Figure 3).
4. Discussion
This prospective, European cohort study provides an initial description of the baseline characteristics, treatments, and outcome of critically ill critically ill COVID-19 patients included in the RISC-19-ICU registry during the peak of the COVID-19 pandemic in early 2020, constituting a near real-time view of a large international cohort. ICU admission and treatment data point to a systemic disease characterized by a cytokine and cellular-driven inflammatory and coagulation activation, severe pulmonary oxygenation deficit, and in approximately 24% of cases progression to multi-organ failure and death. Univariable and multivariable Cox proportional hazards regression modeling identified several prognostic markers for ICU mortality, most notably markers of oxygenation deficit, renal and microvascular dysfunction, and coagulatory activation.
In the present study, the demographics and baseline characteristics of patients who became critically ill due to COVID-19 were predominantly male, middle-aged, and with comorbidities. These findings are in concordance with previous case series, most of which had a predominantly regional or national focus [4,5,7,8,17]. The degree of ARDS severity observed in the present cohort was higher than that reported in other critically ill COVID-19 populations to date [4,5,7,17]. Nevertheless, the incidence of mechanical ventilation in our cohort was lower than that reported in case series from Northern Italy and the United States [7,18]. By contrast, a considerably higher proportion of patients in our cohort received rescue therapies including prone positioning, inhaled nitric oxide and extracorporeal decarboxylation and oxygenation. These differences may reflect the large variability in therapeutic approaches applied in our cohort related to the international scope of this study. Given the relatively low mortality rate in our compared to other reports [4,5,7,8,17,19], together with the lack of clear evidence regarding the optimal respiratory management of critically ill COVID-19 patients, our findings suggest that a wide range of therapeutic approaches—which reflect the particular expertise of the participating hospitals—could be successful strategies in treating critically ill COVID-19 patients. In ICU non-survivors, the extent of forgoing of life-supporting therapies was found to be similar in this cohort of critically ill COVID-19 patients as previously described in European ICUs in a non-pandemic setting [20].
The use of off-label and compassionate use therapies, as well as the combination of multiple empirical drugs, was common in the present cohort, a finding that is consistent with other case series [4,5,19,21]. As evidenced in recent publications, many of these therapies were initially hypothesized to be effective [22,23] and broadly adopted, but subsequently failed to show clear evidence of effectiveness [24,25]. In this regard, our findings add to previous concerns regarding off-label medication, particularly immunosuppressive therapies [26]. The high incidence of bloodstream infections in patients treated with off-label therapies in the present cohort, especially those who received corticosteroids and IL-6 anti-body therapies, underscore the WHO recommendation to limit the use of empirical therapies to the controlled setting of clinical trials [27].
Recently, it has been postulated that multiple parameters may have potential prognostic capacity to discern unfavorable outcomes in general populations of hospitalized COVID-19 patients [4,5,17,28]. The multivariable Cox proportional hazards regression model applied in the present comparatively large, international patient cohort identified several independent predictors of mortality in critically ill COVID-19 patients. While markers of coagulation activation and microvascular dysfunction such as d-dimer and lactate levels, together with markers of renal dysfunction, were positively associated with ICU mortality, an inverse association was found for the P/F ratio as a measure of oxygenation deficit. These findings support previous observations of the presence of severe inflammatory reaction [5] and endothelial dysfunction [6] in these patients, thereby providing a plausible pathophysiological correlate to the severely decreased P/F ratio due to alveolar fluid accumulation. This would explain the initially highly compliant lungs with severely impaired gas diffusion that is pathognomonic for this disease. [29,30] The persistent inflammatory activation and increased recruitment of neutrophils and non-resolving lymphopenia observed in our study—mainly in non-survivors—could explain the transition in certain patients to the classic non-compliant ARDS phenotype later in the course of disease, as previously suggested [29]. Even though the systemic pro-inflammatory state observed in the present cohort confirms previous data [4,5,17], our findings suggest that IL-6 and PCT levels may be less prognostic than previously proposed [4,17,28]. In the present study, our focus on severe cases for which outcome data were available for a high proportion of patients, facilitates the systematic investigation of pathophysiologic processes. By contrast, most previous reports have assessed general hospitalized patient populations with only limited outcome data [4,7]. Ischemic heart disease was the sole predisposing condition assessed in this study that retained an association with ICU mortality on multivariable analysis. Similar d-dimer levels were found in critically ill patients with or without this predisposing condition, presenting no obvious link to coagulatory activation. Ischemic heart disease has been described in previous studies involving general hospitalized cohorts, including non-critically ill patients [31,32], where other conditions such as chronic arterial hypertension, diabetes mellitus, age, and body mass index were also implicated. Prognostic analyses that conjointly model non-critically and critically ill patients to infer hospital mortality without adjusting for disease severity are ultimately at risk of selection bias. The RISC-19-ICU registry provides the prerequisites for the development of risk scores in critically ill patients, and due to its collaborative nature the data presented here could be combined with databases of similar scope for joint data analysis.
The limitations of the present study pertain mainly to the prospective data collection, which was performed in highly variable settings at 93 different collaborating centres at the peak of an unprecedented public health crisis. While missing values due to local differences in laboratory capability or resource availability were present and could potentially have led to effect over- or underestimation, efforts were made to mitigate this variability by rigorous monitoring of data quality and the use of linear mixed model analysis for the descriptive analysis. Further, lead-time bias was moderated by alignment of the data collection time points to the onset of critical disease status. Survival analysis during an ongoing crisis is associated with a potential survivorship bias in favor of patients with a short ICU length of stay with potentially more severe cases still residing in the ICU. However, by including into the outcome analysis only patients that had already been discharged from the ICU, censoring of the patients that were discharged from the ICU alive could be applied in the Cox proportional hazards model to account for the possibility of an unfavorable outcome during the further hospitalization and thus reduce the potential for additional bias. While hospital outcomes and follow-up assessments may be analysed in a future retrospective analysis, the present study is capable of providing insight during the ongoing pandemic. Finally, due to the international study design, the resources, policies and therapeutic approaches utilized in the participating centres and countries presumably were highly heterogeneous, which should be considered when interpreting the results presented here. Correction for clustering was not implemented into the statistical models to prevent an increased risk of type II error in light of the reduced number of patients admitted to certain centres at the time point of analysis as previously described [33]. This heterogeneity, however, could provide the basis to perform regional or resource-centered subgroup analyses in the future.
In conclusion, the European RISC-19-ICU cohort demonstrates a moderate ICU mortality of 24% in critically ill patients with COVID-19. Despite a high degree of ARDS severity, the incidence of mechanical ventilation was low and associated with a higher proportion of rescue therapies, which included prone positioning, inhaled nitric oxide and extracorporeal decarboxylation and oxygenation therapies. In contrast to previously reported risk factors for mortality in hospitalized COVID-19 patients, our findings suggest that only creatinine, d-dimer, lactate, potassium, P/F ratio and alveolar-arterial gradient at admission and ischemic heart disease are predictors of mortality in critically ill patients with COVID-19. The elevated risk of bloodstream infections associated with empirical therapies, especially corticosteroids and tocilizumab, underscores the need to exercise caution with the use of off-label therapies.
Contributors
PDWG, RAS, TF, JM, PG, and MPH conceived, designed and supervised the registry and this study. PDWG, DMH, FRC, and MPH acquired and interpreted the clinical data. PDWG and MPH processed statistical data. PDWG and MPH drafted the manuscript. PDWG, RAS, TF, DMH, JM, PG, FRC, and MPH critically revised the manuscript for important intellectual content. PDWG and MPH had full access to the study data and take full responsibility for the integrity and the accuracy of the data analysis. PDWG had full responsibility for the decision to submit the manuscript for publication in EClinicalMedicine.
Declaration of Competing Interest
The authors declare no conflicts of interest regarding the present study.
Acknowledgments
RISC-19-ICU Investigators
Andorra: Unidad de Cuidados Intensivos, Hospital Nostra Senyora de Meritxell, Escaldes-Engordany (Mario Alfaro Farias, MD; Antoni Margarit, MD; Gerardo Vizmanos-Lamotte, MD). Austria: Department for Anesthesiology and Intensive Care, Johannes Kepler University Linz, Linz (Thomas Tschoellitsch, MD; Jens Meier, MD); Dept. Of Pediatrics, Medical University Vienna, Vienna (Francesco S. Cardona, MD, MSc). Czech Republic: Klinika anesteziologie perioperacni a intenzivni mediciny, Masaryk Hospital, Usti nad Labem (Josef Skola, MD; Lenka Horakova, MD). Ecuador: Unidad de Cuidados Intensivos, Hospital Vicente Corral Moscoso, Cuenca (Hernan Aguirre-Bermeo, MD, PhD; Janina Apolo, BSc). France: SCPARE-Intensive Care Unit, Clinique Louis Pasteur, Essey-lès-Nancy (Geoffrey Jurkolow, MD; Gauthier Delahaye, MD); Department of Anesthesiology and Critical Care Medicine, University Hospital of Nancy, Nancy (Emmanuel Novy, MD; Marie-Reine Losser, MD, PhD). Germany: Department of Medicine III - Interdisciplinary Medical Intensive Care, Medical Center University of Freiburg, Freiburg (Tobias Wengenmayer, MD; Dawid L. Staudacher, MD); Medical Intensive Care, Medical School Hannover, Hannover (Sascha David, MD; Tobias Welte, MD). Greece: Intensive Care Unit, St. Paul General Hospital of Thessaloniki, Thessaloniki (Theodoros Aslanidis, MD). Hungary: Department of Anaesthesia and Intensive Care, Semmelweis University, Budapest (Janos Gal, MD, PhD; Hermann Csaba, MD, PhD); Departement of Anaethesiology and Intensive Care, University of Szeged, Hungary (Barna Babik, MD, PhD; Anita Korsos, MD). Italy: Anesthesia and Intensive Care Unit, Azienda Ospedaliero Universitaria Ospedali Riuniti, Ancona (Abele Donati, MD, PhD; Andrea Carsetti, MD); Anesthesia and Intensive care, Azienda Ospedaliero-Universitaria di Ferrara, Cona (Alberto Fogagnolo, MD; Savino Spadaro, MD, PhD); UO Anestesia e Terapia Intensiva, IRCCS Centro Cardiologico Monzino, Milan (Roberto Ceriani, MD; Martina Murrone, MD); Department of Internal Medicine, ASST Fatebenefratelli Sacco - “Luigi Sacco” Hospital, Milan (Maddalena Alessandra Wu, MD; Chiara Cogliati, MD); Division of Anesthesia and Intensive Care, ASST Fatebenefratelli Sacco - “Luigi Sacco” Hospital, Milan (Riccardo Colombo, MD; Emanuele Catena, MD); Internal Medicine, Azienda Ospedaliera Universitaria di Modena, Modena (Fabrizio Turrini, MD, MSc; Maria Sole Simonini, MD); UOC Anestesia e Rianimazione, Ospedale Infermi, Rimini (Francesca Facondini, MD; Antonella Potalivo, MD); UO Pronto Soccorso Medicina d’Urgenza, Ospedale Infermi, Rimini (Gianfilippo Gangitano, MD; Tiziana Perin, MD); Department of Anesthesiology and Intensive Care Medicine, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome (Maria Grazia Bocci, MD; Massimo Antonelli, MD); Department of Anesthesia and Intensive Care Medicine, Policlinico San Marco, Zingonia (Emanuele Rezoagli, MD, PhD; Giovanni Vitale, MD). Netherlands: Department of Intensive Care Medicine, Erasmus Medical Center, Rotterdam (Diederik Gommers, MD, PhD; Can Ince, PhD). Spain: Intensive Care, Complejo Hospitalario Universitario A Coruña, A Coruña (Raquel Rodriguez Garcia, MD; Jorge Gamez Zapata, MD); Medical Intensive Care Unit, Hospital Clinic de Barcelona, Barcelona (Pedro Castro, MD; Adrian Tellez, MD); Anesthesiology Intensive Care Unit, Hospital Clinic de Barcelona, Barcelona (Adriana Jacas, MD; Guido Muñoz, MD); Acute Critical Cardiac Care Unit, Hospital Clinic de Barcelona, Barcelona (Rut Andrea, MD; Jose Ortiz, MD); Cardiovascular Surgery Critical Care Unit, Hospital Clinic de Barcelona, Barcelona (Eduard Quintana, MD; Irene Rovira, MD); Liver Intensive Care Unit, Hospital Clinic de Barcelona, Barcelona (Enric Reverter, MD; Javier Fernandez, MD); Respiratory Intensive Care Unit, Hospital Clinic de Barcelona, Barcelona (Miquel Ferrer, MD; Joan R. Badia, MD); Servicio de Medicina Intensiva, Hospital General San Jorge, Huesca (Arantxa Lander Azcona, MD; Jesus Escos Orta, MD); Servicio de Medicina Intensiva, Hospital Universitario de Torrejon, Madrid (Maria Cruz Martin Delgado, MD); Servei de Medicina intensiva, Hospital Verge de la Cinta, Tortosa (Eric Mayor-Vázquez, MD); Unidad de Cuidados Intensivos, Hospital Clinico Universitario Lozano Blesa, Zaragoza (Begoña Zalba-Etayo, MD, PhD; Herminia Lozano-Gomez, MD). Switzerland: Klinik für Operative Intensivmedizin, Kantonsspital Aarau, Aarau (Rolf Ensner, MD); Medizinische Intensivstation, Kantonsspital Aarau, Aarau (Marc Philippe Michot, MD; Alexander Klarer); Intensivstation, Kantonsspital Schaffhausen, Schaffhausen (Nadine Gehring, MD); Institut fuer Anesthaesie und Intensivmedizin, Zuger Kantonsspital AG, Baar (Peter Schott, MD; Severin Urech, MD); Department Intensivmedizin, Universitaetsspital Basel, Basel (Martin Siegemund, MD; Nuria Zellweger); Intensivmedizin, St. Claraspital, Basel (Adriana Lambert, MD; Lukas Merki, MD); Interdisziplinaere Intensivmedizin, Lindenhofspital, Bern (Jan Wiegand, MD); Department of Intensive Care Medicine, University Hospital Bern, Inselspital, Bern (Marie-Madlen Jeitziner, RN, PhD; Beatrice Jenni-Moser, RN, MSc); Department Intensive Care Medicine, Spitalzentrum Biel, Biel (Marcus Laube, MD); Interdisziplinaere Intensivstation, Spital Buelach, Buelach (Bernd Yuen, MD; Thomas Hillermann, MD); Intensivstation, Regionalspital Emmental AG, Burgdorf (Petra Salomon, MD; Iris Drvaric, MD); Intensivmedizin, Kantonsspital Graubuenden, Chur (Frank Hillgaertner, MD; Marianne Sieber); Institut fuer Anaesthesie und Intensivmedizin, Spital Thurgau, Frauenfeld (Alexander Dullenkopf, MD; Lina Petersen, MD); Soins Intensifs, Hopital cantonal de Fribourg, Fribourg (Hatem Ksouri, MD, PhD; Govind Oliver Sridharan, MD); Division of Intensive Care, University Hospitals of Geneva, Geneva (Sara Cereghetti, MD; Filippo Boroli, MD; Jerome Pugin, MD, PhD); Division of Neonatal and Pediatric Intensive Care, University Hospitals of Geneva, Geneva (Serge Grazioli, MD; Peter C. Rimensberger, MD); Intensivstation, Spital Grabs, Grabs (Christian Bürkle, MD); Institut für Anaesthesiologie Intensivmedizin & Rettungsmedizin, See-Spital Horgen & Kilchberg, Horgen (Julien Marrel, MD; Mirko Brenni, MD); Soins Intensifs, Hirslanden Clinique Cecil, Lausanne (Isabelle Fleisch, MD; Jerome Lavanchy, MD); Soins intensifs de pédiatrie, CHUV, Lausanne (Anne-Sylvie Ramelet, MD; Marie-Helene Perez, MD); Anaesthesie und Intensivmedizin, Kantonsspital Baselland, Liestal (Anja Baltussen Weber, MD; Peter Gerecke, MD; Andreas Christ, MD); Dipartimento Area Critica, Clinica Luganese Moncucco, Lugano (Romano Mauri, MD; Samuele Ceruti, MD); Interdisziplinaere Intensivstation, Spital Maennedorf AG, Maennedorf (Katharina Marquardt, MD; Karim Shaikh, MD); Institut fuer Anaesthesie und Intensivmedizin, Spital Thurgau, Muensterlingen (Thomas Neff, MD; Tobias Hübner, MD); Intensivmedizin, Schweizer Paraplegikerzentrum Nottwil, Nottwil (Hermann Redecker, MD); Soins intensifs, Groupement Hospitalier de l'Ouest Lémanique, Hôpital de Nyon, Nyon (Thierry Fumeaux, MD; Mallory Moret-Bochatay, MD); Intensivmedizin & Intermediate Care, Kantonsspital Olten, Olten (Michael Studhalter, MD); Intensivmedizin, Spital Oberengadin, Samedan (Michael Stephan, MD; Jan Brem, MD); Anaesthesie Intensivmedizin Schmerzmedizin, Spital Schwyz, Schwyz (Daniela Selz, MD; Didier Naon, MD); Medizinische Intensivstation, Kantonsspital St. Gallen, St. Gallen (Gian-Reto Kleger, MD); Departement of Anesthesiology and Intensive Care Medicine, Kantonsspital St. Gallen, St. Gallen (Miodrag Filipovic, MD; Urs Pietsch, MD); Paediatric Intensive Care Unit, Children’s Hospital of Eastern Switzerland, St. Gallen (Bjarte Rogdo, MD; Andre Birkenmaier, MD); Departement for intensive care medicine, Kantonsspital Nidwalden, Stans (Anette Ristic, MD; Michael Sepulcri, MD); Intensivstation, Spital Simmental-Thun-Saanenland AG, Thun (Antje Heise, MD); Klinik für Anaesthesie und Intensivmedizin, Spitalzentrum Oberwallis, Visp (Friederike Meyer zu Bentrup, MD, MBA); Service d'Anesthesiologie, EHNV, Yverdon- les-Bains (Marilene Franchitti Laurent, MD; Jean-Christophe Laurent, MD); Institute of Intensive Care Medicine, University Hospital Zurich, Zurich (Philipp Bühler, MD; Silvio Brugger, MD, PhD; Daniel Hofmaenner, MD; Simone Unseld, MD; Annelies Zinkernagel, MD, PhD); Interdisziplinaere Intensivstation, Stadtspital Triemli, Zurich (Patricia Fodor, MD; Pascal Locher, MD; Giovanni Camen, MD); Abteilung für Anaesthesiologie und Intensivmedizin, Hirslanden Klinik Im Park, Zurich (Tomislav Gaspert, MD; Marija Jovic, MD); Institut für Anaesthesiologie und Intensivmedizin, Klinik Hirslanden, Zurich (Christoph Haberthuer, MD; Roger F. Lussman, MD). United Kingdom: Harefield Hospital, Royal Brompton & Harefield NHS Foundation Trust, Harefield (Nandor Marczin, MD, PhD; Joyce Wong, MD).
Acknowledgments
This work is funded and endorsed by the Swiss Society of Intensive Care Medicine and funded by the Institute of Intensive Care Medicine at the University Hospital of Zurich with an unrestricted research grant. We thank medical writer Bradley Londres for editorial assistance with this manuscript. Finally, we want to thank all physicians and nurses in our collaborating centers for their tireless and brave efforts in patient treatment and care, without you this health care emergency could not be contained. For Manuel.
Funding
Swiss Society of Intensive Care Medicine & Institute of Intensive Care Medicine, University Hospital Zurich.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100449.
Contributor Information
Pedro David Wendel Garcia, Email: pedrodavid.wendelgarcia@usz.ch.
Thierry Fumeaux, Email: thierry.fumeaux@ghol.ch.
Dorothea Monika Heuberger, Email: dorotheamonika.heuberger@usz.ch.
Reto Andreas Schuepbach, Email: reto.schuepbach@usz.ch.
Matthias Peter Hilty, Email: matthias.hilty@usz.ch.
RISC-19-ICU Investigators:
Mario Alfaro Farias, Antoni Margarit, Gerardo Vizmanos-Lamotte, Thomas Tschoellitsch, Jens Meier, Francesco S. Cardona, Josef Skola, Lenka Horakova, Hernan Aguirre-Bermeo, Janina Apolo, Emmanuel Novy, Marie-Reine Losser, Geoffrey Jurkolow, Gauthier Delahaye, Sascha David, Tobias Welte, Tobias Wengenmayer, Dawid L. Staudacher, Theodoros Aslanidis, Barna Babik, Anita Korsos, Janos Gal, Hermann Csaba, Abele Donati, Andrea Carsetti, Fabrizio Turrini, Maria Sole Simonini, Roberto Ceriani, Martina Murrone, Emanuele Rezoagli, Giovanni Vitale, Alberto Fogagnolo, Savino Spadaro, Maddalena Alessandra Wu, Chiara Cogliati, Riccardo Colombo, Emanuele Catena, Francesca Facondini, Antonella Potalivo, Gianfilippo Gangitano, Tiziana Perin, Maria Grazia Bocci, Massimo Antonelli, Diederik Gommers, Can Ince, Eric Mayor-Vázquez, Maria Cruz, Martin Delgado, Raquel Rodriguez Garcia, Jorge Gamez Zapata, Begoña Zalba-Etayo, Herminia Lozano-Gomez, Pedro Castro, Adrian Tellez, Adriana Jacas, Guido Muñoz, Rut Andrea, Jose Ortiz, Eduard Quintana, Irene Rovira, Enric Reverter, Javier Fernandez, Miquel Ferrer, Joan R. Badia, Arantxa Lander Azcona, Jesus Escos Orta, Philipp Bühler, Silvio Brugger, Daniel Hofmaenner, Simone Unseld, Frank Ruschitzka, Mallory Moret-Bochatay, Bernd Yuen, Thomas Hillermann, Hatem Ksouri, Govind Oliver Sridharan, Anette Ristic, Michael Sepulcri, Miodrag Filipovic, Urs Pietsch, Petra Salomon, Iris Drvaric, Peter Schott, Severin Urech, Adriana Lambert, Lukas Merki, Marcus Laube, Frank Hillgaertner, Marianne Sieber, Alexander Dullenkopf, Lina Petersen, Serge Grazioli, Peter C. Rimensberger, Isabelle Fleisch, Jerome Lavanchy, Katharina Marquardt, Karim Shaikh, Hermann Redecker, Michael Stephan, Jan Brem, Bjarte Rogdo, Andre Birkenmaier, Friederike Meyer zu Bentrup, Patricia Fodor, Pascal Locher, Giovanni Camen, Martin Siegemund, Nuria Zellweger, Marie-Madlen Jeitziner, Beatrice Jenni-Moser, Christian Bürkle, Gian-Reto Kleger, Marilene Franchitti Laurent, Jean-Christophe Laurent, Tomislav Gaspert, Marija Jovic, Michael Studhalter, Christoph Haberthuer, Roger F. Lussman, Daniela Selz, Didier Naon, Romano Mauri, Samuele Ceruti, Julien Marrel, Mirko Brenni, Rolf Ensner, Nadine Gehring, Antje Heise, Tobias Huebner, Thomas A. Neff, Sara Cereghetti, Filippo Boroli, Jerome Pugin, Nandor Marczin, and Joyce Wong
Appendix. Supplementary materials
References
- 1.Chan J.F.-.W., Yuan S., Kok K.-.H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronaviridae Study G. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization W.H. Coronavirus disease 2019 (COVID-19): situation report, 93. 2020.
- 4.Zhou F., Yu T., Du R.H. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C.L., Wang Y.M., Li X.W. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020 doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson S., Hirsch J.S., Narasimhan M. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 10.Organization W.H. World Health Organization; 2020. Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: interim guidance, 2 march 2020. [Google Scholar]
- 11.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilty M.P., Wendel Garcia P.D. hobbes8080/risc-19-icu: registry data transformation v1.0. Zenodo Data Repository. 2020 [Google Scholar]
- 13.Force A.D.T., Ranieri V., Rubenfeld G., Thompson B., Ferguson N., Caldwell E. Acute respiratory distress syndrome. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 14.Group K.W. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2(1):1–138. [Google Scholar]
- 15.Thygesen K., Alpert J.S., Jaffe A.S. Fourth universal definition of myocardial infarction (2018) Eur Heart J. 2019;40(3):237–269. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- Team R.C..R.: A language and environment for statistical computing. 2013.
- 17.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arentz M., Yim E., Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020 doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sprung C.L., Cohen S.L., Sjokvist P. End-of-life practices in European intensive care unitsthe ethicus study. JAMA. 2003;290(6):790–797. doi: 10.1001/jama.290.6.790. [DOI] [PubMed] [Google Scholar]
- 21.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat Publish Group. 2020 doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 23.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao B., Wang Y., Wen D. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. New England J Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grein J., Ohmagari N., Shin D. Compassionate use of remdesivir for patients with severe Covid-19. New England J Med. 2020 doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalil A.C. Treating COVID-19—off-label drug use, compassionate use, and randomized clinical trials during pandemics. JAMA. 2020 doi: 10.1001/jama.2020.4742. [DOI] [PubMed] [Google Scholar]
- 27.Organization W.H. World Health Organization; 2020. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 march 2020. [Google Scholar]
- 28.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intens Care Med. 2020 doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gattinoni L., Chiumello D., Caironi P. COVID-19 pneumonia: different respiratory treatments for different phenotypes. Intens Care Med. 2020:1. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gattinoni L., Coppola S., Cressoni M., Busana M., Chiumello D. Covid-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen R., Liang W., Jiang M. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020 doi: 10.1016/j.chest.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gong J., Ou J., Qiu X. A Tool to Early Predict Severe Corona Virus Disease 2019 (COVID-19): a Multicenter Study using the Risk Nomogram in Wuhan and Guangdong, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Austin P.C., Leckie G. The effect of number of clusters and cluster size on statistical power and Type I error rates when testing random effects variance components in multilevel linear and logistic regression models. J Stat Comput Simul. 2018;88(16):3151–3163. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.